Abstract
Background
Polyomavirus BK (BKV) infection characterized by viruria alone is considered to be of little clinical significance, but this issue has not been systematically studied.
Methods
We studied 230 patients with sustained viruria in whom multiple samples taken after a median of 877 days (range 24–2739) showed no progression to viremia or nephropathy. Biopsies satisfying Banff thresholds for inflammation and tubulitis in the presence of viruria but negative BKV stains were designated as putative T-cell mediated acute rejection.
Results
Compared to no viruria (n=515), sustained viruria was associated with more putative rejection episodes (0.62 versus 0.33 per patient, p=0.006), and greater incidence of steroid resistance (36.2% versus 19.6%, p=0.002). Most putative rejection episodes (52.1%) occurred concurrently with viruria, with a minority prior to (7.8%) or after (40.1%) BKV clearance. Steroid resistance was more frequent in putative rejection with concurrent viruria (48.6%), compared to rejection prior to (9.1%) or after viral clearance (26.0%). These observations remained valid even on separate analysis of patients with BKV load < 1E+07 copies per ml. As assessed by the slope of reciprocal serum creatinine, accelerated deterioration of graft function resulted from rejection episodes occurring >2 years post-transplant.
Conclusions
These observations indicate that intra-renal viral replication in sustained viruria is frequently associated with putative acute rejection. The implications of this association on development of immune tolerance deserve further investigation.
Keywords: Polyomavirus, rejection, viruria, viremia, immune
INTRODUCTION
Polyomavirus BK (BKV) causes ubiquitous infection in early childhood, and 46–94% of the adult population is seropositive (1–4). Primary infection is usually asymptomatic, but leads to viral latency in the genitourinary tract. Reactivation may occur in conditions associated with impaired immunity and is seen in 10–68% of kidney transplant recipients. Infection commences as viruria, which progresses to viremia and histologic nephropathy. In the late 90’s graft loss due to BKV nephropathy ranged from 50 to >80% of cases (5, 6). More recently, widespread screening using urine cytology or PCR has permitted earlier diagnosis, and current graft loss rates have fallen to about 20% (7). To prevent disease progression to an irreversible phase, The Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines suggest reduction of immunosuppression when BK viral load in plasma is persistently greater than 10,000 genomic equivalents per ml (8). There is a widespread belief in the transplant community that viruria alone is a finding of little immediate consequence to the patient. For this reason, many medical centers do not screen for BKV in the urine, and prefer to monitor plasma instead. However, in our current clinical practice, which utilizes regular BKV screening in both urine and plasma, we frequently see patients with prolonged viruria that does not progress to viremia or nephropathy. It is well recognized that viruria per se leads to up regulation of inflammatory markers such as HLA-DR, CD54, IL-6, IL-3 and granzyme B in the urine (9–11). We hypothesized that such persistent inflammation would modulate graft function and possibly affect long-term outcome. Accordingly, we identified 230 patients with sustained viruria to examine the natural history of BKV infection in this clinical setting.
RESULTS
Study material
The inclusion criteria for including a patient in this retrospective cohort study were (a) Recipient of a kidney transplant, (b) urine and plasma PCR performed on at least 4 occasions. The exclusion criteria were (a) insufficient sampling density for inclusion into the study, (b) documented BK viremia at any time point, and (c) biopsy documented BKV nephropathy. A total of 1002 patients satisfied the inclusion criteria out of 1708 who had received a kidney allograft between January 2002 and April 2010 (IRB protocol # 0602155). Testing policy was not uniform: initially only patients with graft dysfunction were tested for BKV infection, but in 2006 a deliberate screening policy was implemented. Data collected retrospectively for this study indicated that samples obtained every 1 to 3 months for 1 year were available for analysis in 705 of 1002 patients. Among 1002 patients initially identified, 102 (10.2%) patients had both viremia and viruria, of which 21 (20.6%) went on to develop biopsy proven viral nephropathy. These viremic subjects were excluded from further analysis as the natural history of viremia in kidney transplant patients is well described. Data from 900 viruric and non-viruric patients was analyzed for the purpose of this study.
Categorization of viral status
In 515/900 patients (57.2%), urine BKV DNA was below the detection threshold of <200 copies/ml laboratory (12). These subjects were designated ‘Negative for BK viruria’. Viruria not complicated by viremia was seen in 385/900 (42.8%) patients. These subjects were sub-classified as:
Indeterminate for BK viruria (n=98): Urine viral load ≥200 and <1,000 copies/ml in one or more samples.
Transient viruria (n=57): viral load ≥1,000 copies/ml in a single sample, taken at a median of 391 days post-transplant.
Sustained viruria (n=230): ≥1,000 copies/ml in 2 or more tests, taken at a median of 118 days post-transplant.
Importantly, none of these patients developed BK viremia during follow up of 877 days (range 24–2739).
Demographic data
Detailed clinical characteristics of patients in different study categories are presented as supplementary material (Supplemental digital content [SDC], Table 1). Briefly, the majority (86%) of patients during the study period received preconditioning with one dose of alemtuzumab induction (Campath 1H, humanized CD52 monoclonal antibody) and two boluses of methylprednisolone (mPSL) followed by post-transplant steroid-free Tacrolimus (Tac) maintenance monotherapy. Mycophenolate mofetil (MMF) or prednisolone (PSL) was added in high risk patients and in those who developed acute rejection. In 628/900 (70.0%), tacrolimus monotherapy was maintained throughout the study period. There were no statistically significant differences in the type of immunosuppression administered to patients who developed BK viruria versus those who did not. The percentage of patients who had only one transplant (labeled as ‘First Graft’ in SDC, Table 1) was lower in the viruria negative and indeterminate groups (p<0.05, Chi-square test). Patients with sustained viruria had an increased number of HLA-B mismatches compared to the subjects with indeterminate and transient viruria (p<0.05). Amongst the 230 patients with sustained viruria, 156 showed a single episode of prolonged viruria varying in duration from 21–377 days (median 146), while the remainder showed multiple episodes. The peak viral loads in the indeterminate, transient and sustained viruria categories were 5.0E+02, 5.8E+03 and 1.2E+07 genomic equivalents per ml.
Table 1.
Frequency, timing of acute rejection episodes, and response to antirejection treatment*
| Negative (n=515) | Indeterminate (n=98) | Transient (n=57) | Sustained (n=230) | |
|---|---|---|---|---|
| Episodes per patient | 0.33 | 0.48 | 0.43 | 0.62 |
| Rejection episode count** | ||||
| None | 392 (76.1%) | 71 (72.4%) | 40 (70.2%) | 159 (69.1%) |
| Once | 84 (16.3%) | 13 (13.3%) | 12 (21.0%) | 28 (12.2%) |
| Twice | 31 (6.0%) | 9 (9.2%) | 2 (3.5%) | 23 (10.0%) |
| 3 times | 8 (1.6%) | 4 (4.1%) | 3 (5.3%) | 13 (5.7%) |
| 4 times | 0 (0.0%) | 1 (1.0%) | 0 (0.0%) | 6 (2.6%) |
| 5 times | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.4%) |
|
| ||||
| Timing of rejection episodes after transplantation | ||||
| <6 mo | 24 (14.1%) | 4 (8.5%) | 6 (24.0%) | 26 (18.3%) |
| 6 mo–1 yr | 39 (22.9%) | 8 (17.0%) | 5 (20.0%) | 22 (15.5%) |
| 1 – 2 yr | 55 (32.4%) | 15 (31.9%) | 6 (24.0%) | 32 (22.5%) |
| >2 yr | 52 (30.6%) | 20 (42.6%) | 8 (32.0%) | 62 (43.7%) |
| Total episodes | 170 (100%) | 47 (100%) | 25 (100%) | 142 (100%) |
|
| ||||
| Number of rejection episodes treated with bolus methylprednisolone | ||||
| 163/170 (95.9%) | 41/47 (87.2%) | 23/25 (92.0%) | 127/142 (89.4%) | |
| Response to treatment | ||||
| CR*** | 85 (52.2%) | 17 (41.5%) | 11 (47.8%) | 44 (34.7%) |
| PR*** | 46 (28.2%) | 13 (31.7%) | 8 (34.8%) | 37 (29.1%) |
| NR | 32 (19.6%) | 11 (26.8%) | 4 (17.4%) | 46 (36.2%) |
CR; complete response, PR; partial response, NR; non-response.
Including bolus mPSL with or without intravenous immunoglobulin, thymoglobulin and adding of MMF.
Episode counts samong 4 temporal categories were compared with Kruskal-Wallis test (p=0.05). Comparison in each two groups by Mann-Whitney U test revealed the significant difference only between sustained and negative groups (p=0.006, significant p<0.05 with Bonferroni correction).
Application of the chi square test to this 3 × 4 contingency table resulted in an overall p-value = 0.03. On further subanalysis, the frequency of complete response (52.2%) and any response (80.4%) was higher in the BKV negative group compared to the rate of complete response (34.7%) and any response ( 63.8%) in the sustained viruria group ( p= 0.003, and p=0.002 respectively, significant p<0.05 with Bonferroni correction).
Evaluation of acute rejection
The cumulative incidence of rejection in BKV-negative, indeterminate, transient and sustained viruria categories were essentially similar at 28.1%, 33.3% 29.0% and 36.7%, respectively (Figure 1). These high rates reflect our immunosuppressive regimen characterized by Campath induction, low dose Tacrolimus monotherapy, and early steroid withdrawal. However, tacrolimus trough levels were similar amongst the study groups, and this argues against intensity of immunosuppression being responsible for differences in rejection rates between the study groups (SDC, Figure 1). When the total number of rejection episodes was enumerated, the BKV negative group had proportionally fewer episodes per patient over a median follow up period of 1069 days (range 131–2926) (Table 1). On pairwise comparison, the frequency of rejection in patients with sustained viruria was higher than what was observed in the BKV negative group (0.62 versus 0.33 episodes per patient, p=0.006). Importantly, this increased frequency of rejection patients was seen even in patients generally considered to have low urine viral load defined as <1E+07 genomic equivalents per ml.
Figure 1.
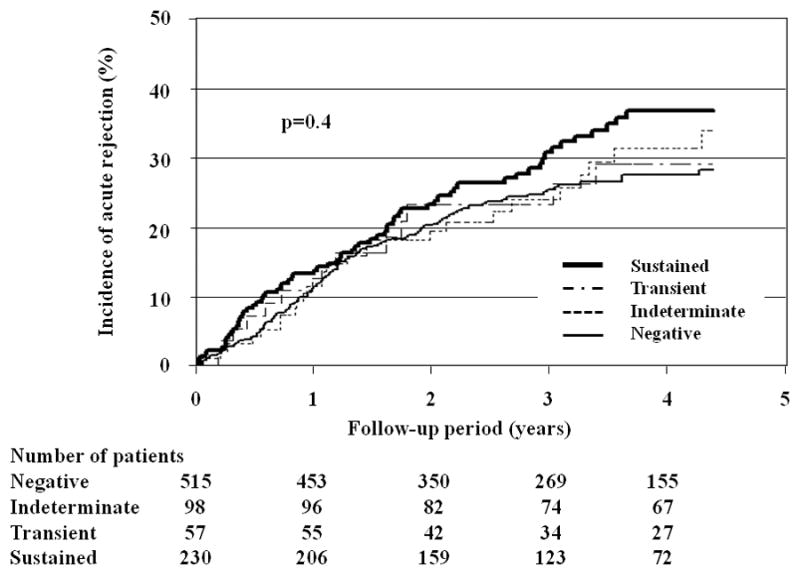
Kaplan-Meier curves showing the incidence of acute rejection in different categories of patients classified by viral status. The curves were truncated at 4.3 years, a time period that corresponds to the 75 percentile of available graft follow-up period.
This increased frequency of rejection in patients with sustained viruria could not be explained by differences in basic demographic information, the type of immunosuppressive protocol used, or mean trough Tacrolimus levels. All 281 episodes of viruria in this study were examined for changes in immunosuppression immediately following detection of viral DNA in the urine. The incidence of acute rejection was not significantly different in patients in whom immunosuppression had been reduced (20/118, 16.9%) compared to those in whom it had been maintained (n= 43/163, 26.3%).
Response to Treatment
Non-response to anti-rejection treatment was seen more often (36.2%) in patients with sustained viruria compared to no viruria (19.6%) (Table 1). On multivariate logistic regression analysis steroid resistant putative T-cells mediated acute rejection could be predicted by the presence of sustained viruria (Odds ratio 2.15, 95% CI 1.30 – 3.54, p = 0.003) and Banff score for interstitial fibrosis (Odds ratio 2.03, 95% CI 1.26 – 3.32, SDC, Table 2). Non-response to steroid treatment was seen more often in putative rejection episodes concurrent with viruria than in rejection episodes occurring prior to onset of or following clearance of viruria (48.6%, 9.1% and 26.0%, respectively) (Table 2).
Table 2.
Response to steroid therapy stratified by temporal relationship to onset of sustained viruria1
| Before viruria | During viruria | After viruria | |
|---|---|---|---|
| No. of episode | 11 | 66 | 50 |
| CR2 | 6 (54.5%) | 17 (25.7%) | 21 (42.0%) |
| PR3, 4 | 4 (36.4%) | 17 (25.7%) | 16 (32.0%) |
| NR | 1 (9.1%) | 32 (48.6%) | 13 (26.0%) |
CR; complete response, PR; partial response, NR; non-response.
Overall p-value for 3 × 3 contingency table = 0.03
P-value for 2 × 2 comparison of CR before (54.5%) vs. during (25.7%) viruria = 0.02
P-value for 2 × 2 comparison of any response (CR or PR) before (90.9%) vs. during (51.4%) viruria = 0.01
P=0.01 significant after Bonferroni correction.
Graft function as measured be serum creatinine in did not significantly change during the observation period (SDC, Figure 2). However, examination of the slope of serum creatinine for rejection episodes occurring >2 years post-transplant in patients with sustained viruria demonstrated accelerated deterioration of graft function (slope −0.058±0.076 before vs. −0.11±0.13 [mg/dl]−1/year after rejection, p=0.02) (Figure 2). There were no group-specific differences in graft survival by Kaplan-Meier analyses (Figure 3).
Figure 2.
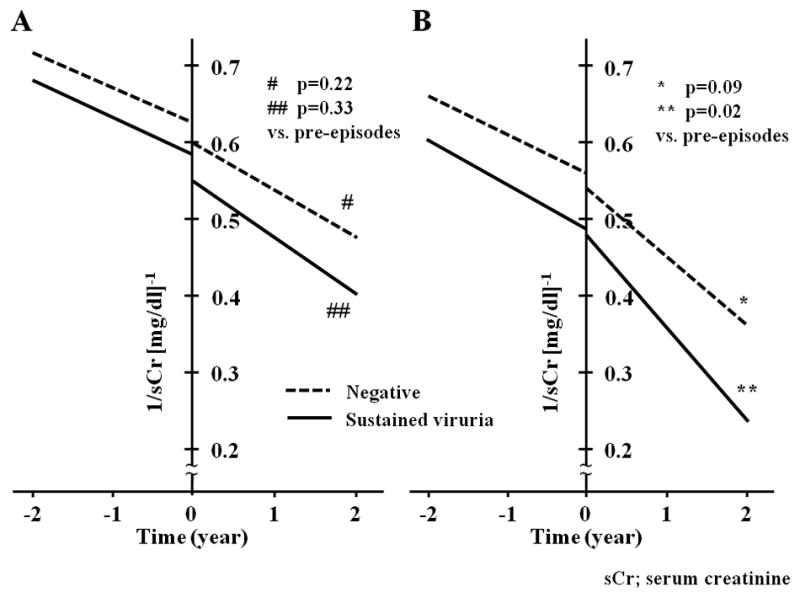
Changes in the slope of serum creatinine in patients with documented acute rejection. Regression lines based on the mean Y-intercept and mean slope of reciprocal serum creatinine are drawn separately for rejection episodes that occurred between 1–2 years (A) or later than 2 years post-transplantation (B). The vertical line at time zero corresponds to the time of biopsy which documented acute rejection. Patients with sustained viruria are represented by the continuous line, while those negative for viruria are depicted by a dashed line. It can be observed that rejection episodes occurring >2 years post-transplant in patients with sustained viruria were associated with accelerated deterioration of graft function (slope −0.058±0.076 before vs. −0.11±0.13 [mg/dl]−1/year after rejection, p=0.02). Rejection in patients without viruria also appeared to have a similar detrimental effect, but it did not reach statistical significance (−0.051±0.055 vs. −0.092±0.14 [mg/dl]−1/year, p=0.09). The mean y-intercept of the slope of reciprocal serum creatinine in sustained viruria patients prior to occurrence of rejection was lower than that observed in the BKV negative group (0.49±0.14 vs. 0.57±0.17 [mg/dl]−1, p=0.02), indicating worse graft function at the time of diagnosis of rejection. The aforementioned differences were not seen on analysis of rejection episodes occurring between 1–2 years post-transplantation.
* p=0.09 slope of BKV negative patients after (−0.092±0.14 [mg/dl]−1/year) versus before (−0.051±0.055 [mg/dl]−1/year) rejection episodes (paired t-test) ** p=0.02 slope of sustained viruria patients after (−0.11±0.13 [mg/dl]−1/year) versus before (−0.058±0.076[mg/dl]−1/year) the rejection episode (paired t-test)
[Explanatory note for Figure 2: The plotted data is derived from a mean of 16±9 serum creatinine measurements before and 13±8 measurements after each rejection episode analyzed (minimum of three measurements in each case spanning a period of 3 or more months). Acute changes in serum creatinine triggered by the rejection episode were excluded from analysis. Rejection episodes that led to graft loss within 3 months (12 in sustained viruria group and 6 in BKV negative group) were also considered to be unsuitable for this analysis. After applying these criteria, data for calculation of slopes was available for 73 of 94 rejection episodes (77.7%) in sustained viruria and 90 of 107 episodes (84.1%) in the BKV negative group. The difference between early and late rejection episodes remained statistically significant after a linear mixed model was used to correct for multiple samples from the same patient.
Figure 3.
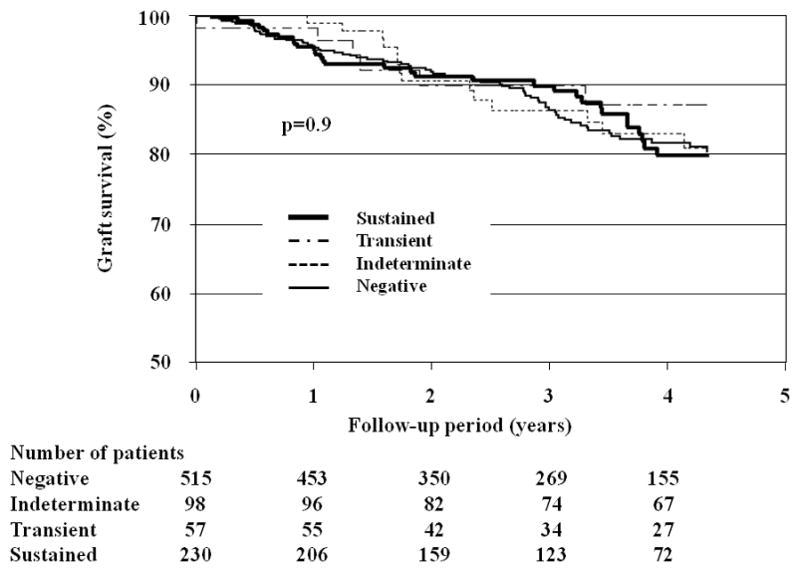
Graft survival in patients classified by BKV status. There were no group-specific differences in either graft or patient survival by Kaplan-Meier analyses. Kaplan-Meier curves were truncated at 4.3 years after the transplantation for graft survival, and 4.5 years for patient survival, because of the small number of patients with follow-up afterwards (75th percentile shown). In the sustained viruria group: 19 grafts failed because of interstitial fibrosis-tubular atrophy (IF/TA). Sixteen (16) patients died with a functioning graft because of heart disease, stroke or malignancy.
The median graft follow up in BKV negative indeterminate, transient and sustained viruria groups were 3.1 (range 0.4–8.1) 3.5 (range 0.5–8.7), 3.6 (range 0.3–8.1) and 3.2 (range 0.4–8.3) years, respectively. Considering patients with functioning grafts, the last serum creatinine (median, range) in the aforementioned groups were also comparable at 1.4 (0.5–12.5), 1.4 (0.4–9.8), 1.4 (0.7–6.2) and 1.6 (0.6–9.8) mg/dl respectively.
Discussion
The spectrum of BKV infection after kidney transplant extends from asymptomatic viral reactivation to tissue destructive disease. The later stages in the evolution of infection, namely BK viremia and biopsy proven viral nephropathy, have been extensively studied in recent years. Lesser attention has been paid to the stage of viruria, on the assumption that it is a condition of no immediate clinical consequence when not accompanied by viremia. However, patients with viruria do show over-expression of inflammatory markers such as HLA-DR, CD54, IL-6, IL-3, and granzyme B in the urine (9–11). Smoldering subclinical inflammation has the potential to result in progressive graft injury. Therefore, we felt it was important to identify a cohort of patients who show persistent viruria and determine their graft function over an extended period of time.
Our data suggests but does not prove that prolonged viruria is associated with enhancement of the immune response, which manifests as inflammatory infiltrates consistent with T-cell mediated acute rejection, as defined by current Banff criteria. Although the overall incidence of acute rejection in the SVU group is 36%, 52% of acute rejection episodes occurred during the period of viruria. It is noteworthy, the median duration of viruria was only 13% of the median observation period for patients in this study. An association between persistent viral excretion and acute rejection has been noted in two smaller series of patients treated with conventional immunosuppression: Babel et al reported an overall acute rejection rate of 29.5% in a series of on 233 patients, of whom 28 developed SVU (13). A higher number of putative acute rejection episodes was seen in persistent viral excretors (1.33 +/− 2.33 episodes per patient) compared to subjects with transient or no BK viruria (0.28+/−1.03 episodes per patient). Hardinger et al reported an acute rejection incidence of 14% in 36 patients with sustained viruria followed up for 5 years (14). Analysis of the national SRTR database revealed that patients treated for BKV infection (viremia or nephropathy) had a 4.8 fold increase of acute rejection 1–12 months after transplantation (15). The specific proportion of rejection episodes that actually occurred during the period of active viral excretion is not specified in either of the aforementioned investigations. This study confirms these observations using a substantially larger cohort of 230 patients with sustained viruria, who did not develop viremia after a median period of observation of 877 days (range 24–2739). Additionally, we have shown that these putative rejection episodes tend to be more steroid resistant, and are associated with demonstrable deterioration of graft function if they occur >2 years post-transplantation. The relationship between viral infection and acute rejection is known to be bidirectional: viral infection can trigger acute rejection or vice versa. In a study by Babel et al, 11/24 (46%) of rejection episodes preceded viral reactivation (13). One could argue that in these cases increased immunosuppression administered to treat acute rejection resulted in viral reactivation. By comparison, 42% of putative acute rejection episodes were detected at the same time as viruria, while the remaining 12% were diagnosed within 3 day time interval of that event. It is likely that in these cases viral replication stimulated anti-viral immunity as well as allogeneic immunity directed against the graft. This phenomenon has been well documented in a murine model of polyomavirus nephropathy (16). In our series, the percentages of acute rejection diagnosed before and concurrently with sustained viruria were 7.8% and 52.1% respectively. Additionally, a substantial percent (40.4%) was diagnosed after clearance of viruria. It is difficult to determine whether the latter cases are related to reduced immunosuppression in response to sustained viruria, or are a manifestation of immune reconstitution by BKV antigen-specific T-cells. While its existence in kidney transplant patients is not well documented, the immune reconstitution syndrome is recognized in the resolution phase of many bacterial, fungal, and viral infections (17–19). It is believed that in this condition the immune-inflammatory response that facilitates clearance of virus from the tissues results in collateral damage by the innocent bystander mechanism. The complex pathogenesis of the inflammatory infiltrates in biopsies taken during the course of sustained viruria explains why many of these cases do not respond to conventional anti-rejection treatment (20, 21).
Previous studies of kidney, lung and liver transplant recipients have documented acute and chronic rejection during and after other viral infections, particularly influenza virus and cytomegalovirus (CMV) infection (22–24). The mechanisms are well-studied in CMV, and include up-regulation of cytokines, such as tumor necrosis factor-alpha or interferon-gamma, up regulation of major histocompatibility complex antigens and adhesion molecules (25–27), and activation of CD4+ and CD8+ T-cells by virus infected allogeneic endothelial cells (28). It is notable that CD4+ and CD8+ T-cells responding to BKV antigens also produce the same cytokines (29–31).
It is important to point out that the diagnosis of putative acute rejection in this study was based on current Banff criteria, wherein any inflammation and tubulitis in the biopsy is interpreted as acute rejection, as long as in-situ hybridization for BKV DNA is negative. This may not be always true: rejection-like infiltrates that are probably a response to viral infection have been noted in viruric patients in other publications (20, 32, 33). Currently, there are no laboratory methods available to conclusively demonstrate that these infiltrates are in fact a response to viral infection. Indeed, it is our belief that many biopsies taken in the context of active BK viruria contain T-cells sensitized to both MHC as well as BKV antigens.
The principal limitation of this study is that it is a retrospective analysis of existing data from a medical center, which has used T-cell depletion and Tacrolimus monotherapy in a majority of the patients studied. The frequency of BKV testing was not uniform and only 1002/1708 (58.7%) of patients had sufficient sampling density to be included in the study. The PCR assay used was targeted at the relatively polymorphic VP-1 gene, and this may have resulted in the underestimation of viral load in less common viral strains. Nevertheless, the prevalence of BK viruria, viremia and nephropathy in this study, which were 38.4%, 8.1% and 2.1%, respectively, are comparable to what has been reported in the literature in patients with diverse immunosuppressive regimens (34–36). Uniform follow up was not available and no protocol biopsies were performed to enable delineation of the tissue pathology associated with sustained viruria. One also needs to consider the possibility that the higher incidence of acute rejection may reflect more careful clinical follow up of patients known to be excreting BK virus.
In summary, sustained BK viruria is associated with multiple and refractory episodes of AR. Graft function is demonstrably compromised in patients who develop an episode of rejection > 2 years post-transplant. Therefore, patients with sustained viruria should be carefully monitored for AR and promptly treated when graft dysfunction develops. Furthermore all patients in this category need continuous monitoring for development of viremia and nephropathy.
METHODS
Clinical acute rejection
T-cell mediated acute rejection (AR), frequently referred to as acute rejection throughout the manuscript, was clinically defined as increase in serum creatinine >15% above baseline with biopsy confirmation (≥Banff grade IA ). Serum creatinine response 4 weeks after treatment was grouped in 3 categories: if treatment led to >70% reversal in the rise of creatinine, it was classified as “complete response (CR)”. A 30–70% reversal qualified for “partial response (PR)”, and <30% reversal or no change or progression was designated as “no response (NR)”.
Laboratory studies
Urine and plasma PCR were performed using a published assay (12). The mean tacrolimus trough level was obtained 1, 3, 6, 9, 12, 18, 24 months after transplantation. Since the majority of patients were on tacrolimus monotherapy, this provided an index of the overall exposure to immunosuppression at each time point.
Patient management and graft outcome
T-cell mediated rejection was initially treated with steroids. Anti-T-cell therapy was given to refractory cases in the BKV negative group. Reduction of tacrolimus dose (25–50%) was effected in patients with BK viruria only if viral load was persistently >1E+07/ml. Graft loss was defined as return to dialysis, re-transplantation or death with a functioning graft. Analysis of the cause of death was based on a perusal of medical records, review of biopsy findings, and examination of allograft nephrectomy specimens, when available.
Histologic studies
Allograft biopsies were performed when patients showed unexplained rise in serum creatinine. The diagnosis of T-cell mediated rejection was based on Banff ‘97criteria for renal allograft pathology (37). This designation was unequivocal in BKV negative patients and in viruric patients where biopsies preceded the first positive PCR test for BKV. However, in biopsies taken concurrent with or following the first documentation of viruria, the term putative acute T-cell mediated rejection was used since routine biopsy evaluation can not determine whether interstitial inflammation and tubulitis in biopsy tissue are a response to viral or donor MHC antigens. Data on donor specific antibodies and C4d staining was not consistently available. However, our earlier studies do not support a relationship between occurrence of BKV infection and antibody mediated rejection (38).
Statistical analysis
JMP version 7 (SAS Institute, Cary, NC, USA) was used for all statistical analyses. Results were presented as mean ± SD, or as median and range as appropriate. Student t-test, Mann-Whitney U test and Kruskal-Wallis test were used to assess differences of unpaired data. Bonferroni corrections were applied for multiple comparisons as indicated in the tables. The chi-square test was used for categorical data. Kaplan-Meier estimates (from day zero to 75thpercentile of the follow up period) and log-rank tests were used to evaluate the incidence of acute rejection, graft survival and patient survival. Tacrolimus trough levels were compared with repeated measure analysis of variance (ANOVA). The paired t-test was used to compare the slopes before and after rejection episodes. P values less than 0.05 were considered statistically significant. The effect of clinical parameters on response to steroid treatment was analyzed by multiple logistic regression using variables that were significant on univariate analysis at the 0.10 level (HLA-B mismatch, history of second transplant, presence of sustained viruria, acute rejection Banff grade 1B or higher, interstitial fibrosis of grade 2 or above in the Banff Schema).
Supplementary Material
Acknowledgments
Supported by NIH grant # RO1 AI 51227 to PR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Institute of Allergy and Infectious Disease. Kosuke Masutani was supported by International Research Fund for Subsidy of Kyushu University School of Medicine Alumni. Jill March provided excellent secretarial assistance.
Abbreviations
- AR
acute rejection
- BKV
Polyomavirus BK
- CR
complete response
- MHC
major histocompatibility complex
- HLA
human leukocyte antigen
- IFTA
interstitial fibrosis and tubular atrophy
- MMF
Mycophenolate mofetil
- mPSL
Methyl Prednisolone
- NR
no response
- PR
partial response
- PSL
Prednisolone
- SVU
sustained viruria
- Tac
Tacrolimus
Footnotes
K.M. collected the clinical data and reviewed the pathology with P.R. Data analysis, and manuscript writing is the work of all the authors, none of whom have any conflict of interest.
References
- 1.Brennan DC, Agha I, Bohl DL, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005;5:582. doi: 10.1111/j.1600-6143.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 2.Hariharan S. BK virus nephritis after renal transplantation. Kidney Int. 2006;69:655. doi: 10.1038/sj.ki.5000040. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch HH, Randhawa P the American Socitety of Transplantation Infectious Disease Community of Practice. BK virus in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S136. doi: 10.1111/j.1600-6143.2009.02904.x. [DOI] [PubMed] [Google Scholar]
- 4.Ramos E, Hirsch HH. Polyomavirus-associated nephropathy: updates on a persisting challenge. Transpl Infect Dis. 2006;8:59. doi: 10.1111/j.1399-3062.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- 5.Randhawa PS, Finkelstein S, Scantlebury V, et al. Human polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation. 1999;67:103. doi: 10.1097/00007890-199901150-00018. [DOI] [PubMed] [Google Scholar]
- 6.Binet I, Nickeleit V, Hirsh HH, et al. Polyomavirus disease under new immunosuppressive drugs: a cause of renal graft dysfunction and graft loss. Transplantation. 1999;67:918. doi: 10.1097/00007890-199903270-00022. [DOI] [PubMed] [Google Scholar]
- 7.Wadei HM, Rule AD, Lewin M, et al. Kidney transplant function and histological clearance of virus following diagnosis of polyomavirus-associated nephropathy (PVAN) Am J Transplant. 2006;6:1025. doi: 10.1111/j.1600-6143.2006.01296.x. [DOI] [PubMed] [Google Scholar]
- 8.Eckardt KU, Kasiske BL. Kidney disease: improving global outcomes. Nat Rev Nephrol. 2009;5:650. doi: 10.1038/nrneph.2009.153. [DOI] [PubMed] [Google Scholar]
- 9.Dadhania D, Snopkowski C, Ding R, et al. Validation of noninvasive diagnosis of BK virus nephropathy and identification of prognostic biomarkers. Transplantation. 2010;90:189. doi: 10.1097/TP.0b013e3181e2a932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SH, Ahn HJ, Kim YS, et al. Urinary HLA-DR and CD54 expression -indicators for inflammatory activity in decoy cell shedding patients. Nephrol Dial Transplant. 2006;21:2601. doi: 10.1093/ndt/gfl253. [DOI] [PubMed] [Google Scholar]
- 11.Sadeghi M, Daniel V, Schnitzler P, et al. Urinary proinflammatory cytokine response in renal transplant recipients with polyomavirus BK viruria. Transplantation. 2009;88:1109. doi: 10.1097/TP.0b013e3181ba0e17. [DOI] [PubMed] [Google Scholar]
- 12.Randhawa P, Ho A, Shapiro R, et al. Correlates of quantitative measurement of BK polyomavirus (BKV) DNA with clinical course of BKV infection in renal transplant patients. J Clin Microbiol. 2004;42:1176. doi: 10.1128/JCM.42.3.1176-1180.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babel N, Fendt J, Karaivanov S, et al. Sustained BK viruria as an early marker for the development of BKV-associated nephropathy: analysis of 4128 urine and serum samples. Transplantation. 2009;88:89. doi: 10.1097/TP.0b013e3181aa8f62. [DOI] [PubMed] [Google Scholar]
- 14.Hardinger KL, Koch MJ, Bohl D, et al. BK-virus and the impact of pre-emptive immunosuppression reduction: 5-year results. Am J Transplant. 2010;10:407. doi: 10.1111/j.1600-6143.2009.02952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schold JD, Rehman S, Kayler LK, et al. Treatment of BK virus: incidence, risk factors and outcomes for kidney transplant recipients in the United States. Transplant Int. 2009;22:626. doi: 10.1111/j.1432-2277.2009.00842.x. [DOI] [PubMed] [Google Scholar]
- 16.Han-Lee E, Kemball CC, Wang J, et al. A Mouse Model for Polyomavirus-Associated Nephropathy of Kidney Transplants. Am J Transplant. 2006;6:913. doi: 10.1111/j.1600-6143.2006.01265.x. [DOI] [PubMed] [Google Scholar]
- 17.Ingram PR, Howman R, Leahy MF, et al. Cryptococcal immune reconstitution inflammatory syndrome following alemtuzumab therapy. Clin Infect Dis. 2007;44:e115. doi: 10.1086/518168. [DOI] [PubMed] [Google Scholar]
- 18.Travis J, Varma A, duPlessis D, et al. Immune reconstitution associated with progressive multifocal leukoencephalopathy in human immunodeficiency virus: a case discussion and review of the literature. Neurologist. 2008;14:321. doi: 10.1097/NRL.0b013e31816e2f13. [DOI] [PubMed] [Google Scholar]
- 19.Sinha S, Sharma SK. Tuberculosis associated immune reconstitution inflammatory syndrome. Indian J Tuberc. 2010;57:177. [PubMed] [Google Scholar]
- 20.Batal I, Franco ZM, Shapiro R, et al. Clinicopathologic analysis of patients with BK viruria and rejection-like graft dysfunction. Hum Pathol. 2009;40:1312. doi: 10.1016/j.humpath.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Kayler LK, Batal I, Mohanka R, et al. Antirejection treatment in kidney transplant patients with BK viruria. Transplantation. 2008;86:797. doi: 10.1097/TP.0b013e3181837802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris WT. Viral infection and renal transplant rejection. Br Med J. 1973;1:355. doi: 10.1136/bmj.1.5849.355-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egli A, Binggeli S, Bodaghi S, et al. Cytomegalovirus and polyomavirus BK posttransplant. Nephrol Dial Transplant. 2007;22(Suppl 8):viii72–viii82. doi: 10.1093/ndt/gfm648. [DOI] [PubMed] [Google Scholar]
- 24.Humar A, Gillingham KJ, Payne WD, et al. Association between cytomegalovirus disease and chronic rejection in kidney transplant recipients. Transplantation. 1999;68:1879. doi: 10.1097/00007890-199912270-00011. [DOI] [PubMed] [Google Scholar]
- 25.Fietze E, Prösch S, Reinke P, et al. Cytomegalovirus infection in transplant recipients. The role of tumor necrosis factor. Transplantation. 1994;58:675. [PubMed] [Google Scholar]
- 26.Tornatore KM, Garey KW, Saigal N, et al. Ganciclovir pharmacokinetics and cytokine dynamics in renal transplant recipients with cytomegalovirus infection. Clin Transplant. 2001;15:297. doi: 10.1034/j.1399-0012.2001.150501.x. [DOI] [PubMed] [Google Scholar]
- 27.Hosenpud JD, Chou SW, Wagner CR. Cytomegalovirus-induced regulation of major histocompatibility complex class I antigen expression in human aortic smooth muscle cells. Transplantation. 1991;52:896. doi: 10.1097/00007890-199111000-00027. [DOI] [PubMed] [Google Scholar]
- 28.Waldman WJ, Knight DA. Cytokine-mediated induction of endothelial adhesion molecule and histocompatibility leukocyte antigen expression by cytomegalovirus-activated T cells. Am J Pathol. 1996;148:105. [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou W, Sharma M, Martinez J, et al. Functional characterization of BK virus-specific CD4+ T cells with cytotoxic potential in seropositive adults. Viral Immunol. 2007;20:379. doi: 10.1089/vim.2007.0030. [DOI] [PubMed] [Google Scholar]
- 30.Randhawa PS, Popescu I, Macedo C, et al. Detection of CD8+ T cells sensitized to BK virus large T antigen in healthy volunteers and kidney transplant recipients. Hum Immunol. 2006;67:298. doi: 10.1016/j.humimm.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 31.Binggeli S, Egli A, Schaub S, et al. Polyomavirus BK-specific cellular immune response to VP1 and large T-antigen in kidney transplant recipients. Am J Transplant. 2007;7:1131. doi: 10.1111/j.1600-6143.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- 32.Petrogiannis-Haliotis T, Sakoulas G, Kirby J, et al. BK-related polyomavirus vasculopathy in a renal-transplant recipient. N Engl J Med. 2001;345:1250. doi: 10.1056/NEJMoa010319. [DOI] [PubMed] [Google Scholar]
- 33.McGilvray ID, Lajoie G, Humar A, et al. Polyomavirus infection and acute vascular rejection in a kidney allograft: coincidence or mimicry? Am J Transplant. 2003;3:501. doi: 10.1034/j.1600-6143.2003.00066.x. [DOI] [PubMed] [Google Scholar]
- 34.Viscount HB, Eid AJ, Espy MJ, et al. Polyomavirus polymerase chain reaction as a surrogate marker of polyomavirus-associated nephropathy. Transplantation. 2007;84:340. doi: 10.1097/01.tp.0000275205.41078.51. [DOI] [PubMed] [Google Scholar]
- 35.Randhawa P, Uhrmacher J. A comparative study of BK and JC virus infections in organ transplant recipients. J Med Virol. 2005;77:238. doi: 10.1002/jmv.20442. [DOI] [PubMed] [Google Scholar]
- 36.Pang XL, Doucette K, LeBlanc B, et al. Monitoring of polyomavirus BK virus viruria and viremia in renal allograft recipients by use of a quantitative real-time PCR assay: one-year prospective study. J Clin Microbiol. 2007;45:3568. doi: 10.1128/JCM.00655-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 38.Batal I, Zainah H, Stockhausen S, et al. The significance of renal C4d staining in patients with BK viruria, viremia, and nephropathy. Mod Pathol. 2009;22:1468. doi: 10.1038/modpathol.2009.118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


