Abstract
Aims
The Insulinogenic Index from 0 to 30 min (ΔI 0-30/ΔG0-30), a measure of insulin secretion derived from the early period of the oral glucose tolerance test, predicts future diabetes. However, there are few data on secretory measures from the late oral glucose tolerance test period. We therefore investigated the association of the ratio of the area under the insulin curve to the area under the glucose curve from 60 to 120 min (I/GAUC 60-120) with incident diabetes.
Methods
Participants were 1540 Mexican Americans and non-Hispanic whites in the San Antonio Heart Study who were free of diabetes at baseline. We analysed indices of sensitivity (Matsuda index) and secretion from the early (ΔI0-30/ΔG0-30) and late oral glucose tolerance test periods (I/GAUC 60-120).
Results
A total of 179 participants developed diabetes after 7.5 years. I/GAUC60-120 was an independent predictor of diabetes [odds ratio × 1 SD unit increase, 0.37 (0.26–0.54)] in a model that also included age, sex, ethnicity, body mass index, family history of diabetes, Matsuda index and (ΔI 0-30/ΔG0-30) as covariates. I/GAUC 60–120 increased the C statistic (a test of discrimination) of the model (0.882 vs. 0.875, P = 0.044). I/GAUC 60–120 correctly reclassified one-fifth of individuals with moderate and strong risks of future diabetes. The net reclassification improvement was 0.13 (P < 0.001) and the integrated discrimination improvement was 0.033 (P < 0.001).
Conclusions
An insulin secretory measure derived from the late oral glucose tolerance test period is useful for classifying individuals at risk of future diabetes independently of other risk factors, including insulin sensitivity and a secretory measure from the early oral glucose tolerance test period.
Keywords: epidemiology, insulin resistance, insulin secretion, oral glucose tolerance test, prediction of Type 2 diabetes mellitus
Introduction
First-phase insulin secretion (acute insulin response) derived from the frequently sampled intravenous glucose tolerance test predict conversion to Type 2 diabetes [1,2]. This test is invasive and costly; therefore, simple surrogate indices of insulin secretion have been developed using insulin and glucose concentrations from the fasting state or the early period of the oral glucose tolerance test (OGTT) (e.g. insulinogenic index from 0 to 30 min (ΔI0–30/ΔG0–30)]. These surrogate indices are also predictors of future diabetes [3–9]. Another early insulin secretory defect reported in individuals with normal glucose tolerance and a first-degree relative with Type 2 diabetes is reduced second-phase insulin release measured by a clamp technique [10]. Stumvoll et al. [11] described insulin secretory indices derived from the OGTT (S1PhOGTT and S2PhOGTT) that have strong correlations with first- and second-phase insulin release measured by the hyperglycaemic clamp. However, the ability of these indices to detect individuals at risk of future diabetes has not been explored.
β-Cell dysfunction needs to be interpreted in the context of concomitant insulin resistance [12–14]. We hypothesized that indices of insulin secretion originated from the late OGTT period could add predictive information to ΔI0–30/ΔG0–30. We used simple strategies, such as the change in insulin concentration relative to the change in glucose concentration [15] and the insulin area under the curve (AUC) relative to the glucose AUC [16], to generate indices of secretion from the late OGTT period: (1) the ratio of relative change in insulin concentration to relative change in glucose concentration from 60 min to 120 min (ΔI60–120/ΔG60–120]; and (2) the ratio of the insulin to glucose concentration areas from 60 to 120 min (I/GAUC 60–120]. The aim of the present study, therefore, was to analyse the association of insulin secretory indices from the early and late OGTT periods with incident Type 2 diabetes.
Patients and methods
Study population
The San Antonio Heart Study is a longitudinal, epidemiological study designed to study Type 2 diabetes and cardiovascular disease among Mexican Americans and non-Hispanic whites living in San Antonio, Texas, USA. Protocols were approved by the Institutional Review Board of the University of Texas Health Science Center at San Antonio. Details of the study design have been previously published [17]. Briefly, all Mexican Americans and non-Hispanic whites (men and non-pregnant women) aged 25–64 years that resided in randomly selected households from low-, middle- and high-income census tracts were invited to participate. All subjects gave written informed consent.
We analysed data from cohort 2 participants, because OGTT sampling times at 30 min and 60 min were not available in cohort 1 participants. Baseline data were collected from January 1984 to December 1988 and follow-up data between October 1991 and October 1996 (mean follow-up of 7.5 years; range 6.3–10.3 years). Incident diabetes was ascertained in 1734 of 2459 [70.5%) participants. Relevant information was missing in 194 participants; therefore, this study presents information on 1540 individuals.
Anthropometric measurements were obtained by trained personnel. Blood specimens were collected before (0 min) and 30, 60 and 120 min after a 75 g oral glucose load (Orangedex; Custom Laboratories, Baltimore, MD, USA) to determine glucose and insulin levels. Serum insulin was measured by a radioimmunoassay (Diagnostic Products Corporation, Los Angeles, CA, USA) that had a high degree of cross-reactivity with proinsulin (70–100%).
We applied the 2003 American Diabetes Association criteria to define diabetes (fasting glucose ≥ 7.0 mmol/l and/or 2-h glucose ≥ 11.1 mmol/l), impaired glucose tolerance (2-h glucose ≥ 7.8 mmol/l and < 11.1 mmol/l), and normal glucose tolerance (2-h glucose < 7.8 mmol/l). Subjects who reported current treatment with glucose-lowering medications were considered to have diabetes.
We used published formulae to calculate homeostasis model assessment of insulin resistance (HOMA IR; basal insulin × basal glucose/22.5) [18] and Matsuda index [104/(basal glucose × basal insulin × mean glucose × mean insulin]0.5) [19]. In this last formula, mean glucose and mean insulin concentrations indicate average glucose (mg/dl) and insulin concentrations (mU/l) based on sampling times at 0, 30, 60 and 120 min. Glucose values at 90 min were not available in the San Antonio Heart Study; however, the Matsuda index based on 0, 30 and 120 min sampling times and the original Matsuda index had similar relationship with clamp-derived insulin sensitivity [20].
We also used published formulas for ΔI0–30/ΔG0–30 (insulin at 30 min − basal insulin/glucose at 30 min − basal glucose) [15], S1PHOGTT [1283 + 1.829 × insulin at 30 min − 138.7 × glucose at 30 min + 3.772 × basal insulin) [11] and S2PHOGTT [287 + 0.4164 × insulin at 30 min − 26.07 × glucose at 30 min + 0.9226 × basal insulin) [11]. The parameter I/GAUC 60–120 was computed as insulin AUC to glucose AUC from 60 to 120 min. The AUCs were calculated by the trapezoidal method. The parameter ΔI60–120/ΔG60–120 was computed as 1 − [(insulin at 60 min − insulin at 120 min/insulin at 60 min)/1 − (glucose at 60 min − glucose at 120 min)/glucose at 60 min]. This formula took into account the negative values resulting from calculating insulin and glucose changes (negative values in 40.3% of the estimates). More importantly, the fit of models with I/GAUC 60–120 (C statistic of a model with the Matsuda index as a covariate, 0.833) was better than that of models with ΔI60–120/ΔG60–120 (C statistic of a model with Matsuda index as a covariate, 0.785, P < 0.001). Thus, I/GAUC 60–120 was used as the index of insulin secretion during the late OGTT period.
Statistical analyses
Statistical analyses were performed with the SAS statistical software (version 9.2; SAS Institute Inc. Cary, NC, USA). Differences in baseline variables by sex and ethnicity were investigated by two-way analysis of covariance for continuous variables or logistic regression for dichotomous variables. Pearson’s partial correlation coefficients were used to analyse the strength of the relationship between variables. Correlation coefficients were compared by the T2 method [21]. The relation of Matsuda index to measures of insulin secretion was also assessed by ordinary least-squares regression as follows: log(secretion measure) = constant + β × log(Matsuda index). We were unable to use a method that accounted for the variability in the measurement of both the dependent and independent variables as previously done for the derivation of both the disposition index (insulin sensitivity index × acute insulin response) on the frequently sampled intravenous glucose tolerance test [13] and insulin secretion-sensitivity index-2 (Matsuda index × insulin AUC to glucose AUC from 0 to 120 min) on OGTT [16]. The risk of future diabetes associated with indices of insulin sensitivity and insulin secretion was determined by logistic regression analysis.
The predictive discrimination was assessed by the C statistic [22]. The C statistic results for different models were compared by bootstrap sampling. We used the Hosmer–Lemeshow goodness-of-fit test, a measure of deviation between observed and expected event rates in deciles of fitted risk values, to assess calibration of a logistic regression model that included age, sex, ethnicity, BMI, family history of diabetes, Matsuda index and ΔI0–30/ΔG0–30 before and after the addition of other indices of secretion. Using these two logistic regression models, we examined measures of reclassification, the net reclassification improvement and the integrated discrimination improvement, to analyse the discriminative value of I/GAUC 60-120. The net reclassification improvement takes into account changes in estimated prediction probabilities that involve a change from one category to another [23]. We used clinically relevant a priori categories (< 1%, 1–5.9%, and ≥ 6% yearly risk of diabetes) based on the yearly risk of developing diabetes among San Antonio Heart Study participants who had normal fasting and 2-h glucose concentrations (0.7% per year) or impaired glucose tolerance (6.1% per year). The integrated discrimination improvement evaluates the change in the estimated prediction probabilities for all possible cut-offs [23]. Log-transformed values of insulin levels and indices of insulin resistance/sensitivity and insulin secretion were used to improve discrimination and calibration of the models and to minimize the influence of extreme observations. We considered a P value < 0.050 significant.
Results
Mexican Americans had higher insulin and glucose levels and more adiposity and family history of diabetes than non-Hispanic whites (Table 1). Indices of insulin resistance and secretion were also higher in Mexican Americans. Men had more insulin resistance and central adiposity and higher fasting and 1-h insulin and glucose concentrations than women. Women had more family history of diabetes and higher 2-h insulin and glucose concentrations. Women also had higher insulin secretion, as measured by ΔI0–30/ΔG0–30, and similar secretion, as measured by the other secretory indices. Ethnicity had an interaction effect on the relation of sex to HOMA IR, Matsuda index, S1PhOGTT and S2PhOGTT.
Table 1.
Age-adjusted baseline characteristics by sex and ethnic origin
| Non-Hispanic whites | Mexican Americans | Ethnicity (P) | Sex (P) | Ethnicity × sex interaction (P) | |||
|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | ||||
| n | 242 | 268 | 425 | 605 | |||
| Age* | 44.1 ± 0.7 | 43.5 ± 0.7 | 42.6 ± 0.5 | 43.7 ± 0.4 | 0.367 | 0.360 | 0.145 |
| BMI (kg/m2) | 26.9 ± 0.3 | 25.5 ± 0.3 | 28.2 ± 0.2 | 28.7 ± 0.2 | < 0.001 | 0.637 | < 0.001 |
| Waist circumference (cm) | 96.0 ± 0.8 | 81.5 ± 0.8 | 94.9 ± 0.6 | 87.2 ± 0.5 | < 0.001 | < 0.001 | < 0.001 |
| Family history of diabetes (%) | 14.4 (10.5–19.5) | 20.1 (15.7–25.3) | 35.1 (30.7–39.7) | 42.2 (38.3–46.9) | < 0.001 | 0.005 | 0.724 |
| Fasting glucose (mmol/l) | 4.82 ± 0.04 | 4.63 ± 0.03 | 4.87 ± 0.03 | 4.72 ± 0.02 | 0.024 | < 0.001 | 0.516 |
| 1-h glucose (mmol/l) | 7.55 ± 0.14 | 6.72 ± 0.13 | 7.90 ± 0.10 | 7.23 ± 0.09 | < 0.001 | < 0.001 | 0.501 |
| 2-h glucose (mmol/l) | 5.21 ± 0.11 | 5.59 ± 0.10 | 5.69 ± 0.08 | 6.25 ± 0.07 | < 0.001 | < 0.001 | 0.349 |
| Fasting insulin (mU/l)† | 9.22 ± 0.52 | 6.94 ± 0.37 | 10.83 ± 0.46 | 10.32 ± 0.46 | < 0.001 | 0.004 | 0.011 |
| 1-h insulin (mU/l)† | 81.0 ± 3.7 | 64.5 ± 2.8 | 104.8 ± 3.6 | 90.5 ± 2.6 | < 0.001 | < 0.001 | 0.291 |
| 2-h insulin (mU/l)† | 39.6 ± 2.5 | 47.9 ± 3.0 | 60.9 ± 2.5 | 79.8 ± 3.3 | < 0.001 | < 0.001 | 0.432 |
| HOMA IR† | 1.96 ± 0.12 | 1.42 ± 0.08 | 2.33 ± 0.10 | 2.15 ± 0.08 | < 0.001 | < 0.001 | 0.011 |
| Matsuda index† | 4.08 ± 0.20 | 5.31 ± 0.25 | 3.16 ± 0.12 | 3.43 ± 0.11 | < 0.001 | < 0.001 | 0.028 |
| ΔI0–30/ΔG0–30† | 1.13 ± 0.06 | 1.30 ± 0.07 | 1.42 ± 0.06 | 1.71 ± 0.06 | < 0.001 | < 0.001 | 0.628 |
| I/GAUC 60–120† | 0.58 ± 0.02 | 0.56 ± 0.02 | 0.75 ± 0.02 | 0.76 ± 0.02 | < 0.001 | 0.924 | 0.440 |
| S1PhOGTT† | 1241 ± 45 | 1170 ± 40 | 1451 ± 39 | 1543 ± 35 | < 0.001 | 0.456 | 0.046 |
| S2PhOGTT† | 331 ± 10 | 307 ± 9 | 385 ± 9 | 397 ± 8 | < 0.001 | 0.777 | 0.042 |
Data are n, mean ± standard error or rates ± 95% confidence intervals.
Non-adjusted values;
Back-transformed from log transformation.
HOMA IR, homeostasis model assessment of insulin resistance; I0–30/G0–30, insulinogenic index from 0 to 30 min; I/GAUC 60–120, ratio of area under the insulin curve to area under the glucose curve from 60 to 120 min; S1PhOGTT and S2PhOGTT, Stumvoll’s first phase and second phase insulin release, respectively.
Correlations between indices of secretion and with other metabolic variables
We found that ΔI0–30/ΔG0–30 had a moderate relationship with I/GAUC 60–120. These two indices had strong correlations with S1PhOGTT and S2PhOGTT (Table 2). In addition, S1PhOGTT was very highly correlated with S2PhOGTT (r = 0.99) and both had similar relationships with all metabolic variables. We found that ΔI0–30/ΔG0–30 was less strongly related to indices of insulin resistance and measures of adiposity than any of the other secretory indices. All secretory indices had direct correlations with insulin levels (moderate for ΔI0–30/ΔG0–30, moderately strong for S1PhOGTT and S2PhOGTT, and strong for I/GAUC 60–120). Secretory indices differed in their relationships with plasma glucose levels (weakly negative for ΔI0–30/ΔG0–30 and weakly positive for I/GAUC 60–120).
Table 2.
Pearson’s partial correlations between indices of secretion and with other metabolic variables*
| ΔI0–30/ΔG0–30† | I/GAUC 60–120† | S1PhOGTT† | S2PhOGTT† | |
|---|---|---|---|---|
| BMI | 0.17 | 0.37‡ | 0.36‡ | 0.39‡ |
| Waist circumference | 0.13 | 0.34‡ | 0.33‡ | 0.35‡ |
| Fasting glucose | −0.08 | 0.05‡ | −0.02‡ | 0.03 § |
| 1-h glucose | −0.38 | 0.16‡ | −0.12‡ | −0.06‡ |
| 2-h glucose | −0.17 | 0.15‡ | 0.01‡ | 0.05‡ |
| Fasting insulin† | 0.24 | 0.58‡ | 0.62‡ | 0.67‡ |
| 1-h insulin† | 0.32 | 0.83‡ | 0.61‡ | 0.65‡ |
| 2-h insulin† | 0.24 | 0.70‡ | 0.49‡ | 0.53‡ |
| HOMA IR† | 0.30 | 0.63‡ | 0.64‡ | 0.69‡ |
| Matsuda index† | −0.31 | −0.78‡ | −0.68‡ | −0.74‡ |
| I/GAUC 60–120† | 0.51 | − | 0.74‡ | 0.77‡ |
| S1PhOGTT† | 0.78 | 0.74§ | - | 0.99‡ |
| S2PhOGTT† | 0.75 | 0.77 | 0.99‡ | – |
Pearson’s partial correlation coefficients were calculated by controlling for age, sex, and ethnic origin;
log transformed variables.
HOMA IR, homeostasis model assessment of insulin resistance; I0–30/G0–30 indicates insulinogenic index from 0 to 30 min; I/GAUC 60–120, ratio of area under the insulin curve to area under the glucose curve from 60 to 120 min; S1PhOGTT and S2PhOGTT, Stumvoll first phase and second phase insulin release, respectively. P-value for the test of difference in the correlation of each secretory index with individual metabolic variables relative to the respective correlation of ΔI0–30/ΔG0–30;
P < 0.001;
P < 0.01
Although curvilinear, the relation of the Matsuda index to ΔI0–30/ΔG0–30 and I/GAUC 60–120 was not rectangular hyperbolic (exponent parameters differed significantly from −1) using an ordinary least-squares regression (see the Supporting Information, Table S1).
Predictive discrimination of measures of secretion
During a 7.5-year follow-up, 179 of the 1540 (11.6%) participants developed diabetes. The predictive discrimination of the Matsuda index (C statistic = 0.766) was increased by adding to the model any of the indices of insulin secretion: ΔI0–30/ΔG0–30 (0.851, P < 0.001), I/GAUC 60–120 (0.832, P < 0.001), S1PhOGTT (0.850, P < 0.001) or S2PhOGTT (0.851, P < 0.001). The predictive discrimination of a model with the Matsuda index and ΔI0–30/ΔG0–30 (C statistic = 0.851) was increased by I/GAUC 60–120 (0.860, P = 0.016), but not by S1PhOGTT (0.853, P = 0.368) or S2PhOGTT (0.854, P = 0.250).
The I/GAUC 60–120 value increased the C statistic of a model that had the Matsuda index and ΔI0–30/ΔG0–30 as independent variables (0.875 vs. 0.882, P = 0.044) (Table 3). Matsuda index, ΔI0–30/ΔG0–30, and I/GAUC 60–120 were independent predictors even after entering IGT into the model. In contrast, the C statistic was not significantly changed by either S1PhOGTT (0.877, P = 0.828) or S2PhOGTT (0.877, P = 0.610).
Table 3.
Odds ratio of indices of insulin sensitivity and insulin secretion for predicting the 7.8-year incidence of diabetes by multiple logistic regression analysis
| Matsuda index | ΔI0–30/ΔG0–30 | I/GAUC 60–120 | S1PhOGTT | S2PhOGTT | C statistic | P | |
|---|---|---|---|---|---|---|---|
| Model 1 | 0.24 (0.18–0.31) | 0.28 (0.22–0.37) | – | – | – | 0.875 | – |
| Model 2 | 0.09 (0.06–0.14) | – | 0.21 (0.15–0.28) | – | – | 0.858 | 0.076 |
| Model 3 | 0.11 (0.07–0.17) | 0.41 (0.31–0.54) | 0.37 (0.26–0.54) | – | – | 0.882 | 0.044 |
| Model 4* | 0.16 (0.10–0.25) | 0.43 (0.32–0.58) | 0.46 (0.31–0.68) | – | – | 0.888 | < 0.001 |
| Model 5 | 0.11 (0.08–0.16) | – | – | 0.24 (0.18–0.32) | – | 0.873 | 0.314 |
| Model 6 | 0.15 (0.10–0.23) | 0.50 (0.32–0.79) | – | 0.46 (0.28–0.76) | – | 0.877 | 0.792 |
| Model 7 | 0.10 (0.07–0.14) | – | – | – | 0.22 (0.16–0.30) | 0.873 | 0.556 |
| Model 8 | 0.14 (0.09–0.22) | 0.51 (0.33–0.80) | – | – | 0.44 (0.25–0.75) | 0.877 | 0.610 |
Odds ratios expressed for 1 standard deviation unit increase. Age, sex, ethnicity, BMI and family history of diabetes were included as covariates in all eight models.
Impaired glucose tolerance (IGT) was also added to Model 4 as a covariate.
I0–30/G0–30, insulinogenic index from 0 to 30 min; I/GAUC 60–120, ratio of area under the insulin curve to area under the glucose curve from 60 to 120 min; and S1PhOGTT and S2PhOGTT, Stumvoll first phase and second phase insulin release, respectively. P-values are for the comparison with Model 1.
Models predicting incident diabetes that contained the product, Matsuda index × I/GAUC 60–120 or Matsuda index × ΔI0–30/ΔG0–30, had similar predictive discrimination to those that contained the individual components (see the Supporting Information, Table S2).
Heterogeneity analyses
In a model with the Matsuda index, ΔI0–30/ΔG0–30 and I/GAUC 60–120 as independent variables, we examined effect modification by testing the statistical significance of the interaction of sex, ethnicity, family history of diabetes and glucose tolerance status on the relation of Matsuda index, ΔI0–30/ΔG0–30 or I/GAUC 60–120 to incident diabetes. All these potential interaction effects had a P-value ≥ 0.16, except for interaction terms ethnicity × ΔI0–30/ΔG0–30 (P = 0.041) and ethnicity × I/GAUC 60–120 (P = 0.086). Interaction terms Matsuda index × ΔI0–30/ΔG0–30, Matsuda index × I/GAUC 60–120, and ΔI0–30/ΔG0–30 × I/GAUC 60–120 were not significant either (P ≥ 0.35). Matsuda index, ΔI0–30/ΔG0–30, and I/GAUC 60–120 were independent predictors of future diabetes in varying categories of age, sex, ethnicity, family history of diabetes, BMI and glucose tolerance (Fig. 1). There were, however, two exceptions: confidence intervals crossed 1.0 with I/GAUC 60–120 in individuals aged 25–44 years [odds ratio (OR) 0.70 (0.36–1.35)] and with ΔI0–30/ΔG0–30 in those with IGT [OR 0.59 (0.34–1.01)].
FIGURE 1.
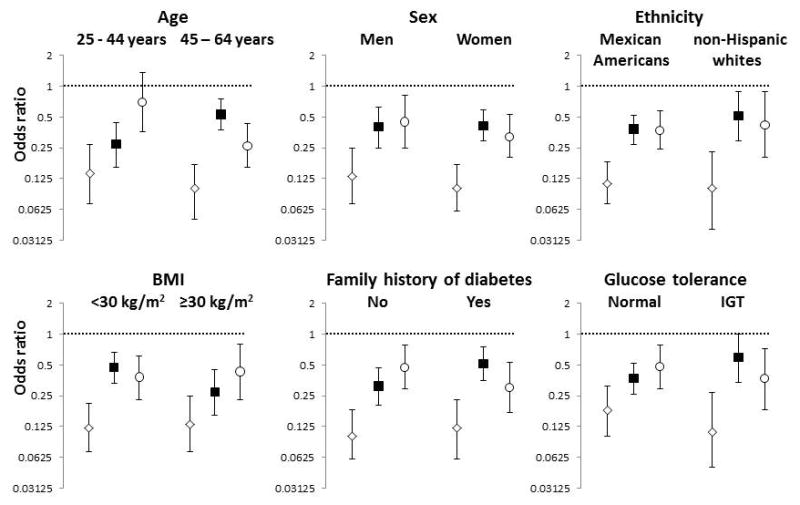
Diabetes risk associated with Matsuda index, ΔI0–30/ΔG0–30 (insulinogenic index from 0 to 30 min) and I/GAUC 60–120 (ratio of area under the insulin curve to area under the glucose curve from 60 to 120 min) stratified by age, sex, ethnicity, BMI, family history of diabetes and glucose tolerance status. Open diamonds, Matsuda index; closed square, ΔI0–30/ΔG0–30; open circle, I/GAUC 60–120. Age, sex, ethnicity, BMI, family history of diabetes, Matsuda index, ΔI0–30/ΔG0–30 and I/GAUC 60–120 were included in all models. The panels present the odds ratio reflecting the change in risk of future diabetes for one standard deviation unit increase. IGT, impaired glucose tolerance.
Discrimination
The Hosmer–Lemeshow test yielded a chi-square of 16.1 (P = 0.041) for the model without I/GAUC 60–120 and 9.1 (P = 0.332) for the model with I/GAUC 60–120. This indicates that the model with I/GAUC 60–120 was better calibrated (higher agreement between observed incidence of diabetes and predictions). Almost one-fifth of individuals with moderate and strong risk of future diabetes were reclassified by the addition of I/GAUC 60–120 (66 and 98 individuals were properly reclassified to a higher and lower risk category, respectively) (Table 4a). The net reclassification improvement was 0.13 (P < 0.001) after the addition of I/GAUC 60–120 and the integrated discrimination improvement was 0.033 (P < 0.001). A statistically significant integrated discrimination improvement indicates that the addition of I/GAUC 60–120 improved the discriminatory property of the model with age, sex, ethnicity, BMI, family history of diabetes, Matsuda index and ΔI0–30/ΔG0–30.
Table 4.
Comparison of predicted and observed risks of 7.5-year incidence of diabetes in models with and without I/GAUC 60–120
| Model A plus I/GAUC 60–120 | ||||
|---|---|---|---|---|
| (a) | < 1% yearly risk | 1–5.9% yearly risk | ≥ 6% yearly risk | % Reclassified |
| (a) Model A* | ||||
| < 1% yearly risk | ||||
| Total, n | 848 | 43 | 0 | – |
| %† | 95.2 | 4.8 | – | 4.8 |
| Observed yearly risk‡ | 0.2 | 1.6 | - | - |
| 1–5.9% yearly risk | ||||
| Total, n | 81 | 408 | 23 | – |
| % | 15.8 | 79.7 | 4.5 | 20.3 |
| Observed yearly risk | 0.7 | 2.9 | 10.4 | – |
| ≥ 6% yearly risk | ||||
| Total, n | 0 | 17 | 75 | – |
| % | – | 18.5 | 81.5 | 18.5 |
| Observed yearly risk | – | 2.4 | 8.4 | – |
| (b)Model B§ | Model B and I/GAUC 60–120 | |||
| < 1% yearly risk | ||||
| Total, n | 907 | 51 | 0 | – |
| %† | 94.7 | 5.3 | – | 5.3 |
| Observed yearly risk‡ | 0.2 | 1.3 | - | – |
| 1–5.9% yearly risk | ||||
| Total, n | 57 | 348 | 15 | – |
| % | 13.5 | 82.9 | 3.6 | 17.1 |
| Observed yearly risk | 0.7 | 3.0 | 8.0 | – |
| ≥ 6% yearly risk | ||||
| Total, n | 0 | 12 | 105 | – |
| % | – | 10.3 | 81.5 | 10.3 |
| Observed yearly risk | – | 2.4 | 8.4 | – |
All estimated and observed risks represent yearly risk of incident diabetes.
Model A included age, sex, ethnicity, family history of diabetes, BMI, Matsuda index and ΔI0–30/ΔG0–30 (insulinogenic index from 0 to 30 min) as independent variables.
Per cent classified in each risk stratum by the model with I/GAUC 60–120 (ratio of area under the insulin curve to area under the glucose curve from 60 to 120 min).
Observed proportion of participants developing diabetes in each category.
Model B included independent variables of Model A plus impaired glucose tolerance.
The addition of I/GAUC 60–120 also had additional value to a different model that included all the independent variables of the previous model plus impaired glucose tolerance (Table 4b). A total of 66 and 69 individuals were correctly reclassified to a higher and lower risk category, respectively. The net reclassification improvement was 0.05 (P = 0.043) and the integrated discrimination improvement was 0.018 (P < 0.001).
Discussion
The Insulinogenic Index, I/GAUC 60–120, predicts incident diabetes independently of ΔI0–30/ΔG0–30. The fit of models is excellent across varying categories of age, sex, family history of diabetes and BMI. Equally good fit is demonstrated in both high-risk Mexican Americans and low-risk non-Hispanic whites as well as in individuals with normal glucose tolerance and in those with impaired glucose tolerance. The index I/GAUC 60–120 has additional value for predicting incident diabetes beyond the predictive discrimination of Matsuda index and ΔI0–30/ΔG0–30.
Most subjects with hyperglycaemia have impaired β-cell function [24]. ΔI0–30/ΔG0–30, the capacity for insulin response during the early OGTT period, has shown to correlate weakly with first- and second-phase insulin secretion (r = 0.25 and r = 0.22, respectively) as measured by hyperglycaemic clamp studies [11] and more strongly with first-and second-phase insulin secretion by intravenous glucose tolerance test (r = 0.58 and r = 0.47, respectively) [20]. ΔI0–30/ΔG0–30 has been described as a predictor of diabetes in multiple studies [6–8]. The current results indicate that ΔI0–30/ΔG0–30 is a strong predictor of incident diabetes independently of a measure of insulin secretion derived from the late OGTT period.
Stumvoll et al. [11] stated that S1PhOGTT and S2PhOGTT had more robust correlations with first- and second-phase insulin release by the hyperglycaemic clamp technique than did ΔI0–30/ΔG0–30 and homeostasis model assessment of β-cell function. In this study, however, both S1PhOGTT and S2PhOGTT had identical relationships with clamp-derived first phase (r = 0.78) and second phase (r = 0.79) insulin release [11]. This suggests that S1PhOGTT and S2PhOGTT are highly correlated, as do our results (r = 0.99 for the relationship between S1PhOGTT and S2PhOGTT). Both indices increase the predictive discrimination of the Matsuda index, but neither of them increases the predictive discrimination of models with ΔI0–30/ΔG0–30.
Measured by C statistic [25,26], I/GAUC 60–120 increases the predictive discrimination of the Matsuda index and ΔI0–30/ΔG0–30. Measures of calibration and reclassification [27,28], which are more sensitive tests of improvement in model discrimination, also indicate that I/GAUC 60–120 adds discriminatory value to the Matsuda index and ΔI0–30/ΔG0–30. This may be relevant for predicting a future outcome in individuals with a moderate or significant risk. For example, the yearly risk of future diabetes is 1.0% in a hypothetical 50-year-old non-Hispanic white woman who has no family history of diabetes, BMI of 25 kg/m2, no impaired glucose tolerance, a Matsuda index of 2 and ΔI0–30/ΔG0–30 of 1.5. The addition of I/GAUC 60–120 to the prediction model changes the estimated yearly risk of future diabetes to 0.6% if I/GAUC 60–120 is 1.5 and to 2.5% if I/GAUC 60–120 is 0.5. A much more relevant change occurs in a 50-year-old non-Hispanic white woman who has no family history of diabetes, BMI of 30 kg/m2, impaired glucose tolerance, a Matsuda index of 2 and ΔI0–30/ΔG0–30 of 1.5. The estimated yearly risk is 4.7% according to the model without I/GAUC 60–120. The addition of this variable changes the yearly risk to 2.3% if I/GAUC 60–120 is 1.5 and to 9.9% if I/GAUC 60–120 is 0.5. Consequently, I/GAUC 60–120 may be useful for the classification of individuals in clinical studies.
The risk estimate that uses I/GAUC 60–120 is a more accurate image of actual risk for all 164 reclassified participants. The proportion of reclassified individuals at low risk (< 1% per year) is relatively small, but a more significant proportion occurs in those at moderate (1–6% per year) and high risks (≥ 6% per year). Thus, I/GAUC 60–120 would have a significant effect in the stratification of individuals in a hypothetical population. For example, if the prediction model with age, sex, ethnicity, BMI, family history of diabetes, Matsuda index and ΔI0–30/ΔG0–30 is applied to a cohort of 100 000 individuals without diabetes from San Antonio, 59 600 of them will be considered at low risk (< 1% yearly risk), 34 200 at moderate risk (1–5.9% yearly risk) and 6200 at high risk (≥ 6% yearly risk) for developing diabetes. The addition of I/GAUC 60–120 will reclassify 10 951 individuals: from the low-risk category, 2861 will now be considered at moderate risk; from the moderate-risk category, 5404 will now be at low risk and 1539 at high risk; and from the high-risk category, 1147 will be at moderate risk. Thus, I/GAUC 60–120 may have discriminatory value in epidemiological studies.
The relationship between acute insulin response and insulin sensitivity index, two measures derived from the frequently sampled intravenous glucose tolerance test, is hyperbolic [13,16]. A similar relationship has been described between two indices derived from the OGTT, insulin AUC to glucose AUC from 0 to 120 min and Matsuda index [16,29]. The product of these two indices, insulin secretion-sensitivity index-2, has a stronger association with the disposition index than products involving ΔI0–30/ΔG0–30 [29]. Unable to take into account the variability in the measurement of Matsuda index and I/GAUC 60–120 [13,16], our study cannot determine whether the relationship between these two indices is rectangular hyperbolic. However, our results indicate that the product, Matsuda index × I/GAUC 60–120 or Matsuda index × ΔI0–30/ΔG0–30, does not improve the ability to predict future diabetes of the individual components, as previously reported using direct measures [30].
In summary, measures of insulin secretion derived from the early and late OGTT periods are independent predictors of Type 2 diabetes. This holds in different subgroups including ethnic and glucose tolerance categories. As the OGTT is relatively easy to perform, insulin secretory indices from the early and late OGTT periods may be useful for understanding the natural history of diabetes.
Supplementary Material
Table S1. Relation of Matsuda index to early and late oral glucose tolerance testing (OGTT) measures of β-cell function
Table S2. C statistic of models predicting the 7.8-year incidence of diabetes by multiple logistic regression analysis. Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Acknowledgments
This work was supported by grants from the National Heart, Lung and Blood Institute (RO1-HL24799 and RO1-HL36820).
Footnotes
Competing interests
Nothing to declare.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med. 1993;329:1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 2.Lorenzo C, Wagenknecht LE, D’Agostino RB, Rewers MJ, Karter AJ, Haffner SM. Insulin resistance, β-cell dysfunction, and conversion to type 2 diabetes in a multiethnic population: The Insulin Resistance Atherosclerosis Study (IRAS) Diabetes Care. 2010;33:67–72. doi: 10.2337/dc09-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skarfors ET, Selinus KI, Lithell HO. Risk factors for developing non-insulin dependent diabetes: a 10-year follow-up of men in Uppsala. BMJ. 1991;303:755–760. doi: 10.1136/bmj.303.6805.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lundgren H, Bengtsson C, Blohme G, Lapidus L, Waldenstrom J. Fasting serum insulin concentrations and early insulin response as risk determinants for developing diabetes. Diabet Med. 1990;7:407–413. doi: 10.1111/j.1464-5491.1990.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 5.Kadowaki T, Miyake Y, Hagura R, Akanuma Y, Kajinuma H, Kuzuya N, et al. Risk factors for worsening to diabetes in subjects with impaired glucose tolerance. Diabetologia. 1984;26:44–49. doi: 10.1007/BF00252262. [DOI] [PubMed] [Google Scholar]
- 6.Kitabchi AE, Temprosa M, Knowler WC, Kahn SE, Fowler SE, Haffner SM, et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54:2404–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haffner SM, Miettinen H, Gaskill SP, Stern MP. Decreased insulin action and insulin secretion predict the development of impaired glucose tolerance. Diabetologia. 1996;39:1201–1207. doi: 10.1007/BF02658507. [DOI] [PubMed] [Google Scholar]
- 8.Chen KW, Boyko EJ, Bergstrom RW, Leonetti DL, Newell-Morris L, Wahl PW, et al. Earlier appearance of impaired insulin secretion than of visceral adiposity in the pathogenesis of NIDDM: 5-year follow-up of initially non-diabetic Japanese American men. Diabetes Care. 1995;18:747–753. doi: 10.2337/diacare.18.6.747. [DOI] [PubMed] [Google Scholar]
- 9.Haffner SM, Miettinen H, Stern MP. Are risk factors for conversion to NIDDM similar in high and low risk populations? Diabetologia. 1997;40:62–66. doi: 10.1007/s001250050643. [DOI] [PubMed] [Google Scholar]
- 10.Pimenta W, Korytkowski M, Mitrakou A, Jenssen T, Yki-Jarvinen H, Evron W, et al. Pancreatic beta-cell dysfunction as the primary genetic lesion in NIDDM. Evidence from studies in normal glucose-tolerant individuals with a first-degree NIDDM relative. JAMA. 1995;273:1855–1861. [PubMed] [Google Scholar]
- 11.Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Järvinen H, Van Haeften T, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000;23:295–301. doi: 10.2337/diacare.23.3.295. [DOI] [PubMed] [Google Scholar]
- 12.Bergman RN, Finegood DT, Kahn SE. The evolution of beta-cell dysfunction and insulin resistance in type 2 diabetes. Eur J Clin Invest. 2002;32(Suppl 3):35–45. doi: 10.1046/j.1365-2362.32.s3.5.x. [DOI] [PubMed] [Google Scholar]
- 13.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 14.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 15.Seltzer HS, Allen EW, Herron AL, Brennan MT. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest. 1967;46:323–335. doi: 10.1172/JCI105534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity (Silver Spring) 2008;16:1901–1907. doi: 10.1038/oby.2008.307. [DOI] [PubMed] [Google Scholar]
- 17.Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004;110:1251–1257. doi: 10.1161/01.CIR.0000140762.04598.F9. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 20.Stancáková A, Javorský M, Kuulasmaa T, Haffner SM, Kuusisto J, Laakso M. Changes in insulin sensitivity and insulin release in relation to glycemia and glucose tolerance in 6,414 Finnish men. Diabetes. 2009;58:1212–1221. doi: 10.2337/db08-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87:245–251. [Google Scholar]
- 22.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 24.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 25.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 26.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–138. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118:2243–2251. doi: 10.1161/CIRCULATIONAHA.108.814251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zethelius B, Berglund L, Sundström J, Ingelsson E, Basu S, Larsson A, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 29.Retnakaran R, Qi Y, Goran MI, Hamilton JK. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet Med. 2009;26:1198–1203. doi: 10.1111/j.1464-5491.2009.02841.x. [DOI] [PubMed] [Google Scholar]
- 30.Lorenzo C, Wagenknecht LE, Rewers MJ, Karter AJ, Bergman RN, Hanley AJG, et al. Disposition index, glucose effectiveness, and conversion to Type 2 diabetes: the Insulin Resistance Atherosclerosis Study (IRAS) Diabetes Care. 2010;33:2098–2103. doi: 10.2337/dc10-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Relation of Matsuda index to early and late oral glucose tolerance testing (OGTT) measures of β-cell function
Table S2. C statistic of models predicting the 7.8-year incidence of diabetes by multiple logistic regression analysis. Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.


