Abstract
Objectives
Considerable interest and controversy over a possible decline in semen quality during the 20th century raised concern that semen quality could have reached a critically low level where it might affect human reproduction. The authors therefore initiated a study to assess reproductive health in men from the general population and to monitor changes in semen quality over time.
Design
Cross-sectional study of men from the general Danish population. Inclusion criteria were place of residence in the Copenhagen area, and both the man and his mother being born and raised in Denmark. Men with severe or chronic diseases were not included.
Setting
Danish one-centre study.
Participants
4867 men, median age 19 years, included from 1996 to 2010.
Outcome measures
Semen volume, sperm concentration, total sperm count, sperm motility and sperm morphology.
Results
Only 23% of participants had optimal sperm concentration and sperm morphology. Comparing with historic data of men attending a Copenhagen infertility clinic in the 1940s and men who recently became fathers, these two groups had significantly better semen quality than our study group from the general population. Over the 15 years, median sperm concentration increased from 43 to 48 million/ml (p=0.02) and total sperm count from 132 to 151 million (p=0.001). The median percentage of motile spermatozoa and abnormal spermatozoa were 68% and 93%, and did not change during the study period.
Conclusions
This large prospective study of semen quality among young men of the general population showed an increasing trend in sperm concentration and total sperm count. However, only one in four men had optimal semen quality. In addition, one in four will most likely face a prolonged waiting time to pregnancy if they in the future want to father a child and another 15% are at risk of the need of fertility treatment. Thus, reduced semen quality seems so frequent that it may impair the fertility rates and further increase the demand for assisted reproduction.
Article summary
Article focus
A paper by Carlsen et al 20 years ago (BMJ 1992;305:609–13) raised controversy with evidence of a decline in semen quality, and several studies on semen quality in human populations have followed.
There has been a lack of larger, prospectively collected quality-controlled data on semen quality in the general population.
Key messages
This study brings good and bad news.
Fifteen years monitoring of semen quality in men of the general population indicated a slight increase in both median sperm concentration and total sperm count.
However, still only a fraction of the men (23%) had optimal sperm concentration and sperm morphology, and the median percentage of abnormal spermatozoa was as high as 93% with no sign of improvement during the study period.
Approximately 15% of the men had a sperm concentration at a level that would indicate a high risk of needing future fertility treatment if they want to father a child, and another 27% of the men will be at risk of a prolonged waiting time to pregnancy.
Strengths and limitations of this study
Large prospective study of semen quality among men of the general population unselected with regard to fertility.
Standardised inclusion and investigation procedures.
Lack of historical, directly comparable data.
Introduction
In the 1990s, a meta-analysis by Carlsen et al,1 showing a decline in human semen quality, initiated a heated scientific debate. The discussion has recently resurfaced in three papers in Epidemiology2–4 and a news article in Science.5 The Carlsen paper, which was a review and meta-analysis of internationally published data on semen quality among healthy men, suggested that there had been a decline in sperm concentration and total sperm count over a period of 50 years. Many were sceptical about the results, and this prompted several researchers to study trends in their own countries, mostly based on data from semen banks or semen donor registries. The resulting papers reported heterogeneous findings (reviewed in Jouannet et al6 and Merzenich et al7), with some confirming a decreasing trend in semen quality, and others not. In 2000, an updated comprehensive meta-analysis was undertaken by Swan et al8 that confirmed the downward trend. During the same period, there is strong evidence for a worldwide increase in the incidence of testicular germ cell cancer, a disease linked to decreased semen quality.9–11
The background for the interest and controversy over trends in semen quality was the obvious concern that semen quality could have reached a critically low level where it might affect fecundity (ie, the ability to reproduce). Therefore, since 1996, we have carried out a prospective quality-controlled study of semen quality in annual cohorts of men from the general Danish population. A total of 4867 individuals have been included in this study.
Methods
The study population was men from the general Danish population from the Copenhagen area examined in 1996–2010. For interpretation of the results, we compared them to published data of two other studies from the Copenhagen area: a recent study of fertile men (male partners of pregnant women) examined in 1996–1998 by our group12 and historical data of male partners from infertile couples examined in 1939–1943 by Dr Richard Hammen.13
Study population: men from the general population examined in 1996–2010
In Denmark, all men, except those with severe or chronic diseases (<15%), are required to attend a medical examination before being considered for military service. Men are called upon to present themselves at the age of 18–19 years, but some postpone this examination until completion of their education. Men attending the medical examinations are therefore considered representative of the general population of young men.
In collaboration with the military health authority, men attending these medical examinations in the greater Copenhagen area of Denmark were asked to participate in the present study, irrespective of whether they were declared fit for military service or not. Further inclusion criteria for this publication were: place of residence in the greater Copenhagen area and both the men and their mothers being born and raised in Denmark. Those men who consented to participate were given an appointment for examination at the Department of Growth and Reproduction at Rigshospitalet (Copenhagen, Denmark). Participants were instructed to abstain from ejaculation for at least 48 h prior to attendance at Rigshospitalet, where each man returned a completed questionnaire, underwent a physical examination and provided a semen sample. Participants received a financial compensation (approximately €65).
Participants in this ongoing study have been included since September 1996. Two of our previous publications have directly focused on the semen quality level of men examined from September 1996 to March 1998.14 15 Other publications have included information based on subpopulations of men examined until the end of 2007,16–35 but no previous trend analysis has been performed on the material from the entire period. Participants examined up until the end of December 2010 were included in the present publication, with 4901 men fulfilling the inclusion criteria. The participation rate among invited men ranged from 19% to 31%, with an overall average of 24%. Data from 34 of the men were excluded: 27 with previous or current use of anabolic steroids, six who had previously received chemotherapy for a malignant disease and one man who failed to deliver a semen sample (he was later diagnosed with testosterone deficiency due to a 46, XX-male karyotype). Thus, results from the remaining 4867 men are reported here. The study comprised annual cohorts of 240–543 men (median 276), 18–29 years of age (median 19). A detailed description of the study population based on questionnaire information and results from the physical examination (see below) is summarised in table 1. The three types of information is presented in table 1, ‘Been diagnosed as having’, ‘Been treated for’ and ‘Has’ are based on questions phrased as ‘Has a doctor ever diagnosed you as having…’, ‘Have you ever been treated for…’ and ‘Have you ever…’, respectively. Within 3 months prior to participation, 601 men (12%) had used medication, mainly antibiotics, painkillers or asthma/allergy medicine.
Table 1.
Physical appearance and self-reported information of young men from the general population in the Copenhagen area, Denmark
| Investigation period |
|||||||||
| 1996–2010, total (N=4867) |
1996–2000 (N=1339) |
2001–2005 (N=2254) |
2006–2010 (N=1274) |
Difference between the three groups, p Value | |||||
| Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | ||
| Physical appearance | |||||||||
| Age (years)* | 19.4 (1.2) | 19.0 (18.4–21.7) | 19.6 (1.4) | 19.0 (18.5–22.4) | 19.3 (1.1) | 18.9 (18.4–21.3) | 19.4 (1.2) | 19.0 (18.4–21.8) | <0.0005¶¶ |
| Height (m) | 1.81 (0.06) | 1.81 (1.71–1.92) | 1.81 (0.07) | 1.81 (1.71–1.92) | 1.81 (0.07) | 1.81 (1.70–1.91) | 1.82 (0.06) | 1.82 (1.71–1.93) | <0.0005¶¶ |
| Weight (kg) | 75.1 (11.5) | 73.5 (59.4–96.4) | 75.2 (11.7) | 73.6 (59.0–97.5) | 74.9 (11.5) | 73.3 (59.2–97.0) | 75.5 (11.2) | 74.1 (60.1–96.0) | 0.15¶¶ |
| BMI (kg/m2) | 22.9 (3.1) | 22.4 (18.7–28.8) | 22.9 (3.2) | 22.4 (18.8–28.9) | 22.9 (3.2) | 22.4 (18.7–29.0) | 22.8 (3.1) | 22.4 (18.7–25.6) | 0.8¶¶ |
| Testis size (ml)† | 20 (5) | 20 (13–28) | 20 (5) | 20 (12–28) | 20 (5) | 20 (13–28) | 22 (5) | 23 (14–29) | <0.0005¶¶ |
| Testis size (ml), US | 15 (4) | 14 (9–22) | 15 (5) | 15 (9–24) | 14 (4) | 14 (9–22) | 14 (4) | 14 (9–21) | <0.0005¶¶ |
| Lifestyle | |||||||||
| Cigarettes daily, all men | 4.1 (6.7) | 0.0 (0.0–20.0) | 5.0 (7.4) | 0.0 (0.0–20.0) | 3.9 (6.5) | 0.0 (0.0–20.0) | 3.7 (6.2) | 0.0 (0.0–18.0) | 0.004¶¶ |
| Cigarettes daily, smokers only | 9.9 (7.1) | 10.0 (1.0–20.0) | 11.7 (7.1) | 10.0 (1.0–20.0) | 9.9 (6.9) | 10.0 (1.0–20.0) | 8.1 (6.9) | 7.0 (0.1–20.0) | <0.0005¶¶ |
| Alcohol consumption (units)‡ | 14 (14) | 11 (0–40) | 13 (13) | 11 (0–38) | 14 (14) | 11 (0–37) | 15 (16) | 12 (0–42) | 0.1¶¶ |
| Ejaculation abstinence (hours) | 81 (117) | 63 (37–155) | 86 (95) | 63 (35–168) | 81 (137) | 62 (38–135) | 77 (96) | 63 (37–134) | 0.1¶¶ |
| % | % | % | % | ||||||
| Taken medication§ | 12.5 | 14.6 | 9.7 | 15.1 | <0.0005¶¶ | ||||
| Smoker | 41.7 | 42.5 | 39.1 | 45.4 | 0.001¶¶ | ||||
| Previous smoker | 3.1 | 2.0 | 2.3 | 5.8 | <0.0005¶¶ | ||||
| Never-smoker | 55.2 | 55.6 | 58.6 | 48.7 | <0.0005¶¶ | ||||
| Mother smoked in pregnancy | 38.0 | 36.2 | 38.3 | 29.1 | <0.0005¶¶ | ||||
| Been diagnosed as having | |||||||||
| Varicocele | 0.6 | 0.5 | 0.7 | 0.4 | 0.4*** | ||||
| Epididymitis | 0.3 | 0.3 | 0.5 | 0.2 | 0.3*** | ||||
| Sexual transmitted disease¶ | 4.3 | 2.2 | 4.6 | 6.2 | <0.0005*** | ||||
| Cystitis | 2.4 | 1.4 | 2.4 | 3.6 | 0.002*** | ||||
| Diabetes | 0.02 | 0.0 | 0.04 | 0.0 | 0.6*** | ||||
| Thyroid disease | 0.04 | 0.0 | 0.05 | 0.08 | 0.001*** | ||||
| Been treated for | |||||||||
| Varicocele | 0.4 | 0.0 | 0.7 | 0.4 | 0.004*** | ||||
| Testicular torsion | 0.8 | 1.0 | 0.6 | 0.9 | 0.4*** | ||||
| Testicular cancer | 0.02 | 0.0 | 0.0 | 0.1 | 0.2*** | ||||
| Cryptorchidism** | 6.1 | 3.9 | 8.0 | 5.0 | <0.0005*** | ||||
| Hypospadias | 0.1 | 0.0 | 0.0 | 0.3 | 0.004*** | ||||
| Phimosis | 3.9 | 5.4 | 2.7 | 4.6 | <0.0005*** | ||||
| Inguinal hernia | 3.4 | 3.8 | 4.8 | 0.5 | <0.0005*** | ||||
| Has | |||||||||
| Had cryptorchidism†† | 8.3 | 4.4 | 11.2 | 6.9 | <0.0005*** | ||||
| Experienced fertility problems‡‡ | 0.6 | 1.7 | 0.2 | 0.2 | <0.0005*** | ||||
| Caused a pregnancy§§ | 6.4 | 7.4 | 5.6 | 6.8 | 0.08*** | ||||
| Subgroup | 80.6 | 84.6 | 77.8 | 81.2 | <0.0005*** | ||||
Calculated as difference between day of attendance in study and self-reported day of birth.
Mean of left and right testes size assessed by palpation. Information of testis size was missing for 3, 9 and 3 men from the 1st, 2nd and 3rd investigation period, respectively.
Sum of intake of beer, wine and strong alcohol recent week prior to participation in study.
Taken any medication recent 3 months prior to participation in study.
Chlamydia or gonorrhoea.
Hormonal, surgical or combination.
Not born with both testicles in scrotum (irrespective of spontaneous descend, treatment or still cryptorchid).
Ever had regular intercourse without use of contraception for at least 1 year, without partner became pregnant.
Ever caused a pregnancy.
Kruskal–Wallis test.
χ2 test.
Subgroup: Men without adverse conditions ‘Been diagnosed as having…’, ‘Been treated for…’ or ‘Has…’. Those that have caused a pregnancy are also included, irrespective of any adverse condition previously. See text for further explanation.
p Value: For comparison of results between the three study periods.
Questionnaires
A standardised questionnaire was developed for this study. In order to ensure the quality of the information regarding previous conditions, the questionnaire was sent to participants before their attendance at the hospital, and they were asked to complete it beforehand and—if possible—in collaboration with their parents. The questionnaire included information on previous or current diseases, including any known history of fertility potential, and some lifestyle factors. The questionnaire has been revised during the course of the study, mainly with new questions being added, and the current publication includes relevant information available from all participants throughout the study.
Semen samples
Semen samples were produced by masturbation. The actual abstinence period was calculated from the self-reported time of previous ejaculation and the time of delivery of semen sample recorded by a technician. The semen samples were produced in the privacy of a room near the laboratory and kept at 37°C.
Semen analysis was performed according to the WHO guidelines.36 In brief, semen volume was estimated by weighing the collection tube with the semen sample and subtracting the predetermined weight of the empty tube, assuming that 1 ml semen =1 g. For sperm motility assessment, 10 μl of well-mixed semen was placed on a clean glass slide kept at 37°C and covered with a 22×22 mm coverslip. The preparation was placed on the heated stage of a microscope at 37°C and immediately examined at ×400 magnification. The sperm were classified as progressively motile, locally motile or immotile.
For the assessment of the sperm concentration, the samples were diluted in a solution of 0.6 mol/l NaHCO3 and 0.4% (v/v) formaldehyde in distilled water. The sperm concentration was subsequently assessed using a Bürker-Türk haemocytometer (Paul Marienfeld GmbH & Co. KG, Lauda-Königshofen, Germany). Only sperm with tails were counted.
Smears were prepared for morphological evaluation, Papanicolaou stained and finally assessed according to ‘strict criteria’.37
The laboratory participates in an external quality control programme for sperm concentration assessment as previously described.15 38–40 The results did not show any temporal trend in assessment level in the Copenhagen laboratory. Therefore, no adjustments according to quality control results were needed. For the first 290 men included in our study, the weight of the empty semen collection tubes was on average overestimated by 0.5 g, giving an underestimation of the semen weight by 0.5 g. The data on semen volume among these men were therefore corrected as follows: Corrected semen volume = Observed semen volume +0.5 ml.
Physical examination
A physical examination of each participant was performed on the day of delivery of his semen sample. Tanner stage of pubic hair was recorded, and testicular size was assessed, all examiners using the same type of wooden orchidometer.
Comparison population: fertile men (partners of pregnant women), examined in 1996–1998
From October 1996 to January 1998, our group also examined the semen quality of 349 fertile men (partners of pregnant women); the results were published previously.12 Pregnant women were approached during routine visits at the antenatal care unit, and their partners were invited to participate in the semen quality study. The eligibility criteria were age 20–45 years at the time of invitation, both the man and his mother being born and raised in Denmark and conception of the current pregnancy by normal sexual relations (not as a result of treatment for subfertility or infertility). Participation of these men was similar to that of the men from the general population: they answered a questionnaire, delivered a semen sample and had a physical examination performed. Both physical examination and semen analysis were performed in the same manner and in the same laboratory as for men from the general population.
A description of the fertile men based on questionnaire information and on the results of the physical examination has previously been published12 and is shown in table 2, which also includes additional information to allow for a better comparison to the men from the general population.
Table 2.
Physical appearance and self-reported information of men from the Copenhagen area, Denmark
| Study population |
|||||
| General population 1996–2010, total (N=4867) |
Fertile 1996–1998 (N=349) |
Infertile couples 1939–1943 (N=839) | |||
| Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | ||
| Physical appearance | |||||
| Age (years)* | 19.4 (1.2) | 19.0 (18.4–21.7) | 31.5 (4.3) | 30.8 (25.4–40.2) | 73% 25–35 years |
| Height (m) | 1.81 (0.06) | 1.81 (1.71–1.92) | 1.83 (6.2) | 1.84 (1.73–1.94) | – |
| Weight (kg) | 75.1 (11.5) | 73.5 (59.4–96.4) | 83.0 (11.2) | 82.0 (67.6–102.2) | – |
| BMI (kg/m2) | 22.9 (3.1) | 22.4 (18.7–28.8) | 24.6 (2.9) | 24.3 (20.6–29.5) | 6.6% ‘moderate obese’ |
| Testis size (ml)† | 20 (5) | 20 (13–28) | 23 (4) | 24 (15–30) | – |
| Testis size (ml), US | 15 (4) | 14 (9–22) | – | – | – |
| Lifestyle | |||||
| Cigarettes daily, all men | 4.1 (6.7) | 0.0 (0.0–20.0) | 4.5 (8.3) | 0.0 (0.0–20) | – |
| Cigarettes daily, smokers only | 9.9 (7.1) | 10.0 (1.0–20.0) | 14.0 (8.9) | 15.0 (0.5–30) | – |
| Alcohol consumption (units)‡ | 14 (14) | 11 (0–40) | 10 (9) | 8 (0–30) | – |
| Ejaculation abstinence (h) | 81 (117) | 63 (37–155) | 81 (65) | 64 (20–182) | – |
| % | % | % | |||
| Taken medication§ | 12.5 | 20.1 | – | ||
| Smoker | 41.7 | 32.5 | – | ||
| Previous smoker | 3.1 | – | – | ||
| Never-smoker | 55.2 | – | – | ||
| Mother smoked in pregnancy | 38.0 | 38.1 | – | ||
| Been diagnosed as having | |||||
| Varicocele | 0.6 | 2.9 | 11.9 | ||
| Epididymitis | 0.3 | 2.6 | 1.9 | ||
| Sexual transmitted disease¶ | 4.3 | 18.6 | 19.4 | ||
| Cystitis | 2.4 | 8.0 | – | ||
| Diabetes | 0.02 | 0.3 | – | ||
| Thyroid disease | 0.04 | 0.0 | – | ||
| Been treated for | |||||
| Varicocele | 0.4 | 0.9 | 0.4 | ||
| Testicular torsion | 0.8 | 1.1 | |||
| Testicular cancer | 0.02 | 0.3 | 0.01 | ||
| Cryptorchidism** | 6.1 | 4.3 | 2.1 | ||
| Hypospadias | 0.1 | 0.0 | 0.0 | ||
| Phimosis | 3.9 | – | – | ||
| Inguinal hernia | 3.4 | 6.0 | 1.5 | ||
| Has | |||||
| Had cryptorchidism†† | 8.3 | – | >5.2 | ||
| Experienced fertility problems‡‡ | 0.6 | 12.3 | 100.0 | ||
| Caused a pregnancy§§ | 6.4 | 100.0 | 26.8 | ||
Infertile couples: 925 men delivered semen samples, however, patient history was only obtained on 839.
Calculated as difference between day of attendance in study and self-reported day of birth.
Mean of left and right testes size assessed by palpation. Information of testis size was missing for 3, 9 and 3 men from the 1st, 2nd and 3rd investigation period, respectively.
Sum of intake of beer, wine and strong alcohol recent week prior to participation in study.
Taken any medication recent 3 months prior to participation in study.
Chlamydia or gonorrhoea.
Hormonal, surgical or combination.
Not born with both testicles in scrotum (irrespective of spontaneous descend, treatment or still cryptorchid). For the Hammen cohort similar information was not obtained, but 5.2% of men were detected as having cryptorchidism when examined.
Ever had regular intercourse without use of contraception for at least 1 year, without partner became pregnant.
Ever caused a pregnancy.
Comparison population: male partners from infertile couples, examined in 1939–1943
Dr Richard Hammen published a doctoral thesis with ground-breaking data on male infertility in 1944.13 From the Copenhagen area in Denmark, he investigated 925 men in ‘childless marriages’, defined as couples where at least 1 year of regular coitus, without use of contraceptives, had not led to a successful pregnancy.
Hammen's data originated from two cohorts (‘material I’ and ‘material II’) that he examined in 1939–1943. Material I comprised 291 male partners attending the Gynaecological Department and Dispensary of the Kommune Hospital, Copenhagen. Material II consisted of 634 men who delivered basic information and semen sample to the General Laboratory of National Health Insurance Physicians, Copenhagen. Hammen stated that information regarding duration of childlessness was somewhat less reliable in this group than in material I but concluded that only a few per cent of these men had a duration of childlessness <1 year.
In his thesis, Hammen provided patient histories of the study populations. The durations of childlessness in the cohorts were 1–2 years (12.7%), 2–3 years (22.7%) and more than 3 years (64.4%). Secondary sterility was ascertained in 26.8%, as 12.0% had children or abortuses with other women and 14.8% with their current partners.
The age distribution of the whole Hammen cohort (materials I and II) was described as 2.7% <25 years, 72.6% 25–35 years, 22.3% 35–45 years and 2.4% >45 years. Therefore, the median age seems to be somewhere in early 30s, similar to that of the fertile men we investigated in 1996–1998.
Some of the information obtained by Hammen was similar to that obtained in our studies of men from the general population and partners of pregnant women; this is summarised in table 2. Information on medical history was obtained from all men in material I, but only from 548 men from material II. Thus, the figures presented in table 2 are calculated based on information from 839 men.
Hammen did not report height or weight, but noted ‘moderate or marked obesity’ in 6.6% of the men. Palpable changes in the epididymis were detected in 11.8%, abnormalities of the testis in 25.2%, cryptorchidism of one or both testes in 5.2% and varicocele in 11.9%.
Previous venereal diseases were reported by 24.1% of men in material I and 16.9% in material II. Other local lesions involving the testes were reported (eg, hernia, cryptorchidism) in 12.1% in material I and 4.0% in material II. Hammen attributed the difference between the two otherwise similar groups to erroneous information given by the questioned patients in material II, as they were not interviewed by Hammen directly. Previous serious diseases (eg, pulmonary tuberculosis, pneumonia and peritonitis) were recorded in 34.7% of the cases. Present chronic conditions (diseases of the stomach and intestines, especially peptic ulcer and gastritis, neurasthenia, chronic otitis media, chronic familial anaemia, osteomyelitis, bronchitis, diabetes mellitus, epilepsy and heart disease) were recorded in 12.7% of the patients.
All men provided a semen sample. They had been instructed to abstain from ejaculation for at least 3 days prior to delivery of the semen sample, which was produced through interrupted coitus at home or by masturbation in a private room in the hospital. Exact information was requested about the period of ejaculation abstinence.
The ejaculates were examined as soon as the specimen was received. Semen was poured from the collection beaker into a graduated container to assess volume. For assessment of sperm concentration, the following procedure was employed: ‘After thorough mixing of the sperm in a shaking-machine or by hand, 0.1 cc. of sperm is added to 1.9 cc. of the diluent (consisting of 190 cc. of physiological salt solution, 7 cc. of a 1% methylene blue solution and 3 cc. of absolute alcohol). The mixing takes place in a dwarf-tube, containing a glass bead, which is shaken for 5 min in a shaking-machine or by hand. The count is carried out with employment of a Bürker-Türk counting-chamber. All sperm heads are counted, also loose heads. Loose tails are not counted’.
Statistical analysis in the present study
Means, medians, SDs, 5–95 percentiles and frequencies were used for basic descriptions. The study subjects were divided into three groups, depending on the investigation periods: 1996–2000, 2001–2005 and 2006–2010. Between-group differences for continuous variables were tested by the non-parametric Kruskal–Wallis test. Between-group differences for categorical variables were tested with Pearson's χ2 test.
The main outcome variables were semen volume, sperm concentration, total sperm count, percentage of motile spermatozoa and percentage morphologically normal spermatozoa. Temporal trend between investigation periods were tested by multiple linear regressions adjusted for confounders. Semen volume, sperm concentration and total sperm count were best normalised by a cubic root transformation before analysis to correct for skewed distribution of residuals. The percentages of motile spermatozoa were logit-transformed. Percentages of morphologically normal spermatozoa were close to normally distributed and entered the model untransformed. Ejaculation abstinence up to 96 h had an increasing effect on semen volume, sperm concentration and total sperm count (all p values<0.05) and was entered as a covariate in the regression analyses of these variables, whereas it had no effect on morphology or motility. Increasing age had a non-significant increasing effect on sperm concentration (p=0.8), but a significant increasing effect on semen volume (p<0.0005), and was also entered as covariate. Season of year was evaluated as a possible confounder for all the semen variables, and duration from ejaculation to assessment was additionally evaluated as a confounder for sperm motility. Both were non-significant and therefore not included in the final models.
Differences in semen quality variables between men from the general population and partners of pregnant women were also tested by linear regressions corrected for the same covariates as stated above. Differences in distribution of sperm concentrations and total sperm counts between men from the general population examined in 1996–2010 and male partners from infertile couples examined in 1939–1943 were tested by χ2 test.
A p value of <0.05 was considered statistically significant. Analyses were performed using PASW GradPack V.18.0 (SPSS Inc.).
Results
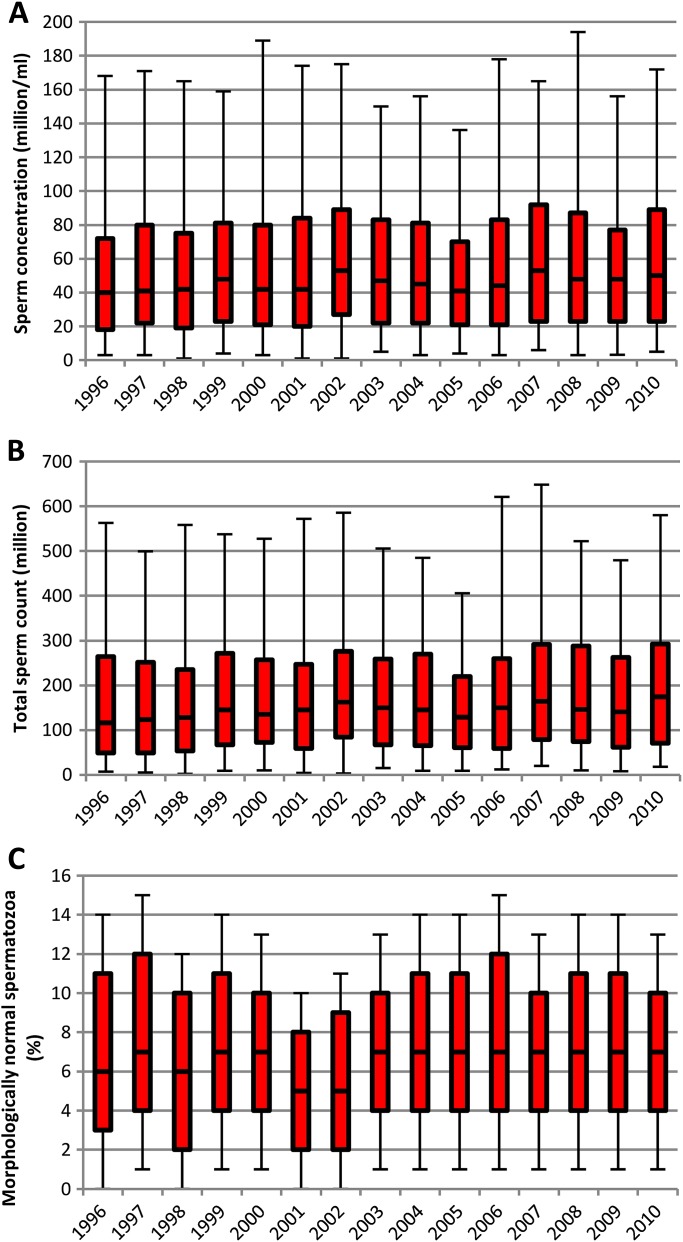
Figure 1 shows sperm concentrations, total sperm counts and percentages of morphologically normal spermatozoa for each year of examination. Grouping results of the 15 years into three 5-year periods showed a temporal increase in the sperm counts (table 3). Men examined in 2006–2010 had higher median sperm concentration, total sperm count and total number of morphologically normal sperm count than men examined in the first 5-year period. Similarly, men examined in 2001–2005 appeared to have higher counts than the previously examined. Estimating the average linear increase over the period confirmed the slightly increasing temporal trends (p=0.02, p<0.0005 and p=0.013, respectively). The median values indicated an increase in semen volume, which was confirmed both when the three 5-year periods were compared and when estimating the annual linear increase (p<0.0005). The percentages of motile and morphologically normal spermatozoa showed no change over time.
Figure 1.
Semen parameters of Danish men from the general population. Red bars show 25th–75th percentiles with median line. Whiskers show 5th–95th percentiles. The sperm concentration (A) and total sperm count (B) increased slightly by year of examination. Percentage of morphologically normal spermatozoa did not show any temporal trend (C).
Table 3.
Semen quality of 4867 young men from the general population in the Copenhagen area in Denmark
| Mean (SD) | Median (5–95) | p Values comparing |
|||
| All periods | 2001–2005 vs 2006–2010 | 1996–2000 vs 2006–2010 | |||
| Semen volume (ml) | |||||
| Investigation period 1996–2010 | 3.4 (2.0) | 3.2 (1.3–6.0) | |||
| Investigation period 1996–2000 | 3.3 (1.5) | 3.1 (1.2–5.8) | |||
| Investigation period 2001–2005 | 3.3 (1.4) | 3.2 (1.4–5.9) | |||
| Investigation period 2006–2010 | 3.6 (3.1) | 3.3 (1.3–6.3) | 0.004 | 0.011 | 0.001 |
| Sperm concentration (million/ml) | |||||
| Investigation period 1996–2010 | 60 (57) | 45 (3–163) | |||
| Investigation period 1996–2000 | 58 (55) | 43 (3–167) | |||
| Investigation period 2001–2005 | 60 (58) | 45 (3–156) | |||
| Investigation period 2006–2010 | 62 (55) | 48 (3–169) | 0.065 | 0.12 | 0.020 |
| Total sperm count (million) | |||||
| Investigation period 1996–2010 | 193 (232) | 143 (9–529) | |||
| Investigation period 1996–2000 | 185 (184) | 132 (6–531) | |||
| Investigation period 2001–2005 | 191 (241) | 146 (8–508) | |||
| Investigation period 2006–2010 | 206 (258) | 151 (13–559) | 0.002 | 0.015 | 0.001 |
| Normal morphology (%) | |||||
| Investigation period 1996–2010 | 7.1 (4.9) | 6.5 (0.5–16.0) | |||
| Investigation period 1996–2000 | 7.3 (5.1) | 7.0 (1.0–17.0) | |||
| Investigation period 2001–2005 | 6.9 (4.8) | 6.0 (0.5–15.5) | |||
| Investigation period 2006–2010 | 7.5 (4.9) | 7.0 (0.5–16.0) | 0.016 | 0.023 | 0.97 |
| Total normal spermatozoa (million) | |||||
| Investigation period 1996–2010 | 16.3 (23.9) | 8.4 (0.0–57.4) | |||
| Investigation period 1996–2000 | 16.5 (24.5) | 7.9 (0.0–60.9) | |||
| Investigation period 2001–2005 | 15.5 (22.6) | 8.0 (0.0–53.8) | |||
| Investigation period 2006–2010 | 17.9 (25.3) | 9.8 (0.1–59.3) | 0.040 | 0.012 | 0.076 |
| Progressively motile (%) | |||||
| Investigation period 1996–2010 | 56 (17) | 59 (23–77) | |||
| Investigation period 1996–2000 | 54 (17) | 57 (22–75) | |||
| Investigation period 2001–2005 | 57 (17) | 60 (22–77) | |||
| Investigation period 2006–2010 | 57 (16) | 59 (25–79) | <0.0005 | 0.30 | 0.005 |
| Motile (%) | |||||
| Investigation period 1996–2010 | 65 (15) | 68 (35–83) | |||
| Investigation period 1996–2000 | 65 (15) | 68 (38–82) | |||
| Investigation period 2001–2005 | 64 (15) | 67 (35–82) | |||
| Investigation period 2006–2010 | 65 (16) | 68 (33–85) | 0.17 | 0.09 | 0.71 |
5–95: 5–95 percentiles.
p Values: Obtained from regression analysis taking confounders into consideration.
As expected, some of the men had previously experienced andrological problems, including cryptorchidism, hypospadias, sexually transmitted diseases and/or other signs or symptoms relating to the reproductive system (table 1). As men with such diseases could be more motivated to participate in the study, we performed a subanalysis on the subgroup of 3921 men (80.6%) who were without previous andrological abnormalities. The main conclusion that impaired semen quality was frequent remained robust. The results described here are based on the entire group, whereas the results from the subgroup are shown in appendix 1.
Comparison population: fertile men, examined in 1996–1998
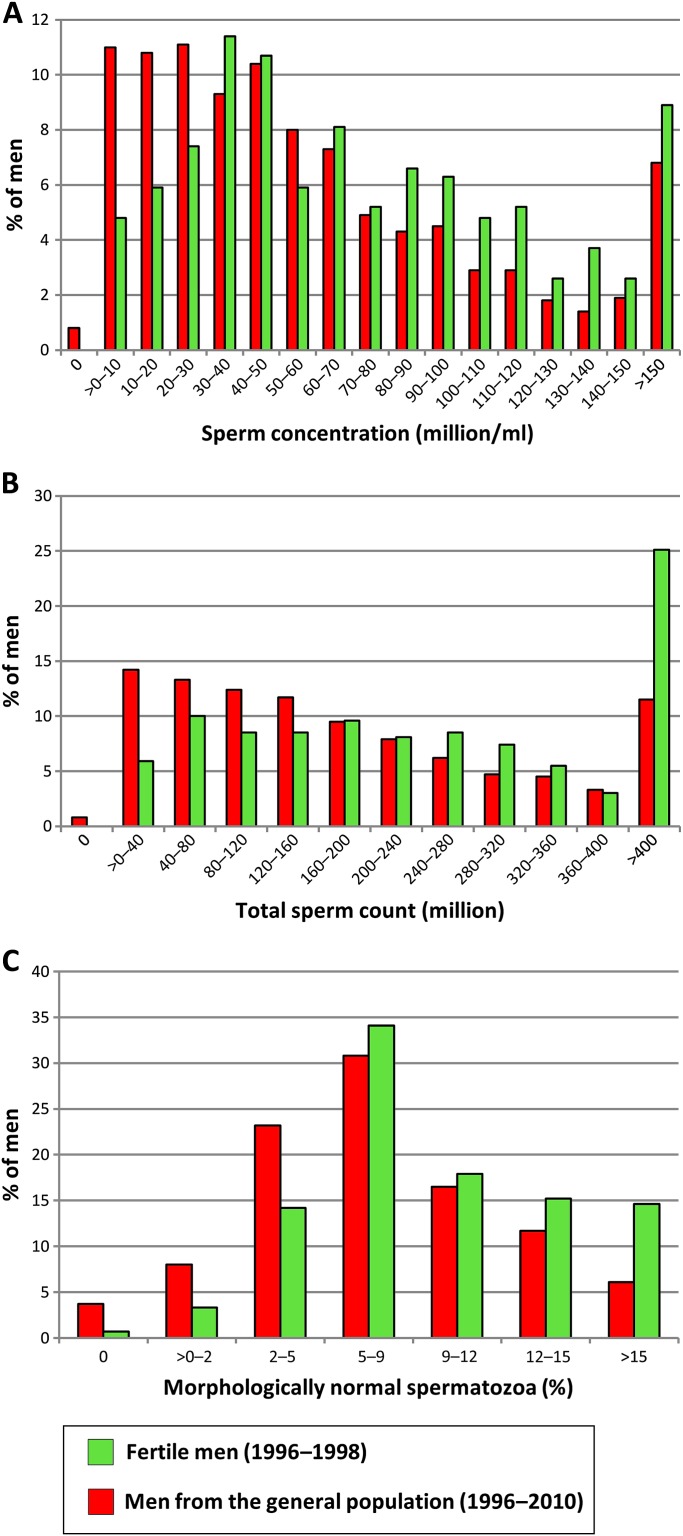
Table 4 summarises the semen results of the 349 fertile men examined previously12 and the men from the general population. The semen variables differed between these groups, with highest semen volume, sperm concentration, total sperm count, total number of morphologically normal spermatozoa and percentage of normal spermatozoa in the fertile men (all p values <0.0005). Motility variables were statistically lower for the fertile men. Approximately 42% of the men from the general population had sperm concentrations below 40 million/ml and 66% had <9% normal forms (figure 2). For 15% and 35% of men, the sperm concentration was below 15 million/ml and the percentage of normal spermatozoa below 5%. For the fertile men, only 8% had a sperm concentration below 15 mill/ml and 18% had <5% normal forms. Only 23% of men from the general population had the optimal sperm concentrations of more than 40 million/ml and more than 9% normal forms, in comparison to 42% of the fertile men.
Table 4.
Semen quality of partners of pregnant women (fertile men) and young men from the general population from the Copenhagen area in Denmark
| Partners pregnant women (N=349) |
General population |
|||||
| Total group (N=4867) |
Subgroup (N=3921) |
|||||
| Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | Mean (SD) | Median (5–95) | |
| Semen volume (ml) | 3.8 (1.7) | 3.6 (1.1–6.7) | 3.4 (2.0) | 3.2 (1.3–6.0) | 3.4 (2.1) | 3.2 (1.3–5.9) |
| Sperm concentration (million/ml) | 77 (66) | 61 (10–207) | 60 (57) | 45 (3–163) | 61 (57) | 47 (4–166) |
| Total sperm count (million) | 276 (240) | 215 (32–795) | 193 (232) | 143 (9–529) | 197 (231) | 146 (10–531) |
| Normal morphology (%) | 9.3 (5.0) | 8.5 (2.0–18.5) | 7.1 (4.9) | 6.5 (0.5–16.0) | 7.2 (4.9) | 6.5 (0.5–16) |
| Total normal spermatozoa (million) | 30 (37) | 18 (1–111) | 16 (24) | 8 (0–57) | 17 (24) | 9 (0.1–59) |
| Progressive motile (%) | 51 (15) | 52 (25–72) | 57 (16) | 60 (24–77) | 57 (16) | 60 (24–77) |
| Motile (%) | 60 (12) | 61 (40–79) | 65 (15) | 68 (35–83) | 65 (15) | 68 (37–83) |
Figure 2.
Distributions of sperm counts and morphologically normal spermatozoa in Danish men from the general population and fertile Danish men (partners of pregnant women). All men had durations of ejaculation abstinence above 48 h. Sperm concentration (A) total sperm counts (B) and percentages of morphologically normal spermatozoa (C) were lower in men from the general population.
Comparison population: male partners from infertile couples, examined in 1939–1943
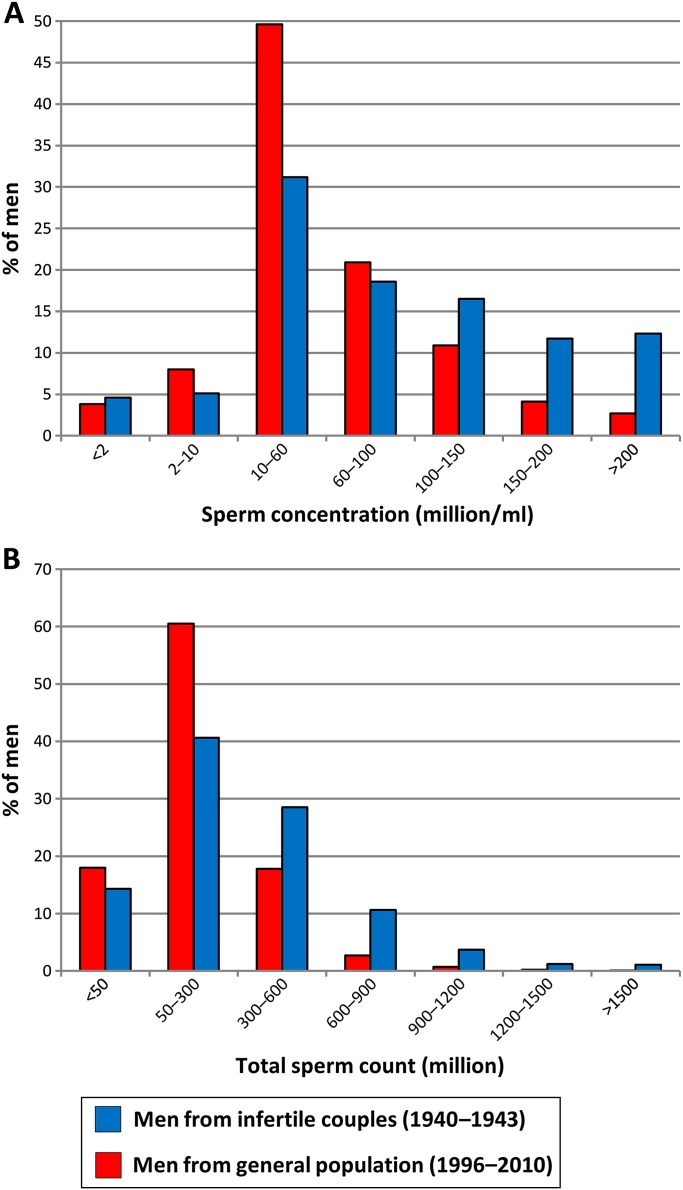
Figure 3 shows the distributions of sperm concentrations and total sperm counts for our study group compared with those of men from infertile couples in Denmark in 1940–1943 (Hammen cohort). As shown, our study group had lower sperm counts than the historical cohort (p<0.0005, for all comparisons).
Figure 3.
Distributions of sperm counts in Danish men from the general population, examined from 1996 to 2010 and Danish men examined in an infertility clinic in the 1940s. All men had durations of ejaculation abstinence above 48 h. Sperm concentration (A) and total sperm counts (B).
Discussion
In this large, prospective and well-controlled study of semen quality of annual cohorts of young men from the general population, statistically significant increases in sperm concentration and total sperm counts over the past 15 years were detected. However, it is of concern that these men from the general population in the new millennium had significantly lower sperm concentrations and total sperm counts than recently examined fertile men and men of a historical cohort of male partners of infertile couples. Both sperm concentration and sperm morphology measures according to strict criteria are known to be informative semen measurements for discriminating between fertile and infertile men.41 Therefore, there is reason to be concerned about future fertility of young Danish men. Smaller cross-sectional studies of men from the general populations in other European countries have shown similar high frequencies of men with poor semen quality.38–40 42 43 Thus, poor semen quality seems to be a widespread phenomenon. This interpretation is in line with the high and increasing need of fertility treatment in Denmark.44
We have considered whether immaturity of the men (with a median age of 19 years) could account for the findings. However, a 4-year longitudinal follow-up with quarterly assessment of semen quality in a subgroup of more than 150 of the men showed no significant change over time in sperm concentration, total sperm count and sperm morphology, suggesting that immaturity does not explain our results.45 It is also possible that our results could be skewed by selection biases. However, during the early stage of our project, we carried out a study on blood samples from the majority of those men who did not volunteer to provide semen samples (N=195, participation rate 79%) and showed that their reproductive hormone levels including the spermatogenesis markers follicle-stimulating hormone and inhibin-b were very similar to those of the participants.14 This suggests that our results are not biased by selection. Furthermore, our results hold true in the subgroup of men without andrological events in their history as presented in appendix 1. It is not likely that the detected temporal trend in sperm count is due to intraobserver or interobserver variations. Our laboratory technicians participated in a quality control study of assessment of sperm concentration, which did not indicate temporal changes in assessment levels. However, a longer observation period is needed to corroborate or refute such a positive tendency. Five observers did 97.6% of all morphology assessments, among which a single observer assessed 91% of samples in the first 5-year period, 14% in the second period and none in the last. One observer assessed 14% of samples from the second period, but none from the first or last period. This observer tended to obtain 1% lower values than other observers (detailed data not shown), which partly explains the lower number of morphologically normal spermatozoa in the second 5-year period. Assessment of semen volume was also controlled and corrected when needed. Effects of potential confounders of semen variables were investigated and accounted for in the statistical analyses. Increasing duration of abstinence up to approximately 96 h had an increasing influence on semen volume, sperm concentration and total sperm count, but no effect on motility or morphology, which is in agreement with our initial findings and with the results of other semen quality studies of men from Europe.38–40 42 From pilot studies in the middle of 1990s, we know that interobserver variation for motility assessment is of significant importance46 and difficult to eliminate. Our results on numbers of motile sperms should therefore be taken with some caution.
The definition of normal semen quality has varied over time. Seventy years ago, the Danish standard for normal sperm concentration set 60 million/ml as a lower cut-off level.13 However, the most recent WHO guidelines adhere to common clinical practice, where the ‘normal’ reference range is defined as the one that covers 95% of a population. The most recent WHO guidelines have reduced the reference limits for sperm concentration from 20 to 15 million/ml.36 Reference limits based on 95% of the population may be relevant in relation to certain clinical tests (eg, levels of sodium or potassium in serum) but are unsuitable for public health issues in which secular changes may affect the whole population (eg, obesity).47 For trend analyses, our data on semen quality of men examined during the past 15 years should therefore rather be compared with data from the previous generations of men. Unfortunately, historical data on semen quality of men from the general population do not exist. Other unique Danish semen data obtained by the pioneer of modern Danish andrology, Dr Richard Hammen, who studied semen quality of men 70 years ago, exists.13 His method for counting sperm concentration by the use of the Bürker-Türk haemocytometer was very similar to that used in our present investigations and in accordance with the current recommendations by the WHO,36 allowing for meaningful comparisons with our new data. Interestingly, sperm numbers among men in the Hammen study from the 1940s were significantly higher than those in the present study, despite the fact that the earlier sample was recruited among male partners in infertile couples. This actually corroborates that semen quality might have decreased temporarily as suggested by the meta-analysis by Carlsen et al.1
Whereas the historical data point to a temporal decrease in sperm concentration and total sperm counts, there is no such data to support a similar trend in the percentages of normal spermatozoa in each ejaculate. A trend may be difficult to detect because different criteria for normality have been applied during the years. In our study, we did not find any trend in sperm morphology despite a slight increase in sperm numbers. However, it is noteworthy that the median percentage of spermatozoa with normal morphology was as low as 6.5%. In contrast to our study, a decrease in the percentage of normal spermatozoa was recently described in a Finnish study, which also reported decreasing trends for sperm concentration and total sperm counts.40
Although only one spermatozoon is needed to fertilise an egg, several studies have shown that the fertilising ability diminishes if the sperm concentration is below 40–50 million/ml or if the percentage of normal spermatozoa is below 9%.48–51 Approximately 42% of the men from the general population had sperm concentrations below 40 million/ml and 66% <9% normal forms. More severe fertility problems may be present when sperm concentration is below 15 million/ml and the percentage of normal spermatozoa is <5%,41 51 which was the case for 15% and 35% of the men from the general Danish population, respectively. It is noteworthy that only 8% and 18% of a group of fertile men in a previous study of partners of pregnant women were below these ‘cut-off’ levels. Sperm concentration, total sperm count and percentage of normal spermatozoa were significantly lower in men from the general population in comparison to fertile men. Only 23% of men from the general population had the optimal sperm concentrations of more than 40 million/ml and more than 9% normal forms in comparison to 42% of the fertile men.
Both clinical practice and animal studies suggest an important role of sperm morphology for conception rates.41 51 Human in vitro fertilisation studies also suggest an important role of sperm morphology for fertilisation rates, which become significantly lower if the percentage of normal spermatozoa is below 5%. In men, the number of morphologically normal spermatozoa is usually reported to be below 10% and in animals above 50%. For example, breeding bulls and boars most often have <10% abnormal spermatozoa,52 and abnormalities are often more subtle than the severe abnormalities frequently seen in human samples. Even with relatively low numbers of normal spermatozoa, humans may still be able to reproduce. In contrast to wild animal species, where survival of the species may depend on a very high conception rate at each coitus, humans in monogamous relationships are not dependent on immediate reproductive success to the same degree. In fact, the current definition of couple infertility in most national health systems is ‘more than 1 year of regular, unprotected sexual relationship without pregnancy’.53 In other words, absence of pregnancy in spite of regular coitus during up to 12 ovulation periods can be considered ‘normal’ from a clinical point of view. However, fecundity may still be reduced compared to couples where conception occurs immediately after unprotected intercourse during the first cycle.
In conclusion, our large prospective study of men of the general population supports previous suggestions of a temporal decrease in semen quality, but it also indicated a recent small increase in sperm concentration and total sperm count. Follow-up studies are needed to detect if the upward trend is a real biological phenomenon or merely random variation. It is noteworthy that only one in four men had optimal semen quality from a fecundity perspective. Approximately 25% had a reduced quality compatible with prolonged waiting time to pregnancy, and another 15% had so severely impaired quality that they have a high risk of the need for fertility treatment to become biological fathers.
Supplementary Material
Appendix 1.
Semen results for men from the general population have been summarised in figure 1 of the main text. Online table A1 summarises the semen results for the subgroup of men without previous andrological abnormalities as well as p values for differences between the three 5-year periods (similar to table 2, which described the entire study population in the main text). Men examined in 2006–2010 had higher median sperm concentration, total sperm count and total number of morphologically normal spermatozoa than men examined in the first 5-year period. In analyses using year of examination as a continuous variable, the significant trends were also confirmed for the subgroup: p=0.02, p=0.001 and p=0.004.
Table 3 in the main text summarised the semen results of the 349 fertile men examined previously.12 The semen variables differed between these men and men from the general population, with highest semen volume, sperm concentration, total sperm count, total number of morphologically normal spermatozoa and percentage of normal spermatozoa in the fertile men. These differences were all highly significant at p<0.0005, irrespective of the comparisons being made between the fertile men and the entire study group of men from the general population; the subgroup of men from the general population without any andrological event in their history examined in 1996–2010 or the smaller subgroup examined in 2006–2010. The differences are shown in online figure A1.1 and A1.2, which show the distributions of sperm concentrations, total sperm count and number of morphologically normal spermatozoa for men from the general population (red bars), and partners of pregnant women (green bars). For these variables, we show data based on the entire study population, data based on only those with an ejaculation abstinence of at least 48 h, data based on only those having an ejaculation abstinence period of at least 48 h and without any andrological event in their history (subgroup) and finally data based on the same subgroup of men from the general population examined in the period 2006–2010. The tendency that men from the general population have lower semen volume, sperm concentration, total sperm count, total number of morphologically normal spermatozoa and percentage of normal spermatozoa than partners of pregnant women is seen irrespective of which of the four groupings are evaluated.
Online figure A2 shows the distributions of sperm concentrations and total sperm counts for the men from the general population (grouped as in online figure A1) compared with men from infertile couples in Denmark, 1940–1943.13 Here, too, it can be seen that the recent general population has lower sperm counts than the historical cohort (p<0.0005, for all comparisons).
Footnotes
To cite: Jørgensen N, Joensen UN, Jensen TK, et al. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open 2012;2:e000990. doi:10.1136/bmjopen-2012-000990
Contributors: Substantial contributions to conception and design: NJ, TKJ, A-MA, JT and NES. Acquisition of data: NJ, UNJ, TKJ, MBJ, IAO, AJ and EC. Analysis of data: NJ, UNJ, KA, JHP and NES. Interpretation of data: all authors. Drafting the article: NJ, UNJ and NES. Revising the article critically for important intellectual content: all authors. Final approval of the version to be published: all authors.
Funding: This study has been supported economically by several grants: the European Union (contract numbers BMH4-CT96-0314, QLK4-CT-1999–01422, QLK4-CT—2002–00603 and most recently FP7/2007–2013, DEER Grant agreement no. 212844), the Danish Research Council (grants numbers 9700833 2107-05-0006), the Danish Agency for Science, Technology and Innovation (Grant number 271070678), Rigshospitalet (Grant number 961506336), the University of Copenhagen (Grant number 211-0357/07-3012), the Danish Ministry of Health and the Danish Environmental Protection Agency, A.P. Møller and wife Chastine McKinney Møllers foundation and Svend Andersens Foundation. The funding organisations played no role in the design and conduct of the study; in collection, management, analysis and interpretation of the data; or in the presentation, review or approval of the manuscript.
Competing interests: None.
Ethics approval: The local Science Ethical Committee had approved the study (June 1996, the Science Ethical Committee for the Copenhagen and Frederiksberg municipalities, reference number KF01-117/96, and most recently June 2011, the Capital Region of Denmark, reference number H-KF-289428), and all participants had given their informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no additional data available.
References
- 1.Carlsen E, Giwercman A, Keiding N, et al. Evidence for decreasing quality of semen during past 50 years. BMJ 1992;305:609–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilcox AJ. On sperm counts and data responsibility. Epidemiology 2011;22:615–16 [DOI] [PubMed] [Google Scholar]
- 3.Bonde JP, Ramlau-Hansen CH, Olsen J. Trends in sperm counts, the saga continues. Epidemiology 2011;22:617–19 [DOI] [PubMed] [Google Scholar]
- 4.Skakkebæk NE, Andersson AM, Juul A, et al. Sperm counts, data responsibility, and good scientific practice. Epidemiology 2011;22:620–1 [DOI] [PubMed] [Google Scholar]
- 5.Vogel G. Danish sperm counts spark data dispute. Science 2011;332:1369–70 [DOI] [PubMed] [Google Scholar]
- 6.Jouannet P, Wang C, Eustache F, et al. Semen quality and male reproductive health: the controversy about human sperm concentration decline. APMIS 2001;109:333–44 [DOI] [PubMed] [Google Scholar]
- 7.Merzenich H, Zeeb H, Blettner M. Decreasing sperm quality: a global problem. BMC Public Health 2010;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934-1996. Environ Health Perspect 2000;108:961–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmiedel S, Schüz J, Skakkebæk NE, et al. Testicular germ cell cancer incidence in an immigration perspective, Denmark, 1978 to 2003. J Urol 2010;183:1378–82 [DOI] [PubMed] [Google Scholar]
- 10.Chia VM, Quraishi SM, Devesa SS, et al. International trends in the incidence of testicular cancer, 1973-2002. Cancer Epidemiol Biomarkers Prev 2010;19:1151–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huyghe E, Matsuda T, Thonneau P. Increasing incidence of testicular cancer worldwide: a review. J Urol 2003;170:5–11 [DOI] [PubMed] [Google Scholar]
- 12.Jørgensen N, Andersen AG, Eustache F, et al. Regional differences in semen quality in Europe. Hum Reprod 2001;16:1012–19 [DOI] [PubMed] [Google Scholar]
- 13.Hammen R. Studies on impaired fertility in man with special reference to the male. Acta Obstet Gynecol Scand 1944;24(Suppl 3):1–205 [Google Scholar]
- 14.Andersen AG, Jensen TK, Carlsen E, et al. High frequency of sub-optimal semen quality in an unselected population of young men. Hum Reprod 2000;15:366–72 [DOI] [PubMed] [Google Scholar]
- 15.Jørgensen N, Carlsen E, Nermoen I, et al. East-West gradient in semen quality in the Nordic-Baltic area: a study of men from the general population in Denmark, Norway, Estonia and Finland. Hum Reprod 2002;17:2199–208 [DOI] [PubMed] [Google Scholar]
- 16.Krausz C, Quintana-Murci L, Rajpert-De Meyts E, et al. Identification of a Y chromosome haplogroup associated with reduced sperm counts. Hum Mol Genet 2001;10:1873–7 [DOI] [PubMed] [Google Scholar]
- 17.Krausz C, Rajpert-De Meyts E, Frydelund-Larsen L, et al. Double-blind Y chromosome microdeletion analysis in men with known sperm parameters and reproductive hormone profiles: microdeletions are specific for spermatogenic failure. J Clin Endocrinol Metab 2001;86:2638–42 [DOI] [PubMed] [Google Scholar]
- 18.Jensen TK, Jørgensen N, Punab M, et al. Association of in utero exposure to maternal smoking with reduced semen quality and testis size in adulthood: a cross-sectional study of 1,770 young men from the general population in five European countries. Am J Epidemiol 2004;159:49–58 [DOI] [PubMed] [Google Scholar]
- 19.Jensen M, Leffers H, Petersen JH, et al. Frequent polymorphism of the mitochondrial DNA polymerase gamma gene (POLG) in patients with normal spermiograms and unexplained subfertility. Hum Reprod 2004;19:65–70 [DOI] [PubMed] [Google Scholar]
- 20.Jensen TK, Andersson AM, Jørgensen N, et al. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril 2004;82:863–70 [DOI] [PubMed] [Google Scholar]
- 21.Bay K, Hartung S, Ivell R, et al. Insulin-like factor 3 serum levels in 135 normal men and 85 men with testicular disorders: relationship to the luteinizing hormone-testosterone axis. J Clin Endocrinol Metab 2005;90:3410–18 [DOI] [PubMed] [Google Scholar]
- 22.Bang AK, Carlsen E, Holm M, et al. A study of finger lengths, semen quality and sex hormones in 360 young men from the general Danish population. Hum Reprod 2005;20:3109–13 [DOI] [PubMed] [Google Scholar]
- 23.Jensen TK, Jørgensen N, Asklund C, et al. Fertility treatment and reproductive health of male offspring: a study of 1,925 young men from the general population. Am J Epidemiol 2007;165:583–90 [DOI] [PubMed] [Google Scholar]
- 24.Blomberg Jensen M, Leffers H, Petersen JH, et al. Association of the polymorphism of the CAG repeat in the mitochondrial DNA polymerase gamma gene (POLG) with testicular germ-cell cancer. Ann Oncol 2008;19:1910–14 [DOI] [PubMed] [Google Scholar]
- 25.Jensen TK, Jørgensen N, Asklund C, et al. Self-rated health and semen quality among 3,457 young Danish men. Fertil Steril 2007;88:1366–73 [DOI] [PubMed] [Google Scholar]
- 26.Aksglæde L, Jørgensen N, Skakkebæk NE, et al. Low semen volume in 47 adolescents and adults with 47, XXY Klinefelter or 46, XX male syndrome. Int J Androl 2009;32:376–84 [DOI] [PubMed] [Google Scholar]
- 27.Joensen UN, Bossi R, Leffers H, et al. Do perfluoroalkyl compounds impair human semen quality? Environ Health Perspect 2009;117:923–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asklund C, Jensen TK, Main KM, et al. Semen quality, reproductive hormones and fertility of men operated for hypospadias. Int J Androl 2010;33:80–7 [DOI] [PubMed] [Google Scholar]
- 29.Jørgensen N, Rajpert-De Meyts E, Main KM, et al. Testicular dysgenesis syndrome comprises some but not all cases of hypospadias and impaired spermatogenesis. Int J Androl 2010;33:298–303 [DOI] [PubMed] [Google Scholar]
- 30.Jensen TK, Swan SH, Skakkebæk NE, et al. Caffeine intake and semen quality in a population of 2,554 young Danish men. Am J Epidemiol 2010;171:883–91 [DOI] [PubMed] [Google Scholar]
- 31.Frederiksen H, Jørgensen N, Andersson AM. Correlations between phthalate metabolites in urine, serum, and seminal plasma from young Danish men determined by isotope dilution liquid chromatography tandem mass spectrometry. J Anal Toxicol 2010;34:400–10 [DOI] [PubMed] [Google Scholar]
- 32.Frederiksen H, Jørgensen N, Andersson AM. Parabens in urine, serum and seminal plasma from healthy Danish men determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). J Expo Sci Environ Epidemiol 2011;21:262–71 [DOI] [PubMed] [Google Scholar]
- 33.Ravnborg TL, Jensen TK, Andersson AM, et al. Prenatal and adult exposures to smoking are associated with adverse effects on reproductive hormones, semen quality, final height and body mass index. Hum Reprod 2011;26:1000–11 [DOI] [PubMed] [Google Scholar]
- 34.Blomberg Jensen M, Nielsen JE, Jørgensen A, et al. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum Reprod 2010;25:1303–11 [DOI] [PubMed] [Google Scholar]
- 35.Blomberg Jensen M, Bjerrum PJ, Jessen TE, et al. Vitamin D is positively associated with sperm motility and increases intracellular calcium in human spermatozoa. Hum Reprod 2011;26:1307–17 [DOI] [PubMed] [Google Scholar]
- 36.World Health Organisation WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th edn 2010. http://whqlibdoc.who.int/publications/2010/9789241547789_eng.pdf [Google Scholar]
- 37.Menkveld R, Stander FS, Kotze TJ, et al. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod 1990;5:586–92 [DOI] [PubMed] [Google Scholar]
- 38.Paasch U, Salzbrunn A, Glander HJ, et al. Semen quality in sub-fertile range for a significant proportion of young men from the general German population: a co-ordinated, controlled study of 791 men from Hamburg and Leipzig. Int J Androl 2008;31:93–102 [DOI] [PubMed] [Google Scholar]
- 39.Fernandez MF, Duran I, Olea N, et al. Semen quality and reproductive hormone levels in men from Southern Spain. Int J Androl 2012;35:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jørgensen N, Vierula M, Jacobsen R, et al. Recent adverse trends in semen quality and testis cancer incidence among Finnish men. Int J Androl 2011;34:e37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kruger TF, Menkveld R, Stander FS, et al. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril 1986;46:1118–23 [DOI] [PubMed] [Google Scholar]
- 42.Jørgensen N, Asklund C, Carlsen E, et al. Coordinated European investigations of semen quality: results from studies of Scandinavian young men is a matter of concern. Int J Androl 2006;29:54–61 [DOI] [PubMed] [Google Scholar]
- 43.Axelsson J, Rylander L, Rignell-Hydbom A. No secular trend over the last decade in sperm counts among Swedish men from the general population. Hum Reprod 2011;26:1012–16 [DOI] [PubMed] [Google Scholar]
- 44.Jensen TK, Sobotka T, Hansen MA, et al. Declining trends in conception rates in recent birth cohorts of native Danish women: a possible role of deteriorating male reproductive health. Int J Androl 2008;31:81–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlsen E, Swan SH, Petersen JH, et al. Longitudinal changes in semen parameters in young Danish men from the Copenhagen area. Hum Reprod 2005;20:942–9 [DOI] [PubMed] [Google Scholar]
- 46.Jørgensen N, Auger J, Giwercman A, et al. Semen analysis performed by different laboratory teams: an intervariation study. Int J Androl 1997;20:201–8 [DOI] [PubMed] [Google Scholar]
- 47.Skakkebæk NE. Normal reference ranges for semen quality and their relations to fecundity. Asian J Androl 2010;12:95–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonde JP, Ernst E, Jensen TK, et al. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet 1998;352:1172–7 [DOI] [PubMed] [Google Scholar]
- 49.Slama R, Eustache F, Ducot B, et al. Time to pregnancy and semen parameters: a cross-sectional study among fertile couples from four European cities. Hum Reprod 2002;17:503–15 [DOI] [PubMed] [Google Scholar]
- 50.Jedrzejczak P, Taszarek-Hauke G, Hauke J, et al. Prediction of spontaneous conception based on semen parameters. Int J Androl 2008;31:499–507 [DOI] [PubMed] [Google Scholar]
- 51.Guzick DS, Overstreet JW, Factor-Litvak P, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med 2001;345:1388–93 [DOI] [PubMed] [Google Scholar]
- 52.Blom E. On the Evaluation of Bull Semen with Special Reference to its Employment for Artificial Insemination (Ed: A/S Carl Fr. Mortensen, 1950, Copenhagen).
- 53.Crosignani PG, Rubin BL. Optimal use of infertility diagnostic tests and treatments. The ESHRE Capri Workshop Group. Hum Reprod 2000;15:723–32 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.