Abstract
Objectives
To describe the major characteristics of reported notifiable gastrointestinal illness (NGI) data in the Northwest Territories (NWT) from January 1991 through December 2008.
Design
Descriptive analysis of 708 reported cases of NGI extracted from the Northwest Territories Communicable Disease Registry (NWT CDR).
Setting
Primary, secondary and tertiary health care centres across all 33 communities of the NWT.
Population
NWT residents of all ages with confirmed NGI reported to the NWT CDR from January 1991 through December 2008.
Main outcome measure
Laboratory-confirmed NGI, with a particular emphasis on campylobacteriosis, giardiasis and salmonellosis.
Results
Campylobacteriosis, giardiasis and salmonellosis were the most commonly identified types of NGI in the territory. Seasonal peaks for all three diseases were observed in late summer to autumn (p<0.01). Higher rates of NGI (all 15 diseases/infections) were found in the 0–9-year age group and in men (p<0.01). Similarly, rates of giardiasis were higher in the 0–9-year age group and in men (p<0.02). A disproportionate burden of salmonellosis was found in people aged 60 years and older and in women (p<0.02). Although not significant, the incidence of campylobacteriosis was greater in the 20–29-years age group and in men (p<0.07). The health authority with the highest incidence of NGI was Yellowknife (p<0.01), while for salmonellosis and campylobacteriosis, it was Tlicho (p<0.01) and for giardiasis, the Sahtu region (p<0.01). Overall, disease rates were higher in urban areas (p<0.01). Contaminated eggs, poultry and untreated water were believed by health practitioners to be important sources of infection in cases of salmonellosis, campylobacteriosis and giardiasis, respectively.
Conclusions
The general patterns of these findings suggest that environmental and behavioural risk factors played key roles in infection. Further research into potential individual and community-level risk factors is warranted.
Article summary
Article focus
To date, there are very little baseline data on Notifiable Gastrointestinal Illness (NGI) in the Northwest Territories (NWT), where Aboriginal people constitute a majority of the population. The demographic, socio-cultural and health conditions of northern Aboriginal people are markedly different from those of other Canadian populations.
There is a clear need to identify the major characteristics of reported NGI in order to generate hypotheses, guide future studies and help public health officials target resources, interventions or increased surveillance to areas of greatest need in the NWT.
Key messages
The annual average rate of NGI over the study period was 95.5 cases per 100 000 with increased risk in the 0–9-year age group and men.
Reported rates of NGI declined from 1991 to 2008; however, seasonal peaks were observed in late summer and autumn.
There was variability in the rates of NGI with higher notifications in the southern urban areas compared with the northern rural/remote areas of the territory suggesting the possible involvement of geographical risk factors and/or bias in the surveillance data.
Strengths and limitations of this study
The study provides a historical portrait of NGI as the NWT CDR broadly covered the entire territory over 18 years, therefore allowing comparisons across communities and time periods.
Due to under-reporting, the rates of infections reported in this study are likely underestimates of the true incidence of diseases and therefore should be interpreted as reporting rates rather than as incidence rates.
Suspected sources of infection are infrequently confirmed by microbiological testing; therefore, the results regarding ‘suspected exposure’ must be viewed with caution and be thought of as hypotheses.
Background
Notifiable gastrointestinal illness (NGI) is an important global public health issue and a growing concern in the Northwest Territories (NWT), where Aboriginal people constitute a majority of the population.1 The Aboriginal population of the NWT maintains strong ties to the environment, continually adapting and learning to use available resources to provide food and other necessities, sustain livelihoods and reinforce social relations.2 Foods obtained by harvesting, hunting, fishing and trapping are referred to as traditional or country foods. About 40-60% of NWT residents living in remote and/or isolated communities rely on country foods for 75% or more of their meat and fish consumption.3
Country foods in the NWT vary by geographic area, season, climate and availability and include items such as caribou, moose, ducks, geese, seals, hare, grouse, ptarmigan, lake trout, char, inconnu, white fish, pike and burbot.4 5 Due to the harsh climate, animal products are the staple, and fresh vegetables and fruits provide additional nutrients when available. During the short summers, items such as blueberries, cranberries, blackberries and cloudberries are gathered, both for eating fresh and for drying or freezing to eat during the winter.4 The consumption of untreated water from lakes, creeks and rivers in the summer or from melted ice or snow in winter and spring is also common practice during subsistence activities.6
A well-balanced diet is primarily achieved by consuming muscle meat and other parts of the animals (raw or with minimal processing) such as the stomach, liver and fat, which contain iron, calcium and a range of vitamins.7 Common traditional meats are also an excellent source of protein and are lower in fat compared with meats eaten in Southern Canada. Seal and whale are good sources of omega-3 fatty acids, which help reduce the risk of chronic conditions such as cardiovascular disease.7 Although the traditional diet is nutritious, it is also very high in calories. High caloric intake is an adaptation feature that enables residents of the North to keep warm through the long frigid winters.5
Sharing food is a key element of the Aboriginal culture in the NWT. Traditionally, when hunters return to communities with fresh game or fish, it is distributed according to social rules or convention.2 Meals are communal and fresh, uncooked animal-derived foods are first given out to people who are cold or hungry, then to the rest of the community and, finally, the remaining portion is shared within the household. The distribution and consumption of raw meats can occur several times in a week.2
Activities such as hunting, fishing and trapping as well as the traditional preparation, storage and consumption of wild game, seafood and untreated water can increase exposure to pathogenic agents in the environment.8 Illness can result from the ingestion of microorganisms in contaminated food or water, through contact with animals or other contaminated objects and some infections can be further spread by person-to-person transmission.9 Symptoms can include loss of appetite, abdominal cramps, diarrhoea of variable severity, nausea, vomiting and fever.10 Estimates of the overall morbidity and identification of potential risk factors for NGI in the NWT have not been previously published in the literature and hence, there are very little baseline data to inform policies and guide public health interventions in the territory. Using data elements extracted from cases of NGI in the Northwest Territories Communicable Disease Registry (NWT CDR), this study provides a descriptive analysis of reported NGI in the NWT from January 1991 through December 2008.
Methods
Study area
The NWT is located in Northern Canada with a majority Aboriginal population (50.3%).11 As of the 2006 Census, the population was 41 464, an increase of 11% from 2001.3 There are 33 officially recognised communities across 1 140 835 km2 of land; the smallest is Kakisa with 52 residents and the largest is Yellowknife with 18 700 residents.12 The NWT population density is 0.03 people per square kilometre. There is a high proportion of children under 15 years of age (23.9%) and a low proportion of people over 65 years of age (4.7%).12 The median age for both sexes is 31 years; men comprise a majority of the population (51.2%).12
Data sources
Data on reported cases of NGI for the period January 1991 through December 2008 were obtained from the NWT CDR. Reported NGI is an umbrella term for 15 enteric, foodborne and waterborne conditions that were reportable under the NWT Public Health Act during the study period: amoebiasis, botulism, brucellosis, campylobacteriosis, cryptosporidiosis, infection with Escherichia coli, food poisoning, giardiasis, hepatitis A, listeriosis, salmonellosis, shigellosis, tapeworm, tularemia and yersiniosis. Ethics approval was obtained from the University of Guelph Research Ethics Board, the Government of the Northwest Territories (GNWT) and the Aurora Research Institute.
The NWT Communicable Disease Manual provides guidelines to assist public health practitioners with decision making about specific situations and to support consistency of territorial public health practice13; therefore, the general procedures for notification remained consistent over the study period. Upon symptomatic presentation of NGI as described in the Manual, health practitioners send the patient's clinical specimen to the laboratory for confirmation and serotyping. The patient's demographic information, food and water histories are collected by the health practitioner and manually entered into the foodborne and waterborne illness investigation form. The paper form is submitted to the Population Health Division of the GNWT Department of Health and Social Services (DHSS). Health practitioners and laboratories are required to report patients with confirmed NGI to the Population Health Division within 24 h. Once the paper form is received, disease registry officers at the territorial level collate, verify, enter and disseminate illness investigation data electronically through the Integrated Public Health Information System for inclusion into the NWT CDR and the National Notifiable Disease Database at the Public Health Agency of Canada.13
Case notification data, stripped of personal identifiers, were received for 15 diseases/infections and associated fields listed in table 1; none of these fields were considered mandatory at the time of notification. A geographical conversion database was used to assign case–patients to their respective census subdivision (community), Health and Social Services Authority (HSSA) as well as assign them a status of rural or urban location; cases were classified as urban if reported at a health centre located in a community of at least 1000 persons and 400 persons people per square kilometre, and others were classified as rural.3 12
Table 1.
Notifiable gastrointestinal illness (NGI) and associated per cent missing or unspecified values, by field and disease, Northwest Territories, Canada, 1991–2008
| Disease/agent (number of reported cases 1991–2008) | Notifiable disease report form fields—per cent missing values |
|||||||
| Age | Gender | Community | Health unit | Report date | Etiologic agent | Subtype | Suspected exposure | |
| Amoebiasis (n=10) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 60.0 |
| Botulism (n=8) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 12.5 | 25.0 |
| Brucellosis (n=3) | 0.0 | 0.0 | 0.0 | 33.3 | 0.0 | 0.0 | 66.7 | 66.7 |
| Campylobacteriosis (n=175) | 0.0 | 0.0 | 2.3 | 0.6 | 0.0 | 0.0 | 0.0 | 79.4 |
| Cryptosporidiosis (n=18) | 0.0 | 0.0 | 11.1 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 |
| Escherichia coli (VTEC) (n=40) | 0.0 | 0.0 | 12.5 | 0.0 | 0.0 | 0.0 | 0.0 | 62.5 |
| Food poisoning* (n=10) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | 10.0 |
| Giardiasis (n=205) | 0.0 | 0.0 | 3.9 | 0.0 | 0.0 | 0.0 | 0.0 | 73.7 |
| Hepatitis A (n=10) | 0.0 | 0.0 | 10.0 | 0.0 | 0.0 | 0.0 | 0.0 | 90.0 |
| Listeriosis (n=1) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 |
| Salmonellosis (n=202) | 0.0 | 0.0 | 0.0 | 4.5 | 0.0 | 0.0 | 0.0 | 70.8 |
| Shigellosis (n=12) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 83.3 |
| Tapeworm (n=7) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 57.1 |
| Tularemia (n=1) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 |
| Yersiniosis (n=6) | 0.0 | 0.0 | 16.7 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 |
| Total NGI cases (n=708) | 0.0 | 0.0 | 2.6 | 0.1 | 0.0 | 0.0 | 1.8 | 73.2 |
Includes five cases due to clostridium and five cases due to bacillus. Infections from these agents are not notifiable unless they are from food poisoning.
Data quality evaluation and descriptive analyses
Data quality evaluation involved manually checking data associated with each case for completeness and internal consistency. Missing values were replaced with the term ‘unspecified’. The numbers and percentages related to ‘unspecified’ values were calculated for each field.
Population denominators for each year were obtained from the NWT Bureau of Statistics and the mean annual age-specific rates of disease were calculated for the territory. The average annual number of cases was calculated using the total number of notifications divided by 18 years. Data manipulation and statistical analyses were conducted in SPSS V.17 (SPSS Inc.), and choropleth maps of disease rates by health authority were created in ArcView GIS V.3.1 (ESRI). Means and medians were used to describe the data; medians were used when dealing with highly skewed distributions. A least squares regression analysis was used to determine the rate of change over time. Fischer's exact tests were used to determine statistical significance (p<0.05 (two-tailed)) for categorical variables. Community-level risk factors for NGI are reported elsewhere.14
Results
The percentages of missing or unspecified values for the nine fields considered in the analysis are shown in table 1.
From the 708 case–patients with NGI from all years, 458 (64.7%) had bacterial infections, 240 (33.9%) had parasitic infections and 10 (1.4%) had viral (hepatitis A) infections. The three largest contributors to the total number of notifications were giardiasis with 205 cases (29.0%), salmonellosis with 202 cases (28.5%) and campylobacteriosis with 175 cases (24.7%). Too few cases were attributed to other agents (<6% each) to draw inferences; therefore, the focus of the rest of this paper was on the three most commonly notified diseases.
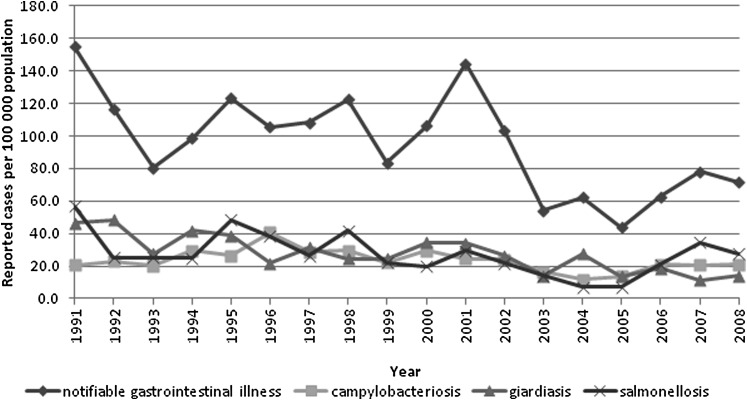
The annual reported incidence rates of NGI (total and cause specific for giardiasis, salmonellosis and campylobacteriosis) are shown in figure 1. A least squares regression analysis indicated that the incidence of NGI decreased by 3.7 (p<0.01) cases per 100 000 per year over the study period. Giardiasis and salmonellosis decreased by 1.7 (p<0.01) and 1.2 (p<0.01) cases per 100 000 per year, respectively, but there was no significant (p<0.13) linear change in incidence of campylobacteriosis. A majority of campylobacteriosis (85.7%), giardiasis (62%) and salmonellosis (58.4%) cases were reported from health facilities in urban areas (p<0.01).
Figure 1.
Annual incidence rates of notifiable gastrointestinal illness (total and cause specific for giardiasis, salmonellosis and campylobacteriosis), Northwest Territories, Canada, 1991–2008.
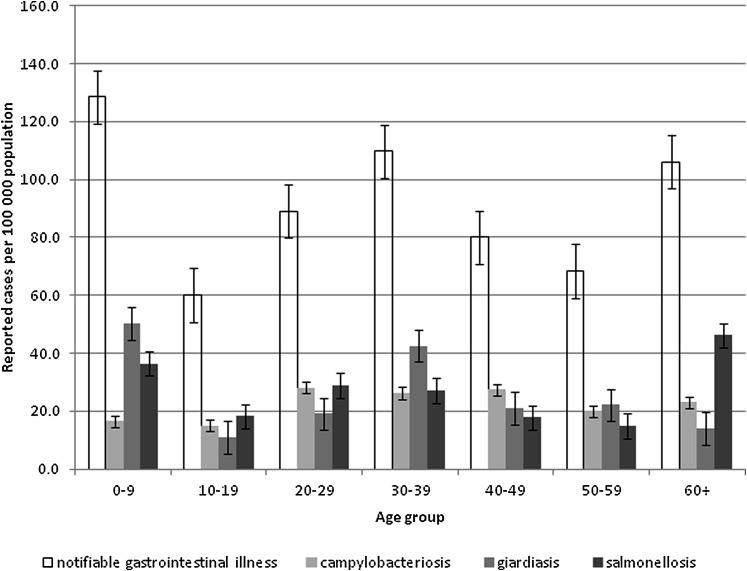
The average annual incidence of NGI (total and cause specific for giardiasis, salmonellosis and campylobacteriosis) by age group are shown in figure 2. The highest rates of NGI (128.5 cases per 100 000) were observed in the 0–9-year age group with 56% of cases occurring in men (p<0.01). The highest rates of giardiasis (50.4 cases per 100 000) were also found in the 0–9-year age group with 57% of cases occurring in men (p<0.02). The highest rates of salmonellosis (46.1 cases per 100 000) were found in the 60+-year age group with 51% occurring in women (p<0.02). Although not significant (p<0.07), the highest rates of campylobacteriosis were observed in the 20–29-year age group for campylobacteriosis (28.2 cases per 100 000) with 53% of cases occurring in men.
Figure 2.
Incidence of notifiable gastrointestinal illness (total and cause specific for giardiasis, salmonellosis and campylobacteriosis) by age group, Northwest Territories, Canada, 1991–2008.
Table 2 shows that the most frequently suspected vehicle for NGI was contaminated food (p<0.01). The probable source of giardiasis was most often attributed to untreated water, whereas for campylobacteriosis and salmonellosis, it was poultry and eggs, respectively (p<0.01).
Table 2.
Percentage distribution of reported suspected sources of infection for notifiable gastrointestinal illness (NGI), campylobacteriosis, giardiasis and salmonellosis, Northwest Territories, Canada, 1991–2008
| Suspected exposure (%) | Per cent of cases attributed to suspected exposure |
|||
| NGI | Campylobacteriosis | Giardiasis | Salmonellosis | |
| Beef | 6.8 | 2.8 | 3.7 | 3.4 |
| Caribou | 6.3 | 2.8 | 5.6 | 5.1 |
| Fish/seafood | 3.2 | 11.1 | 0.0 | 1.7 |
| Muktuk (whale) | 1.6 | 0.0 | 0.0 | 0.0 |
| Pork | 4.7 | 2.8 | 0.0 | 13.6 |
| Poultry/eggs | 18.9 | 38.9 | 1.9 | 33.9 |
| Seal | 0.5 | 0.0 | 0.0 | 0.0 |
| Foodborne unknown | 28.4 | 41.7 | 5.6 | 37.3 |
| Untreated water | 27.9 | 0.0 | 81.5 | 0.0 |
| Waterborne unknown | 0.5 | 0.0 | 1.9 | 5.1 |
| Perinatal transmission | 0.5 | 0.0 | 0.0 | 0.0 |
| Person-to-person | 0.5 | 0.0 | 0.0 | 0.0 |
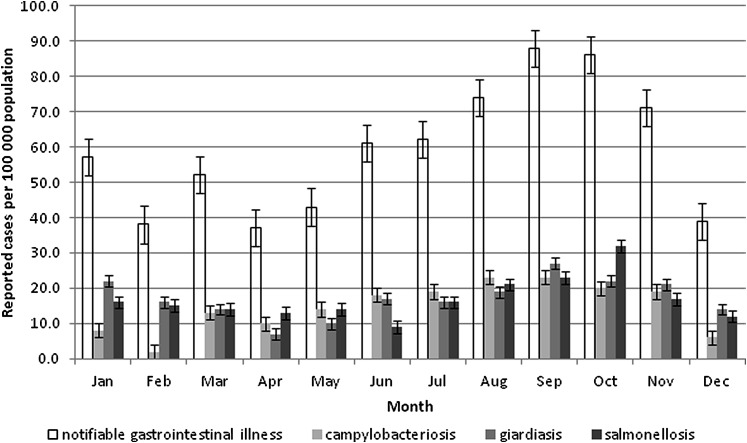
Figure 3 shows that cases of NGI (p<0.01) and more specifically campylobacteriosis (p<0.01) and salmonellosis (p<0.04) occurred more frequently in the late summer and early fall. Although not significant (p<0.07), giardiasis showed a similar trend on visual inspection of the data.
Figure 3.
Incidence of notifiable gastrointestinal illness (total and cause specific for giardiasis, salmonellosis and campylobacteriosis) by month, Northwest Territories, Canada, 1991–2008.
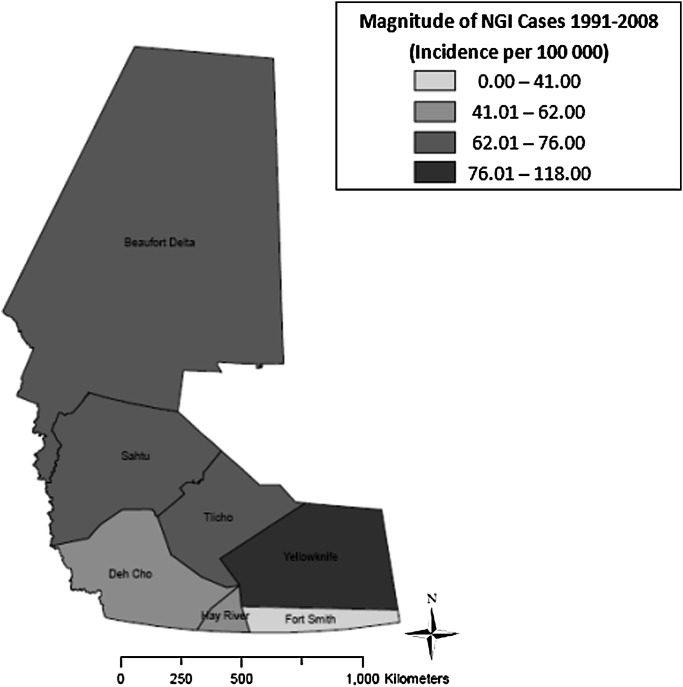
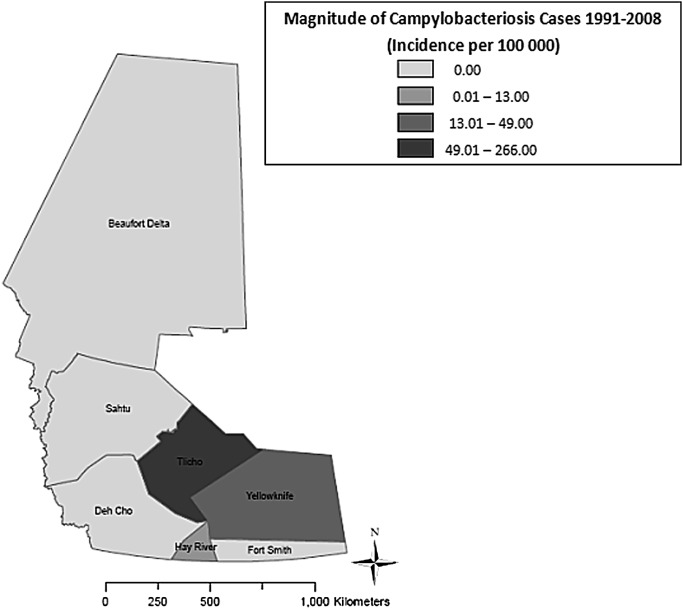
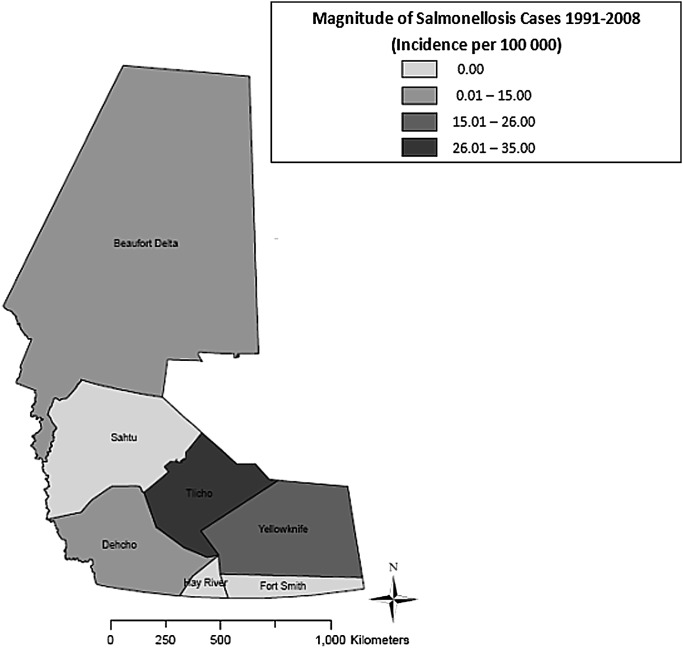
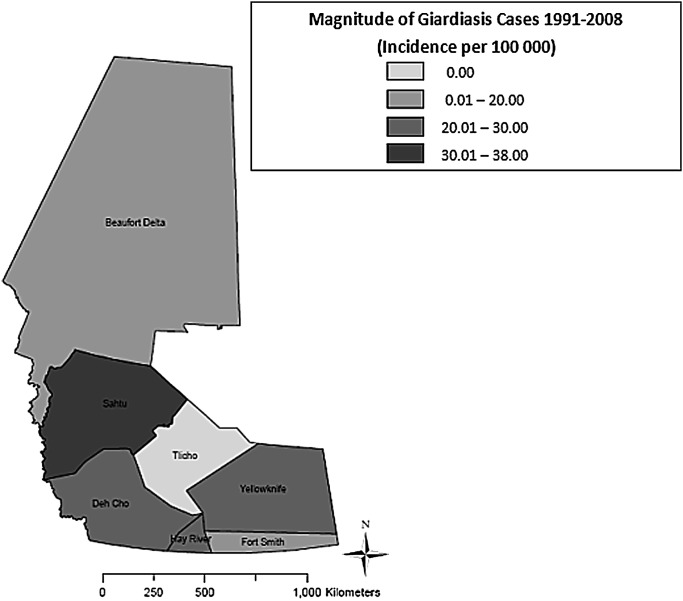
As shown in figure 4, the highest median annual incidence of NGI (118.0 cases per 100 000) was observed in Yellowknife HSSA (p<0.01), whereas the lowest median annual incidence (41.0 cases per 100 000) was found in Fort Smith HSSA (p<0.01). Figure 5 shows that the highest median annual incidence of campylobacteriosis (265.5 cases per 100 000) was found in Tlicho HSSA (p<0.01), whereas the lowest median annual incidence (0.0 cases per 100 000) was found in Beaufort Delta, Dehcho, Fort Smith and Sahtu HSSAs (p<0.01). Figure 6 shows the highest median annual incidence of salmonellosis (35.0 cases per 100 000) was also found in Tlicho HSSA (p<0.01); however, the lowest median annual incidence (0.0 cases per 100 000) was found in Fort Smith, Hay River and Sahtu HSSAs (p<0.01). Figure 7 shows highest median annual incidence of giardiasis (38.0 cases per 100 000) was found in the Sahtu HSSA (p<0.01), whereas the lowest median annual incidence (0.0 cases per 100 000) was found in Tlicho HSSA (p<0.01).
Figure 4.
Map of incidence rates per 100 000 population for reported cases of notifiable gastrointestinal illness (NGI), Northwest Territories, Canada, 1991–2008.
Figure 5.
Map of incidence rates per 100 000 population for reported cases of campylobacteriosis, Northwest Territories, Canada, 1991–2008.
Figure 6.
Map of incidence rates per 100 000 population for reported cases of salmonellosis, Northwest Territories, Canada, 1991–2008.
Figure 7.
Map of incidence rates per 100 000 population for reported cases of giardiasis, Northwest Territories, Canada, 1991–2008.
Discussion
The results of this study suggest that NGI is an important health problem in the NWT and that giardiasis, salmonellosis and campylobacteriosis account for the great majority (82.2%) of reported NGI in the territory. The mean annual reported rate of these three enteric diseases in the NWT was 78.0 cases per 100 000, which is less than reported for Ontario (87.0 cases per 100 000) and British Columbia (145.8 cases per 100 000) based on notifiable disease data from 1991 through 2008.9 This may suggest that compared with some southern areas as of Canada, NWT residents may be at decreased risk of infection or alternatively, there may be a higher degree of under-reporting in the territory15 16; further investigation is required. Previous studies have shown that about one of 313 (Ontario) to 350 (British Columbia) cases of acute gastrointestinal illness are captured by surveillance systems.17 18 Using these adjustment factors from Ontario and British Columbia, we estimate that between 182 748 and 204 282 cases of campylobacteriosis, giardiasis and salmonellosis, collectively, may have occurred in the NWT over the 18 years.10 11 19 Several explanations for under-reporting have been proposed, such as cases not presenting to medical facilities, health workers not submitting clinical samples to laboratories, laboratory test sensitivity issues, absence or delay of reporting from local to territorial health authorities. Patients may not seek medical attention because symptoms are mild and self-limiting, they may be too ill to travel or they may prefer to seek treatment from local healers.19 These tendencies are exacerbated in rural/remote communities of the NWT due to the relative paucity of available health services, facilities and health professionals. Increased distances to health facilities and transportation problems further aggravate other barriers to accessing the health systems in rural/remote settings in northern communities.15 16 There are no data addressing possible geographical reporting biases in the NWT; therefore, research to characterise and quantify reporting bias in the NWT CDR is needed. Reduction of under-reporting and differential reporting (if it does exist) would require increased awareness of community health practitioners about the potential usefulness of surveillance data and therefore the need to improve their quality.
In the NWT, seasonal peaks over the study period may have been attributed to social environmental factors such as higher ambient temperatures, frequent travel for subsistence activities, centralised outdoor meal preparation as well as the consumption of country foods and surface water.4 6 20–23 Control strategies, such as regular, coordinated public education and communication about known risk factors of the disease (eg, drinking contaminated water, safe food preparation), would therefore need to be targeted during these seasons. Such public health programmes need to take into account the wide geographic distribution of these communities, their cultural diversity and the number of languages used.24 Community-oriented media, such as local television and radio, have proven to be successful methods of reaching rural/remote populations by providing a forum for which health issues can be identified and discussed thus, increasing general awareness.25–27
Fluctuations in rates of NGI over the 18 years are likely to be explained, at least in part, by random variation due to a small number of cases. The peaks in 1995 and 2001 also coincide with known outbreaks of salmonellosis and cryptosporidiosis, respectively.28 The incidence of NGI, however, declined over the last few years of the study period (since 2002), which is consistent with observed trends in Southern Canada and the USA. The decline may be attributed to effective ongoing efforts to improve food and water quality or an artefact of diagnostic procedures, reporting practices or changes in population demographics.29 30 The extent to which these factors may have contributed to a decrease in incidence is unknown but it is an important topic for future research. The statistically significant decreasing trend of NGI incidence, however, is inconsistent with the predicted temperature-driven increase of enteric disease in the North.31 Since the 1940s (when record collection began), the average annual temperature in the NWT has increased by about 2°C and scientists predict that temperatures will continue to warm due to climate change.32 The potential impact of warmer temperature on the incidence of NGI in the NWT should be further explored.
Spatial analysis revealed that the incidence of campylobacteriosis, giardiasis and salmonellosis varied substantially between health authorities. Higher or lower than expected rates in health authorities could be a result of disparities in the geographical distribution of risk factors and behaviours,33 suggesting that further studies on population-level risk factors are warranted. Overall, NGI was reported more frequently in urban than rural areas, but the underlying reasons could not be evaluated with the available data. In theory, higher reporting rates in urban areas could reflect greater propensity for person-to-person transmission; however, this is more commonly seen with organisms with human reservoirs.34 Other possibilities include greater accessibility, affordability and/or reliance on store-purchased foods, restaurant meals and foreign travel as well as other population-level risk factors such as community water systems.35 It is also possible that some infections were acquired in rural/remote areas of the NWT but were reported at health facilities in urban areas.36 We expected exposure to these environmental or zoonotic pathogens to be more common in rural/remote areas, through contact with animals, their feces, as well as contaminated surface water and raw foods compared with urbanised areas.37 Furthermore, higher disease rates could also be an artefact of differential reporting of cases or methods of data collection that vary by area or practitioners. Several studies have demonstrated that higher reporting rates in urban areas are often a function of the amount and type of available health services, rather than the occurrence of illness itself.38–40
Giardiasis was the most commonly reported infection in this study, reflecting its importance as an enteric pathogen in the territory. Giardiasis commonly occurs through the ingestion of infective cysts found in contaminated water, food or infected persons by the fecal–oral route. The cysts can be present in contaminated wells and water systems, particularly those sourced from surface water such as fresh water lakes and streams. Person-to-person transmission also accounts for many Giardia infections and is usually associated with poor hygiene and sanitation. In the Arctic, cysts of Giardia spp. have been found in water, sewage and fecal samples of marine mammals harvested for food.23 Our findings of higher rates in infants and children in the NWT could be related to reporting bias, poor hygiene, more frequent exposure to communal facilities or recreational water, lack of protective immunity, or a combination of factors.41 42 High rates in patients 30–39 years of age may also be at least partially attributed to contact with infected children as parents or as caregivers, and these persons are possibly more likely to seek medical care and therefore more likely to be captured by the surveillance system.43 The higher rate of giardiasis in men is unexplained but has also been noted in other studies.44 In the NWT, gender may act as a surrogate for true causal variables related to exposure, such as the consumption of untreated surface water or contaminated traditional foods, particularly while carrying out subsistence activities in northern areas of the NWT. Consistent with previous research, the incidence of giardiasis in this study was higher in the late summer and autumn months, which may be related to greater environmental exposure during leisure and subsistence activities, potentially greater likelihood of infectious levels of cysts in water at this time of year, or exposure to contaminated recreational water that favours indirect person-to-person transmission.45
Salmonellosis, the second most frequently reported enteric infection, is commonly acquired from consuming contaminated food of animal origin, mainly meat, poultry, eggs and milk, but also contaminated fruit and vegetables.36 In the NWT, poultry/eggs were identified by those reporting illness as the most probable sources of this infection. Other suspected food vehicles included pork, caribou, beef and fish/seafood; however, we do not know whether these vehicles were identified through epidemiological investigation, follow-up microbiological testing or speculation by the health practitioner. Moreover, we do not know whether suspect foods were obtained through individual subsistence activities, community freezers or retail locations making it difficult to hypothesize the source of microbial contamination; however, outbreaks of verotoxin-producing E coli O157:H7 (fourth highest notification) in the NWT have been attributed to frozen minced beef and caribou obtained from grocery stores and homes.46 47 Higher observed rates of salmonellosis in infants and children (0–9-year age group) and the elderly (60+-year age group) in this study have been noted in a previous study and may be related to lack of protective immunity or other factors mentioned for giardiasis.41 48 49 Higher rates of disease in women are so far unexplained, but further research considering differences in food handling practices and hygiene as well as the types of foods consumed may indicate their role in apparent gender differences.50 Higher rates of infection in the late summer and autumn months may be attributable to environmental and social factors. These may include higher ambient temperatures, frequent travel as well as higher prevalence in food animal populations, centralised outdoor meal preparation and consumption related to large social gatherings.20 51
Campylobacteriosis, the third most frequently reported infection, commonly occurs through the poor handling of raw poultry and consumption of undercooked poultry, unpasteurised milk and contaminated drinking water. Campylobacter is also common in migratory birds and the consumption of fresh water from surface contaminated with bird feces could be a seasonal driver of this disease in the North.52 In the NWT, the predominant mode of transmission was believed to be foodborne; poultry/eggs, pork, caribou, beef and fish/seafood from unspecified sources were once again identified as probable exposures for infection. Incidence rates were highest in adults 20–29 years of age. The relatively higher rates in young males noted in other studies have been thought to reflect poor hygiene and food handling practices.53 As with other studies on campylobacteriosis, disease occurred more frequently in the late summer and autumn months.54 Traditionally, in northern communities, hunting activities and the collection of plants, berries and bird's eggs as well as the consumption of surface water occur more frequently during this time period.4 Campylobacter, however, are more susceptible to freezing than other bacteria; therefore, it is tempting to speculate that the colder northern climate may play a role in reducing exposure in food and water.
Cryptosporidium infections in humans may be from either human or animal origin, and no attempts were made to differentiate among strains in this study. The apparent low incidence of pathogens such as Cryptosporidium (2.4 cases per 100 000) in the NWT may be due to the lack of exposure to agricultural animals in the North.55 Domestic livestock including beef and dairy cattle as well as sheep are often perceived to be the leading environmental source of waterborne pathogens,56 although contamination from human sewage also occurs. Animals shed oocysts through manure contributing to the Cryptosporidium load of drinking water sources.57 Several studies have shown that concentrations of Cryptosporidium are significantly higher in agricultural rather than non-agricultural watersheds.58 59 The role of wildlife as a source of Cryptosporidium is less clear in published literature. A study conducted over a 4-year period in Northern Alaska found that the prevalence of Cryptospordium spp. in fecal samples of marine mammals from subsistence hunts was highest in ringed seals (22.6%) followed by right whales (24.5%) and bowhead whales (5.1%).60 A study in Nunavik (Quebec, Canada) also found a prevalence of 9% in fecal samples of ringed seals.61 These studies suggest that some animals used in traditional foods may be reservoirs for the disease in the North. In this study, caribou, muktuk and seal were also suspected sources of infection for 8.4% of NGI cases; therefore, further evaluations of environmental risk factors in the NWT are warranted.
This study demonstrates the usefulness of surveillance data to guide epidemiological research and public health practice in northern communities. Of the nine reporting fields in the NWT CDR, eight had <5% of data missing; however, the field ‘suspected exposure’, unknown (missing) for 73.2% of the records, is a source of potential bias. Exposure information is frequently ascertained through an interview or questionnaire; thus, it is difficult to assess the extent to which recall or reporting bias has occurred and there are obvious limitations on the quality of exposure data obtained in this fashion. In addition, suspected sources are infrequently confirmed by microbiological testing; therefore, the results regarding the ‘suspected exposure’ must be viewed with considerable caution and can be thought of as hypotheses. For the data to be useful, particularly for risk factor identification, it is essential that the completeness of fields and hence, quality be improved. From 1991 to 2008, there were no mandatory fields enforced by the GNWT. Due to the contextual challenges of conducting surveillance in northern rural/remote communities, the NWT CDR is based around the minimum data set concept, where the focus is on collecting the most essential data fields; however, these fields must be standardised and sufficiently detailed to support the delivery, planning and monitoring of public health initiatives. Although issues related to data quality are not unique to surveillance systems serving northern rural/remote areas, they may be exacerbated when the systems serve sparse populations and have inadequate infrastructure, human and financial resources.62 The implementation of electronic-based platforms for reporting has been shown to improve data quality and completeness in low-resource settings.63 64
Published knowledge on surveillance in rural/remote areas is sparse; as a result, very little has been recommended in terms of cohesive and effective approaches to enhance surveillance in these communities. The gap in the literature suggests that the development of a comprehensive public health surveillance system for rural/remote communities, which takes into account local realities and needs, is a priority area for research; however, this will require a collaborative effort from stakeholders, partners and knowledge users of the system. Suggestions for moving forward include a collaborative design of suitable data elements, data collection protocols, data quality assurance, research and evaluation training, and procedures for confidential data entry and transfer. The existing literature recommends several strategies to augment insufficient data from traditional health surveillance. Andresen et al65 suggest methodological approaches such as aggregation, spatial smoothing, small area estimation and exact statistics. Sentinel surveillance, population-based sample surveys, community-based observations and syndromic surveillance can also be used as surrogates for more widespread surveillance.65 66 The capacity to generate high-quality surveillance data in northern rural/remote populations, such as those in the NWT, may exist if innovative, informal and population-specific approaches are considered and applied to public health surveillance.
In 2011, the DHSS, GNWT, introduced a new electronic tool to improve surveillance for NGI. The application, called DHSS Tools, is a restricted-access site which includes a case reporting module (environmental health—foodborne and waterborne illness investigation) that can be used by community public health officers, disease consultants, epidemiologists and environmental health officers to ensure better communication, follow-up, decision making and completeness of information.
In summary, the results of the study indicate that giardiasis, salmonellosis and campylobacteriosis were the most important enteric diseases in the NWT from 1991 through 2008, and the incidence declined in later years of the study period. There was increased risk of NGI in the late summer and early fall, in infants and children, men and urban residents. The geographical distribution of case–patients varied by disease, suggesting that environmental and behavioural risk factors played key roles in infection and may provide opportunities for prevention. For future study, multivariable regression and spatial analyses at the community level are necessary for valid risk factor identification as well as for implementing specific and geographically appropriate risk reduction and control strategies. It is anticipated that this information will guide future research as well as the allocation of resources for prevention, promotion and control initiatives.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the Government of the Northwest Territories Department of Health and Social Services and Bureau of Statistics for providing the data to complete this study.
Footnotes
To cite: Pardhan-Ali A, Wilson J, Edge VL, et al. A descriptive analysis of notifiable gastrointestinal illness in the Northwest Territories, Canada, 1991–2008. BMJ Open 2012;2:e000732. doi:10.1136/bmjopen-2011-000732
Contributors: AP-A contributed to the manuscript through study design and planning, data collection, analysis and interpretation of results, drafting the manuscript and response to editorial comments and preparation of the final manuscript for submission. JW, VLE, CF, RR-S and SAM contributed to the manuscript through study design and planning, consultation on study progress, troubleshooting, data analysis and interpretation of results, reviewing and commenting on manuscript drafts. MS contributed to the manuscript through data collection, interpretation of results and reviewing and commenting on manuscript drafts.
Funding: This work was supported by Nasivvik Centre for Inuit Health and Changing Environments.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The data set may be requested from Population Health, Department of Health and Social Services, Government of the Northwest Territories (http://www.hlthss.gov.nt.ca).
References
- 1.Lambden J, Receveur O, Kuhnlein H. Traditional food attributes must be included in studies of food security in the Canadian Arctic. Int J Circumpolar Health 2007;66:308–19 [DOI] [PubMed] [Google Scholar]
- 2.Fienup-Riordan A. The Nelson Island Eskimo: Social Structure and Ritual Distribution. Anchorage, AK: Alaska Pacific University Press, 1983 [Google Scholar]
- 3.Statistics Canada Aboriginal Peoples in Canada in 2006: Inuit, Métis and First Nations, 2006 Census. Ottawa, ON: Statistics Canada, 2008 [Google Scholar]
- 4.Kuhnlein HV, Turner NJ. Traditional Plant Foods of Canadian Indigenous Peoples: Nutrition, Botany, and Use. Volume 8, Food and Nutrition in History and Anthropology. Philadelphia, PA: Gordon and Breach, 1991 [Google Scholar]
- 5.Kuhnlein H. Global Nutrition and the Holistic Environment: The Path to Healing. Ottawa, ON: Royal Commission on Aboriginal Peoples: The Path to Healing, 1993 [Google Scholar]
- 6.Anctil M. Nunavik Inuit Health Survey 2004, Qanuippitaa? How Are We? Survey Highlights. Quebec, ON: Institut national de santé publique du Québec & Nunavik Regional Board of Health and Social Services, 2008 [Google Scholar]
- 7.Berti PR, Hamilton SE, Receveur O, et al. Food use and nutrient adequacy in Baffin Inuit children and adolescents. Can J Diet Pract Res 1990;60:63–70 [PubMed] [Google Scholar]
- 8.Ross P, Olpinski S, Curtis M. Relationships between dietary practice and parasite zoonoses in northern Québec Inuit. Etud Inuit 1989;13:33–47 [Google Scholar]
- 9.Epstein P. Climate and health. Science 1999;285:347–8 [DOI] [PubMed] [Google Scholar]
- 10.Majowicz SE, Doré K, Flint JA. Magnitude and distribution of acute, self-reported gastrointestinal illness in a Canadian community. Epidemiol Infect 2004;132:607–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacDougall L, Majowicz S, Dore K, et al. Under-reporting of infectious gastrointestinal illness in British Columbia, Canada: who is counted in provincial communicable disease statistics?. Epidemiol Infect 2008;136:248–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Statistics Canada 2001 Aboriginal Peoples Survey Community Profiles. Ottawa, ON: Statistics Canada, 2004. http://www12.statcan.ca/english/profil01aps/home.cfm (accessed 28 Apr 2010). [Google Scholar]
- 13.Department of Health and Social Services, Government of the Northwest Territories Communicable Disease Manual. Yellowknife, NWT: Government of the Northwest Territories, 2007 [Google Scholar]
- 14.Pardhan-Ali A. An Investigation of Notifiable Gastrointestinal Illness in the Northwest Territories [PhD Thesis]. Guelph, ON: University of Guelph, 2011 [Google Scholar]
- 15.Inuit Tapiriit Kanatami Backgrounder on Inuit Health: For Discussion at Health Sectoral Meeting, November 4th and 5th, 2004. Ottawa, ON: Inuit Tapiriit Kanatami, 2004 [Google Scholar]
- 16.Marrone S. Understanding barriers to health care: a review of disparities in health care services among Indigenous populations. Int J Circumpolar Health 2007;66:188–98 [DOI] [PubMed] [Google Scholar]
- 17.Thomas MK, Majowicz SE, MacDougall L, et al. Population distribution and burden of acute gastrointestinal illness in British Columbia, Canada. BMC Public Health 2006;6:307–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majowicz SE, McNab WB, Socket P, et al. Burden and cost of gastroenteritis in a Canadian community. J Food Prot 2006;69:651–9 [DOI] [PubMed] [Google Scholar]
- 19.Flint JA, Doré K, Majowicz SE, et al. From stool to statistics: reporting of acute gastrointestinal illnesses in Canada. Can J Public Health 2004;95:309–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Souza RM, Becker NG, Hall G, et al. Does ambient temperature affect foodborne disease? Epidemiology 2004;15:86–92 [DOI] [PubMed] [Google Scholar]
- 21.Leclair D, Forbes LB, Suppa S, et al. A preliminary investigation on the infectivity of Trichinella larvae in traditional preparations of walrus meat. Parasitol Res 2004;93:507–9 [DOI] [PubMed] [Google Scholar]
- 22.Olson ME, Roach PD, Stabler M, et al. Giardiasis in ringed seals from the western arctic. J Wildl Dis 1997;33:646–8 [DOI] [PubMed] [Google Scholar]
- 23.Roach PD, Olson ME, Whitley G, et al. Waterborne Giardia cysts and Cryptosporidium oocysts in the Yukon, Canada. Appl Microbiol 1993;59:67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spencer D, Schlemmer E. Crossing the cultural divide. Aust Nurs J 1997;4:20–2 [PubMed] [Google Scholar]
- 25.de Bocanegra HT, Gany F. Good provider, good patient: changing behaviors to eliminate disparities in healthcare. Am J Manag Care 2004;10:20–8 [PubMed] [Google Scholar]
- 26.DeBuono B. Health literacy: a hidden and critical challenge to effective health care. AHIP Cover 2004;45:38–40 [PubMed] [Google Scholar]
- 27.Richardson D. Proceedings of the Don Snowden Rural Telecommunications Conference: October 26, 1998. Guelph, ON: University of Guelph, 1998 [Google Scholar]
- 28.Government of the Northwest Territories Health and Social Services: The Northwest Territories Epidemiology Newsletter - EpiNorth 8(3) Yellowknife, NWT: Government of the Northwest Territories; 1996 [Google Scholar]
- 29.Department of Health and Social Services, Government of the Northwest Territories Health and Social Services System Annual Report 2007/2008. Yellowknife, NWT: Government of the Northwest Territories, 2009. http://www.assembly.gov.nt.ca/_live/documents/content/09-11-05%20NWT%20Health%20and%20Social%20Services%20System%20Annual%20Report%202007-2008.pdf (accessed 9 Jan 2011). [Google Scholar]
- 30.Department of Municipal and Community Affairs, Government of the Northwest Territories GNWT Report on Drinking Water Quality 2006. Yellowknife, NWT: Government of the Northwest Territories, 2007 [Google Scholar]
- 31.Furgal C, Seguin J. Monitoring as a community response for climate change and health. Int J Circumpolar Health 2005;64:498–509 [DOI] [PubMed] [Google Scholar]
- 32.Department of Environment and Natural Resources, Government of the Northwest Territories NWT Climate Change Impact Report 2008. Yellowknife, NWT: Government of the Northwest Territories, 2008 [Google Scholar]
- 33.Crimmins E, Seeman T. Integrating Biology into the Study of Health Disparities. New York, NY: Population Council, Inc., 2005 [Google Scholar]
- 34.Swift L, Hunter PR, Lees AC, et al. Wildlife trade and emergence of infectious diseases. EcoHealth 2007;4:25–30 [Google Scholar]
- 35.Green CG, Krause DO, Wylie JL. Spatial analysis of campylobacter infection in the Canadian province of Manitoba. Int J Health Geogr 2006;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas MK, Majowicz SE, Sockett PN. Estimated numbers of community cases of illness due to Salmonella, Campylobacter and Verotoxigenic Escherichia Coli: pathogen-specific community rates. Can J Infect Dis Med Microbiol 2006;17:229–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kabore H, Levallois P, Michel P, et al. Association between potential zoonotic enteric infections in children and environmental risk factors in Québec, 1999-2006. Zoonoses Public Health 2000;57:195–205 [DOI] [PubMed] [Google Scholar]
- 38.Hunter JM, Arbona S. Disease rate as an artifact of the health care system: tuberculosis in Puerto Rico. Soc Sci Med 1984;19:997–1008 [DOI] [PubMed] [Google Scholar]
- 39.Seidel JE, Beck CA, Pocobelli G. Location of residence associated with the likelihood of patient visit to the preoperative assessment clinic. BMC Health Serv Res 2006;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arcury TA, Gesler WM, Preisser JS, et al. The effects of geography and spatial behavior on health care utilization among the residents of a rural region. Health Serv Res 2005;40:135–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kendall P, Medeiros LC, Hillers V, et al. Food handling behaviors of special importance for pregnant women, infants and young children, the elderly, and immune-compromised people. J Am Diet Assoc 2003;103:1646–9 [DOI] [PubMed] [Google Scholar]
- 42.Kramer MH, Herwaldt BL, Craun GF, et al. Surveillance for waterborne-disease outbreaks—United States, 1993–1994. MMRW 1996;45:1–33 [PubMed] [Google Scholar]
- 43.Pickering LK, Woodward WE, DuPont HL, et al. Occurrence of Giardia lamblia in children in day care centers. J Pediatr 1984;104:522–6 [DOI] [PubMed] [Google Scholar]
- 44.Espelage W, an der Heiden M, Stark K, et al. Characteristics and risk factors for symptomatic Giardia lamblia infections in Germany. BMC Public Health 2010;10:1471–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasley JN, Daly JJ, McCullogh D. Circannual incidence of Giardia lamblia. Chronobiol Int 1989;6:185–9 [DOI] [PubMed] [Google Scholar]
- 46.Orr P, Lorencz B, Brown R, et al. An outbreak of diarrhea due to verotoxin-producing Escherichia coli in the Canadian Northwest Territories. Scand J Infect Dis 1994;26:675–84 [DOI] [PubMed] [Google Scholar]
- 47.Government of the Northwest Territories Health and Social Services: The Northwest Territories Epidemiology Newsletter - EpiNorth 10(4) Yellowknife, NWT: Government of the Northwest Territories; 1997 [Google Scholar]
- 48.Olsen SJ, Bishop R, Brenner FW, et al. The changing epidemiology of Salmonella: trends in serotypes isolated from humans in the United States, 1987-1997. J Infect Dis 2001;183:753–61 [DOI] [PubMed] [Google Scholar]
- 49.Gradel KO, Schonheyder HC, Dethlesfsen C, et al. Morbidity and mortality of elderly patients with zoonotic Salmonella and Campylobacter: a population-based study. J Infect 2008;57:214–22 [DOI] [PubMed] [Google Scholar]
- 50.Patil S, Cates S, Morales R. Consumer food safety knowledge, practices, and demographic differences: findings from a meta-analysis. J Food Prot 2005;68:1884–94 [DOI] [PubMed] [Google Scholar]
- 51.Calvert N, Stewart WC, Riley WJ. Salmonella typhimurium DT104 infection in people and animals in Scotland: a collaborative epidemiological study 1993-96. Vet Rec 1998;143:351–4 [DOI] [PubMed] [Google Scholar]
- 52.Jones K. Campylobacter in water, sewage and the environment. Symp Ser Soc Appl Microbiol 2001;90:68–79 [DOI] [PubMed] [Google Scholar]
- 53.Stafford RJ, Schluter PJ, Wilson AJ, et al. Population-attributable risk estimates for risk factors associated with Campylobacter infection, Australia. Emerg Infect Dis 2008;14:895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones K. The Campylobacter conundrum. Trends Microbiol 2001;9:365–6 [DOI] [PubMed] [Google Scholar]
- 55.Riedlinger D. Climate change and the Inuvialuit of Banks Island, NWT: using traditional environmental knowledge to complement Western science. InfoNorth 1999;52:430–2 [Google Scholar]
- 56.Dawson D. Foodborne protozoan parasites. Int J Food Microbiol 2005;103:207–27 [DOI] [PubMed] [Google Scholar]
- 57.Neumann NF, Smith DW, Belosevic M. Waterborne disease: an old foe re-emerging? J Environ Eng 2005;4:155–71 [Google Scholar]
- 58.Graczyk TK, Evans BM, Shiff CJ, et al. Environmental and geographical factors contributing to watershed contamination with Cryptosporidium parvum oocysts. Environ Res 2000;82:263–71 [DOI] [PubMed] [Google Scholar]
- 59.Atwill ER, Pereira MD, Alonso LH, et al. Environmental load of Cryptosporidium parvum oocysts from cattle manure in feedlots from the central and western United States. J Environ Qual 2006;35:200–6 [DOI] [PubMed] [Google Scholar]
- 60.Hughes-Hanks JM, Rickard LG, Panuska C, et al. Prevalence of Cryptosporidium spp. and Giardia spp. in five marine mammal species. J Parasitol 2005;91:1225–8 [DOI] [PubMed] [Google Scholar]
- 61.Dixona BR, Parringtona LJ, Parenteaub M, et al. Giardia duodenalis and Cryptosporidium spp. in theintestinal contents of ringed seals (Phoca hispida) and bearded seals (Erignathus barbatus) in Nunavik, Quebec, Canada. J Parasitol 2008;94:1161–3 [DOI] [PubMed] [Google Scholar]
- 62.Anderson MJ, Smylie JK. Health systems in Canada: how well do they perform in first nations, Inuit and Metis contexts? Pimatisiwin 2009;7:99–115 [PMC free article] [PubMed] [Google Scholar]
- 63.Nado P, Stinson KW, Coggin W, et al. Electronic tuberculosis surveillance systems: a tool for managing today's TB programs. Int J Tuberc Lung Dis 2008;12:8–16 [PubMed] [Google Scholar]
- 64.Robertson C, Sawford K, Samson DLA, et al. Mobile phone-based infectious disease surveillance system, Sri Lanka. Emerg Infect Dis 2010;16:1524–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andresen E, Diehr P, Luke D. Public health surveillance of low frequency populations. Annu Rev Public Health 2004;25:25–52 [DOI] [PubMed] [Google Scholar]
- 66.Oum S, Chandramohan P, Cairncross S. Community-based surveillance: a pilot study from rural Cambodia. Trop Med Int Health 2005;10:689–97 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.