Abstract
This description pertains to a previously healthy gentleman aged 54 years who developed symptoms coherent with Raynaud’s phenomenon. The patient never had any prior episodes of peripheral cyanosis. The patient’s first presentation was in summer and the paroxysms of peripheral cyanosis were not associated with any specific aggravating factor. The paroxysms went on to become more severe and painful across a span of 6 months, when he also developed non-radiating pain in the right lateral chest-wall, which would aggravate after episodes of cough. A chest roentgenogram then demonstrated the presence of a mass lesion in the right lung and a fine-needle-aspiration cytology confirmed malignancy- an adenocarcinoma. There was a dramatic relief in pain and a reduction in the intensity and duration of paroxysms of peripheral cyanosis within 2-weeks of initiation of chemotherapy for lung cancer.
Background
Raynaud’s phenomenon is a very common condition in the society. Though majority of the cases can be considered as ‘primary Raynaud’s phenomenon’, a small but significant proportion of cases may have underlying aetiologies such as connective tissue disorders and hyperviscosity syndromes. Paraneoplastic Raynaud’s phenomenon is a very rare but documented phenomenon wherein underlying malignancies of a large variety can be the basis behind the symptomatology. This report describes the case of a patient who developed abrupt and quickly progressive symptoms of Raynaud’s phenomenon which did not benefit from conservative measures and oral nifedepine. Then, since thoracic pain ensued, a subsequent chest-roentgenogram and cytology were confirmatory for adenocarcinoma of the lung. The treatment with chemotherapy for lung cancer also drastically and rapidly resulted in an improvement in peripheral cyanosis. This case demonstrates the validity for suspicion for malignancy when an abrupt onset of Raynaud’s phenomenon occurs in an otherwise healthy person. This case also demonstrates that the treatment of the underlying malignancy can also alleviate paraneoplastic Raynaud’s phenomenon.
Case presentation
A 54-year-old gentleman, a policeman by profession, was born, raised and situated in a town geographically elevated at 2600 meters above mean sea level in the Himalayan foothills. He who never had any episode of peripheral cyanosis for the first 53 years of his life had a sudden onset of bilateral peripheral cyanosis involving all four limbs, symmetrically, and not associated with any specific precipitating or aggravating factor. In fact, the symptoms of peripheral cyanosis had first manifested in mid-August, when cold is not an issue. He reported ignoring the condition for the first 2 months. In the subsequent November, he noticed increasing intensity of symptoms, which now also included a sharp pain in the tips of his fingers and toes. He also noticed an increasing duration in the length of the paroxysms of peripheral cyanosis. As per his records from a private practitioner, he was advised measures such as ‘avoidance of cold’, ‘warm water dips’ and even oral nifedepine- all of which the patient claims of having had no effect whatsoever. After another month, he noticed pain in the right side of the chest, more-so in the lateral aspects. As per his history, the pain in the right-lateral chest would aggravate temporarily after episodes of coughing.
The patient had no prior history of trauma, cardiovascular illnesses, limb discolorations, neuromuscular disorders, snake-bite, haematological disorders, rheumatologic disorders, hypertension or diabetes. He gave no history of having visited very high altitudes, or being exposed to very low temperatures which could have caused frostbite. The patient gave no history of recent drug intake for other illnesses.
The patient had quit smoking at the age of around 35 years and had not smoked for the last two decades. There was no history of calf-pains, or the typical ‘night-cries’ which could have been associated with Buerger’s disease.
The patient was well built and well nourished. There was visible cyanosis in the fingers and toes, more-so over the distal phalanges. There was an erythematous appearance which extended proximally to the dorsal aspects of the hands and feet (figure 1). There was no elicitable tenderness, and the patient claimed that- ‘the fingers and toes hurt all the time’. All peripheral pulses were easily felt, with no diminution in the volume of any peripheral pulses. Bilateral radial pulses, as well as dorsalis-pedis pulsations were intact. The blood-pressure measurements in the upper and lower limbs were equal (systolic values within 134–140 mm Hg and diastolic values within 80–90 mm Hg). Fundoscopic evaluation was normal. Sensory and motor examination of the nervous system was within normal limits. There was no central cyanosis.
Figure 1.
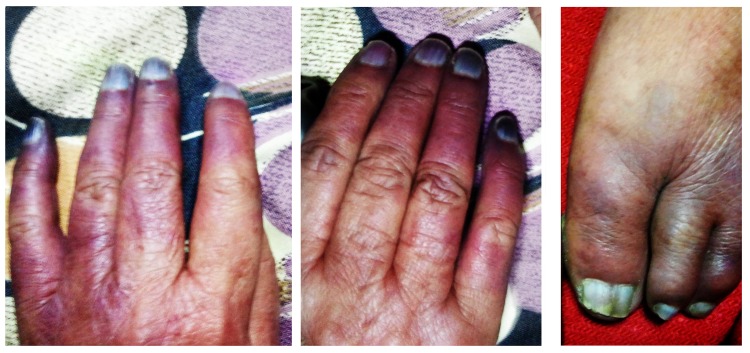
Peripheral cyanosis involving the fingers and the toes.
The thorax appeared symmetrical with equal movements on respiration. There were no evidences of any engorged or visible veins. There was no evidence of any external injuries over the thorax. The patient reported tenderness over palpation of the right infra-axillary area. On percussion, there was an impaired note over the right infra-axillary area. There was no clinical evidence of effusion.
Investigations
In view of the recent onset of right-sided chest pain and cough, a chest-radiograph was procured, which showed a peripheral opacity in the right hemi-thorax, which was abutting the chest-wall (figure 2), coinciding with the clinical locus of chest-wall tenderness. A fine-needle aspirate (figure 3) revealed an adenocarcinoma.
Figure 2.
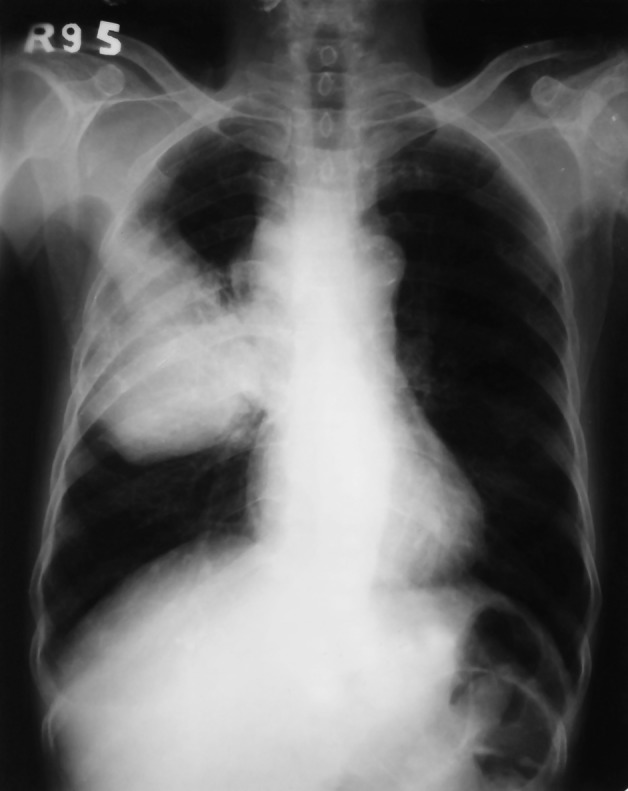
Chest radiograph showing a peripheral opacity in the right lung, abutting the chest wall.
Figure 3.
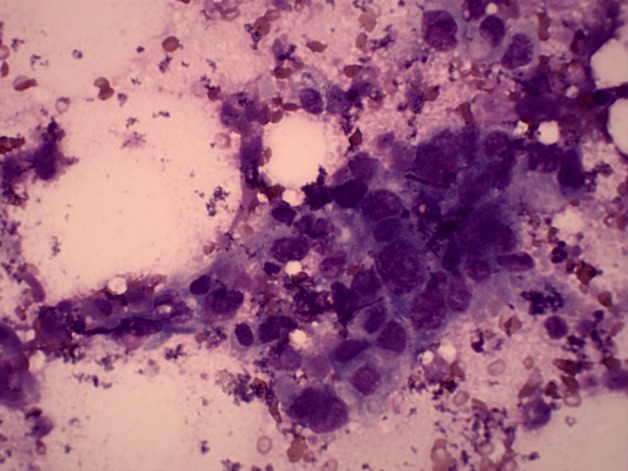
Cytology of needle aspirate from the lung mass depicting adenocarcinoma.
The patient’s ECG and echocardiography were normal. The measured haemoglobin was 12.3 grams per decilitre. The total leucocyte count was 6800 cells per microliter. On a differential count, neutrophils made up 64%, lymphocytes made up 35%, and eosinophils made up 1%. The erythrocyte sedimentation rate was 19 mm in the 1st h.
There were also no abnormalities detected on serum protein levels, albumin:globulin ratio and in the compliment profile. Further, there was no yield on testing for hepatitis B, hepatitis C, antinuclear antibodies, rheumatoid-factor, cryoglobulins and cold-agglutinins. Abdominal ultrasonography was normal.
The patient’s pulmonary adenocarcinoma on CT scan was seen to be invading the chest-wall and there were multiple enlarged ipsilateral hilar lymph-nodes, the largest measuring 2.6 cm in its short-axis diameter. The patient was staged T3N1M0 (stage-IIIA) as per the seventh edition of the TNM-AJCC staging system.1
Differential diagnosis
The following differential diagnoses were considered at some or any time during the evaluation of the patient, however, all of which were ruled out via history and investigations.
Primary idiopathic Raynaud’s phenomenon.
Thromboangitis obliterans (Buerger’s disease).
Auto-immune/connective tissue disorders such as rheumatoid arthritis, systemic lupus erythematosus, scleroderma, Sjogren’s syndrome, etc.
Drug-induced vasculitis.
Viral hepatitis associated vasculitis.
Hepatitis C associated cryoglobulinaemia.
Atherosclerosis.
Snake-bite.
Frost-bite.
Treatment
In view of the patient’s refusal for radical surgery, the patient was offered chemotherapy upfront (owing to a lack of treatment slots for upfront initiation of chemo-radiotherapy). Chemotherapy was based on gemcitabine and cisplatin (gemcitabine 1600 mg on days 1 and 8; and cisplatin 140 mg on day 1, of a 21 day cycle).
Outcome and follow-up
After the completion of the second cycle of chemotherapy, the patient has currently been initiated on concurrent chemo-radiotherapy. There has been an objective response in terms of about 50% reduction of the pulmonary opacity, as well as in the near-complete shrinkage of mediastinal lymphadenopathy. The patient has enjoyed marked reduction in chest-pain and there has been a marked reduction in peripheral cyanosis in terms of intensity and the associated pain, quoted as more than 75% as per the patient-quoted estimate.
Discussion
Raynaud’s phenomenon is a common condition occurring in up to 10% of the general population, characterised by episodes or paroxysms of digital vasospasm in the hands and/or feet. Often, these symptoms can be precipitated or aggravated by exposure to cold, emotional stress or vibrations. A typical episode of this vasogenic phenomenon encompasses a sequence of initial pallor, subsequent cyanosis followed by rubor and dolor (redness and pain).2–4
Raynaud’s phenomenon most often is idiopathic- an isolated phenomenon to which no underlying illness can be linked with. Up to 90% of all cases of Raynaud’s phenomenon can be idiopathic/primary.5 6
Among the aetiologies of secondary Raynaud’s phenomenon, the predominant aetiology would lie among the large spectrum of connective tissue and auto-immune disorders such as scleroderma, mixed connective tissue disorder and rheumatoid arthritis. Another prominent cause, especially in the chronic smoker would thromboangitis-obliterans, also called as ‘Buerger’s disease’.7
Further causes include frost-bite, vibrational trauma (in occupational drillers), snake-bite (common in the agricultural/forestry workers), peripheral atherosclerosis, hyper-viscosity syndromes such as cryoglobulinaemia, etc.8
Raynaud’s phenomenon associated with malignancies is a rarely reported clinical entity. Termed as ‘paraneoplastic Raynaud’s phenomenon’, though the first such case was reported as way back as in 1884,9 there has been a paucity in published cases, with two largest reviews in the subject having been able to collect only 31 cases8 and 33 cases,10 respectively. Also, one can speculate that the condition is under-reported, partly owing to the bias against case-reports by modern medical journals.
The underlying malignancies among patients with paraneoplastic Raynaud’s phenomenon can be highly varied, including carcinomas of the lung, breast, ovary, testes, skin and thyroid. Sarcomas and haematological malignancies too have been associated.11–16
As varied are the possible malignancies underlying cases of paraneoplastic Raynaud’s phenomenon, the potential pathways of pathogenesis for paraneoplastic Raynaud’s phenomenon are vast. Hsu et al8 in their review of literature mention various potential pathogenic mechanisms- such as tumour invasion of sympathetic nerves, hyper-viscosity, hyper-coagulability, vasoactive tumour-secreted substances, generalised vasospasm and spontaneous platelet aggregation.8
Paraneoplastic Raynaud’s phenomenon also differs from idiopathic Raynaud’s phenomenon in that the paraneoplastic variant affects a population which is older and also has an increased incidence in males. With paraneoplastic Raynaud’s phenomenon, the progression is quick and more than 80% of the affected patients progress to ischaemic complications such as necrosis and gangrene.17 18
Hsu et al identified three temporal patterns in the association of paraneoplastic Raynaud’s phenomenon with malignancy. The first type included patients in whom peripheral ischaemia leads to the diagnosis of underlying unresectable malignancy, the second type included patients in whom peripheral ischaemia preceded the diagnosis of malignancy and resolved after tumour resolution with therapy, and the third type included patients in whom peripheral ischemia heralded relapse of a previously treated malignancy.8
With paraneoplastic Raynaud’s phenomenon, benefit cannot be uniformly expected with modalities traditionally used in idiopathic Raynaud’s phenomenon (nifedipine, surgical sympathetectomy, calcitonin, anticoagulants and prostacyclins). This could be because of the vast heterogeneity with the possible underlying pathogenic mechanisms.11 19–23
As for all paraneoplastic syndromes, the treatment of the underlying malignancy can be expected to alleviate the co-existing paraneoplastic Raynaud’s phenomenon. Various prior case-reports have demonstrated that treatment of the malignancy also causes improvement with regards to the paraneoplastic Raynaud’s phenomenon.6 15 24
In concluding, we state that a fresh onset of rapidly progressive Raynaud’s phenomenon in the middle-aged and the older people should alert the clinician to search for an underlying malignancy, especially if other more common causes of Raynaud’s phenomenon cannot be supported by investigative work-up.
Learning points.
Paraneoplastic Raynaud’s phenomenon is a heterogeneous condition, with various possible underlying malignancies.
A sudden onset of Raynaud’s phenomenon should raise suspicions of its character being paraneoplastic.
Paraneoplastic Raynaud’s phenomenon if not addressed can adversely affect quality of life.
Like all other paraneoplastic symptomatology, paraneoplastic Raynaud’s phenomenon can be expected to abate after treatment against the underlying malignancy is initiated.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer (AJCC) cancer staging manual. Seventh Edition Chicago: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 2.Tolosa-Vilella C, Ordi-Ros J, Vilardell-Tarres M, et al. Raynaud’s phenomenon and positive antinuclear antibodies in a malignancy. Ann Rheum Dis 1990;49:935–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maricq HR, Weinrich MC, Keil JE, et al. Prevalence of Raynaud phenomenon in the general population. A preliminary study by questionnaire. J Chronic Dis 1986;39:423–7. [DOI] [PubMed] [Google Scholar]
- 4.Silman A, Holligan S, Brennan P, et al. Prevalence of symptoms of Raynaud’s phenomenon in general practice. BMJ 1990;301:590–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spencer-Green G. Outcomes in primary Raynaud phenomenon: a meta-analysis of the frequency, rates, and predictors of transition to secondary diseases. Arch Intern Med 1998;158:595–600. [DOI] [PubMed] [Google Scholar]
- 6.Schildmann EK, Davies AN. Paraneoplastic Raynaud’s phenomenon–good palliation after a multidisciplinary approach. J Pain Symptom Manage 2010;39:779–83. [DOI] [PubMed] [Google Scholar]
- 7.Lazarides MK, Georgiadis GS, Papas TT, et al. Diagnostic criteria and treatment of Buerger’s disease: a review. Int J Low Extrem Wounds 2006;5:89–95. [DOI] [PubMed] [Google Scholar]
- 8.Hsu ST, Lee YY, Lie MF. Symmetrical peripheral gangrene of sudden onset- a paraneoplastic syndrome? – a case report and review of the literature. Dematol Sinica 1996;14:82–8. [Google Scholar]
- 9.O’Connor B. Symmetrical gangrene. BMJ 1884;1:640. [Google Scholar]
- 10.Poszepczynska-Guigné E, Viguier M, Chosidow O, et al. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol 2002;47:47–52. [DOI] [PubMed] [Google Scholar]
- 11.Domz CA, Chapman CG. Pseudo-Raynaud’s: cryoglobulinemia secondary to occult neoplasm. Calif Med 1961;95:391–3. [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett TI, Poulton EP. Raynaud’s disease associated with cancer of the stomach. Am J Med Sci 1928;176:656. [Google Scholar]
- 13.Hamilton WF. Carcioma of the esophagus and Raynaud’s disease. Can Med Assoc J 1920;10:670–1. [PMC free article] [PubMed] [Google Scholar]
- 14.Hawley PR, Johnston AW, Rankin JT. Association between digital ischaemia and malignant disease. Br Med J 1967;3:208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen D, Robinson D, Mittoo S. Paraneoplastic Raynaud’s phenomenon in a breast cancer survivor. Rheumatol Int 2010;30:789–92. [DOI] [PubMed] [Google Scholar]
- 16.Auboire L, Landy S, Perrot JY, et al. [A negative first-line work-up of Raynaud’s phenomenon: And what if it were cancer?]. J Mal Vasc 2010;35:35–7. [DOI] [PubMed] [Google Scholar]
- 17.Wong AS, Hon Yoon K. Paraneoplastic Raynaud phenomenon and idiopathic thrombocytopenic purpura in non-small-cell lung cancer. Am J Clin Oncol 2003;26:26–9. [DOI] [PubMed] [Google Scholar]
- 18.DeCross AJ, Sahasrabudhe DM. Paraneoplastic Raynaud’s phenomenon. Am J Med 1992;92:571–2. [DOI] [PubMed] [Google Scholar]
- 19.Friedman SA, Bienenstock H, Richter IH. Malignancy and arteriopathy. A report of two cases. Angiology 1969;20:136–43. [DOI] [PubMed] [Google Scholar]
- 20.Scheinfeld N. A review of the cutaneous paraneoplastic associations and metastatic presentations of ovarian carcinoma. Clin Exp Dermatol 2008;33:10–15. [DOI] [PubMed] [Google Scholar]
- 21.Taylor LM, Jr, Hauty MG, Edwards JM, et al. Digital ischemia as a manifestation of malignancy. Ann Surg 1987;206:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thoelke A, Schmid HP, Figl R, et al. Jo-1 positive paraneoplastic systemic sclerosis in a patient with metastatic melanoma. Eur J Dermatol 2006;16:428–30. [PubMed] [Google Scholar]
- 23.Albin G, Lapeyre AC, 3rd, Click RL, et al. Paraneoplastic digital thrombosis: a case report. Angiology 1986;37:203–6. [DOI] [PubMed] [Google Scholar]
- 24.Maier C, Baron R, Loose R, et al. [Endoscopic transthoracic sympathectomy in a paraneoplastic Raynaud’s syndrome]. Dtsch Med Wochenschr 1994;119:1162–6. [DOI] [PubMed] [Google Scholar]


