Abstract
A 22-year-old woman with features suggestive of Cushing’s syndrome was found to have right adrenal mass on imaging studies. She had paradoxical rise in basal cortisol on dexamethasone suppression testing. Black adenoma of the right adrenal cortex, a pigmented adenoma consisting of compact cells with numerous pigments suggestive of melanin and lipofuscin was laproscopically removed from this patient. This case illustrates that in the setting of unilateral adrenal mass with paradoxical cortisol response with dexamethasone suppression testing, pigmented adrenal adenomas should be also suspected in addition to primary pigmented nodular adrenocortical disease.The decision to go with either unilateral or bilateral adrenalectomy should be based on the attributes of contralateral adrenal gland.
Background
The common causes of adrenocorticotropic hormone (ACTH) independent endogenous Cushing’s syndrome include the adrenal causes like adenoma, carcinoma and hyperplasia. While adrenal adenoma and carcinoma are usually unilateral, hyperplasias are commonly bilateral. Rarely an entity involving bilateral adrenal glands called primary pigmented nodular adrenocortical disease (PPNAD) occurs as a cause of Cushing’s syndrome. It is characterised by paradoxical cortisol rise with dexamethasone suppression testing (DST) along with a pathological feature of bilateral multiple nodules and internodular atrophy. However, the paradoxical cortisol response with DST is not specific to PPNAD, but can rarely occur in some adrenal adenomas also.
Case presentation
A 22-year-old woman was admitted in Orthopedic ward of our institute with history of difficulty in standing and walking due to fracture of right femoral neck with non-union. She had sustained injury to right hip 4 months ago following trivial trauma when she slipped while walking. During her evaluation, she was found to be hypertensive with a blood pressure of 180/100 mm Hg and was referred to our department for evaluation of ‘hypertension in young’.
On evaluation, she had several characteristic features of overt Cushing’s syndrome, which had progressed insidiously since adolescence. Her guiding signs on examination included truncal obesity, protuberant abdomen with livid striae (figure 1), buffalo hump, excessive vellous facial hair, moon facies with facial plethora. She had thinning of skin but no hyperpigmentation or proximal weakness before injury. There was no visual disturbance, headache, changes in her personality, polyuria, polydipsia or easy bruising. She was married for 7 years with three children and had amenorrhea since 1½ years. She did not have phenotype of Carney’s complex and there was no intake of exogenous steroids in the past.
Figure 1.
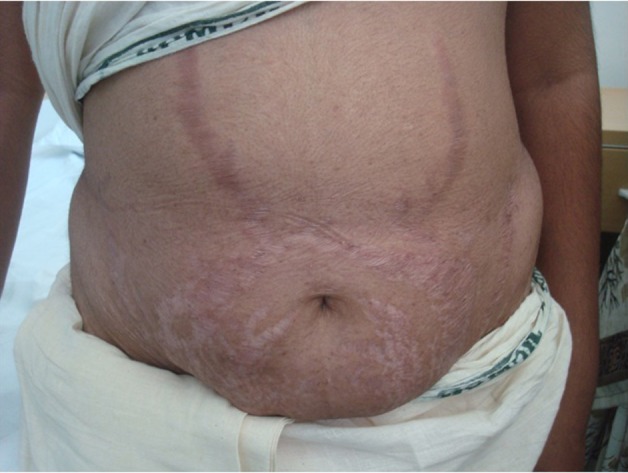
Protuberant abdomen showing livid striae of Cushing’s syndrome along with striae atrophicae following her past pregnancies.
Investigations
Her circadian rhythm of cortisol was altered with basal level of 662 nmol/l (normal range 138–662 nmol/l) and midnight level of 634 nmol/l (normal range <50 nmol/l). Her cortisol levels postlow dose DST (oral dexamethasone 0.5 mg 6th hourly for 48 h) was paradoxically raised to 938 nmol/l (46% above basal cortisol). Subsequent posthigh dose DST (oral 2 mg dexamethasone 6th hourly for 48 h) cortisol was also paradoxically elevated to1021 nmol/l (58% above basal cortisol). Her plasma ACTH level was 1.8 pmol/l (normal range 2–11.4 pmol/l). Glucose tolerance was well maintained. Abdominal CT revealed a well-defined right adrenal tumour 2.5 cm in diameter (figure 2). The contralateral adrenal gland was atrophic. DXA scan revealed osteoporosis with Z-score of −3.44 at lumbar spine.
Figure 2.
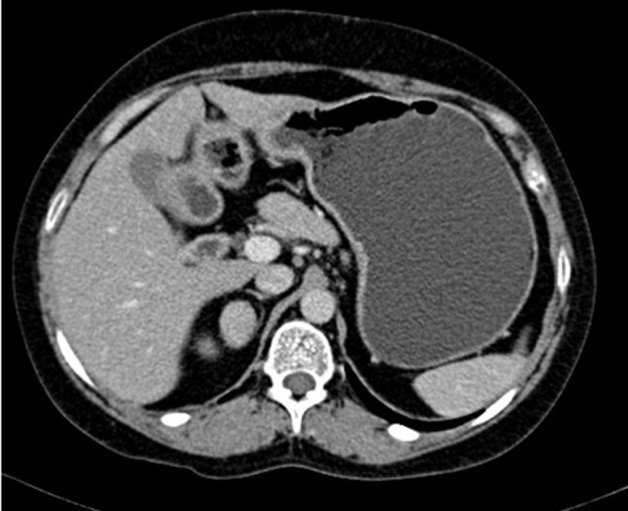
CT abdomen showing right adrenal adenoma.
Diagnosis
On the basis of the symptoms, laboratory findings and imaging studies, she was diagnosed as endogenous ACTH independent Cushing’s syndrome, probably due to an unilateral adrenal adenoma. The paradoxical response of cortisol to DST kept the possibility of PPNAD open. However, its likelihood was decreased by the finding of the removed gland showing single unilateral adrenal nodule with atrophy of adjacent adrenal tissue of the involved gland and of the contralateral full adrenal gland.
Treatment
She underwent laparoscopic total right adrenalectomy, following which her basal cortisol levels became very low (day 5 cortisol of 38 pmol/l). Therefore, supplementary treatment with prednisolone and fludrocortisone was initiated and continued.
Outcome and follow-up
Postadrenalectomy, she lost 3 kgs and her hypertension resolved. She developed severe body ache and joint pains which subsided with steroid replacement. Currently, she continues to require supplementary steroids 6 months postoperatively.
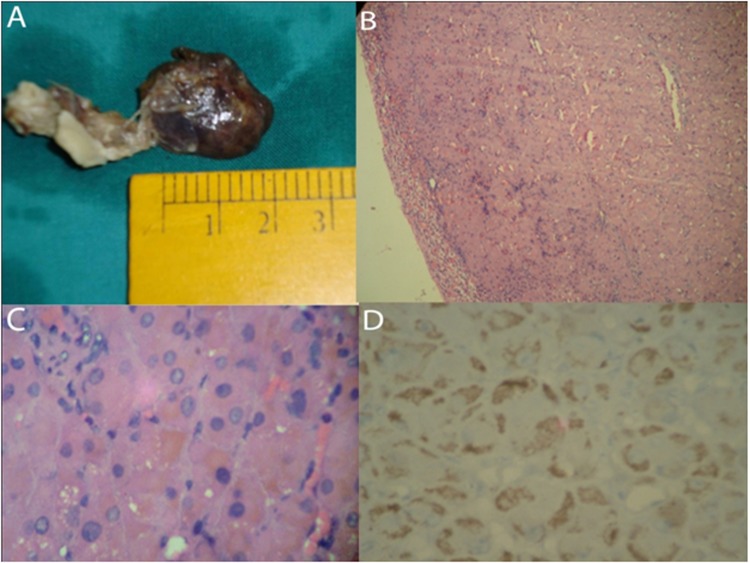
Histopathologically, the removed right adrenal gland, weighing 12 grams, contained a single, well-circumscribed black adenoma measuring 2.5×2.2×1.5 cm (figure 3A). The residual adrenal grand adjacent to the adenoma showed atrophic change of the cortex (figure 3B). Microscopically, the tumour was homogeneous and consisted of compact cells with eosinophilic cytoplasm which was filled with numerous pigment granules. Dark to golden brown colour pigments were seen on H&E staining (figure 3C). These pigment granules were negative for Schmorl, Congo red, iron and mucin staining but they were positive for Periodic Acid-Schiff, modified Ziehl–Neelsen and Fontana staining. These features supported the lipofuscin nature of the pigment. Immunohistochemistry for melanin (HMB 45) was positive (figure 3D). Tumour cells showed neither necrosis nor mitosis and there was no finding of vascular invasion.
Figure 3.
(A) Macroscopic appearance of the right adrenal gland showing a black coloured circumscribed lesion 2.5 cm in maximum dimension. (B) Low power view of the tumour showing a well-circumscribed tumour with thinned out adrenal cortex in the periphery (arrow) (H&E x100). (C) High power view of the cells demonstrating the fine brown coloured pigment in the cytoplasm of individual cells (H&E x400). (D) Cytoplasmic HMB 45 positivity in the compact cells (IHC- HMB x400).
Discussion
The common causes of ACTH-independent Cushing’s syndrome are adrenal adenoma/carcinoma and ACTH independent adrenal hyperplasias. In our case,the possibility of PPNAD was also considered in differential diagnosis despite unfavourable imaging characteristics on account of her age, slowly smouldering clinical features and paradoxical response with DST. But we decided for unilateral right laparoscopic adrenalectomy considering atrophic contralateral adrenal gland and patient’s considerations. The histology features of pigmented adenoma as opposed to hyperplasia and presence of only a single adenoma without other contour irregularities in the involved gland favour the diagnosis of black adrenal adenoma. The patient’s postoperative clinical response also favours black adrenal adenoma over PPNAD.
Pigmented or black adenomas of the adrenal cortex were first described by Baker in the English literature in 1938.1 They were initially regarded as non-functional neoplasms of unknown clinical significance, being documented mainly in autopy studies across the world.2 They were later found to be associated with different forms of hyperfunction of adrenal cortex such as subclinical or overt Cushing’s syndrome3–6 and primary aldosteronism.7 8 Cushing’s syndrome is the most common presentation of adrenocortical hyperfunction in black adrenal adenoma.
Black adrenal adenoma and PPNAD, both can have paradoxical response with DST. The cause of paradoxical response with dexamethasone suppression tests for black adrenal adenomas is not clearly defined as in PPNAD. The pathophysiology of such response in black adrenal adenoma may be speculated to be similar to that established in PPNAD. It is attributed to intense expression of glucocorticoid receptors in the nodules of PPNAD.9 It is also ascribed to the paradoxical stimulation of cortisol release through a glucocorticoid receptor-mediated effect on protein kinase A (PKA) catalytic subunits.10 The exact aetiopathogenesis of black adrenal adenoma still remains to be ascertained. Mitochondrial injury causing impaired excretion of lipoid containing pigments is probably responsible for pigmentation in black adrenal adenoma.11
The present case, thus emphasises that the paradoxical response with DST is restricted to PPNAD and to pigmented adrenal adenomas. There are many cases in literature documenting paradoxical cortisol rise with DST in black adrenal adenoma causing Cushing’s syndrome.3 4 12 Currently bilateral adrenalectomy is the treatment of choice for cases of PPNAD.13 Its preoperative diagnosis is largely based on paradoxical responses with DST in appropriate clinical setting and its probability increases with magnitude of paradoxical response. However, it may be prudent to go with unilateral adrenalectomy despite a paradoxical response with DST if we have an unilateral adenoma with atrophic contralateral adrenal gland. There are documented reports in medical literature of initially diagnosed cases of unilateral adrenal adenoma (based on radiology) with paradoxical response with DST being relabelled as PPNAD following histopathology of adrenalectomy specimen.14 But in our case, the histopathology was unequivocally suggestive of single adenoma with adjacent cortical atrophy.
The question of whether black adrenal adenoma is a separate entity of its own or is a part of pathological spectrum of PPNAD remains to be answered. Nevertheless, in situations of unilateral adrenal adenoma with paradoxical response with DST, it would be logical to decide on surgical plan depending on imaging characteristics of contralateral adrenal gland. Atrophic contralateral adrenal gland can necessitate only a unilateral adrenalectomy conjecturing the involved gland to have an adenoma while a normal/multi-nodular contralateral adrenal gland calls for bilateral adrenalectomy presuming the baseline condition to be PPNAD. Further genetic studies on black adrenal adenoma can be expected to give useful insights into it and its relation to PPNAD in future.
Learning points.
Conventionally, when paradoxical rise in cortisol occurs in response with dexamethasone suppression testing in a case of Cushing’s syndrome, a condition of bilateral adrenal involvement, primary pigmented nodular adrenocortical disease is considered.
This paradoxical response of cortisol with dexamethasone suppression testing can occur also in unilateral pigmented adenomas like black adrenal adenoma.
Management decision to go for either unilateral or bilateral adrenalectomy in Cushing’s syndrome with unilateral adrenal adenoma and paradoxical cortisol response with dexamethasone suppression testing should be based on the status of contralateral adrenal gland.
It is judicious to opt for unilateral adrenalectomy when the contralateral adrenal gland is atrophic.
Acknowledgments
The authors acknowledge the contributions of Dr Girish Parthan and Dr Karthik Balachandran, senior residents in department of Endocrinology for preparation of the manuscript.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Baker MR. A pigmented adenoma of the adrenal. Arch Pathol 1938;26:845–52. [Google Scholar]
- 2.Robinson MJ, Pardo V, Rywlin AM. Pigmented nodules (black adenomas) of the adrenal. An autopsy study of incidence, morphology, and function. Hum Pathol 1972;3:317–25. [DOI] [PubMed] [Google Scholar]
- 3.Zaniewski M, Sheeler LR. Cushing’s syndrome associated with functional black adenoma of the adrenal cortex. South Med J 1980;73:1410–2. [DOI] [PubMed] [Google Scholar]
- 4.Tseng CH, Chang GK, Wong QY, et al. Cushing’s syndrome and functioning adrenal black adenoma. South Med J 1978;71:1166–8. [DOI] [PubMed] [Google Scholar]
- 5.Tsuji A, Takao M, Asano T, et al. [A case of Cushing’s syndrome caused by an adrenal black adenoma]. Hinyokika Kiyo 1987;33:738–42. [PubMed] [Google Scholar]
- 6.González Moraleja J, Arce Mainzhausen A, Brea Hernando AJ, et al. [Cushing’s syndrome caused by black adrenal gland adenoma]. Rev Clin Esp 1992;191:227–8. [PubMed] [Google Scholar]
- 7.Caplan RH, Virata RL. Functional black adenoma of the adrenal cortex. A rare cause of primary aldosteronism. Am J Clin Pathol 1974;62:97–103. [DOI] [PubMed] [Google Scholar]
- 8.Cohen RJ, Brits R, Phillips JI, et al. Primary hyperaldosteronism due to a functional black (pigmented) adenoma of the adrenal cortex. Arch Pathol Lab Med 1991;115:813–5. [PubMed] [Google Scholar]
- 9.Bourdeau I, Lacroix A, Schürch W, et al. Primary pigmented nodular adrenocortical disease: paradoxical responses of cortisol secretion to dexamethasone occur in vitro and are associated with increased expression of the glucocorticoid receptor. J Clin Endocrinol Metab 2003;88:3931–7. [DOI] [PubMed] [Google Scholar]
- 10.Louiset E, Stratakis CA, Perraudin V, et al. The paradoxical increase in cortisol secretion induced by dexamethasone in primary pigmented nodular adrenocortical disease involves a glucocorticoid receptor-mediated effect of dexamethasone on protein kinase A catalytic subunits. J Clin Endocrinol Metab 2009;94:2406–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balázs M. Functioning “black adenoma” of the adrenal gland with emphasis on ultrastructural studies. Zentralbl Pathol 1991;137:151–6. [PubMed] [Google Scholar]
- 12.Inomoto C, Sato H, Kanai G, et al. Black adrenal adenoma causing preclinical Cushing’s syndrome. Tokai J Exp Clin Med 2010;35:57–61. [PubMed] [Google Scholar]
- 13.Jameson JL, Groot LJD. Endocrinology: Adult and Pediatric. Sixth Edition Philadelphia, PA: Saunders Elsevier; 2010. [Google Scholar]
- 14.Sarlis NJ, Chrousos GP, Doppman JL, et al. Primary pigmented nodular adrenocortical disease: reevaluation of a patient with carney complex 27 years after unilateral adrenalectomy. J Clin Endocrinol Metab 1997;82:1274–8. [DOI] [PubMed] [Google Scholar]