Abstract
Although the deep sea is the largest ecosystem on Earth, its infaunal ecology remains poorly understood because of the logistical challenges. Here we report the morphology of relatively large burrows obtained by in situ burrow casting at a hydrocarbon-seep site and a non-seep site at water depths of 1173 and 1455 m, respectively. Deep and complex burrows are abundant at both sites, indicating that the burrows introduce oxygen-rich sea water into the deep reducing substrate, thereby influencing benthic metabolism and nutrient fluxes, and providing an oxic microhabitat for small organisms. Burrow castings reveal that the solemyid bivalve Acharax johnsoni mines sulphide from the sediment, as documented for related shallow-water species. To our knowledge, this is the first study to examine in situ burrow morphology in the deep sea by means of burrow casting, providing detailed information on burrow structure which will aid the interpretation of seabed processes in the deep sea.
Keywords: benthos, ichnology, Acharax, bioturbation, bioirrigation, resin casting
1. Introduction
Burrowing organisms are particularly important in seafloor environments, because they mix sediments, disrupt microstratigraphy, influence the biogeochemistry of sea-floor sediment, and produce burrows that harbour other organisms and microbes [1–3]. The deep sea is the largest single marine ecosystem on Earth and contains abundant benthic fauna living on and in the sea floor sediment: understanding their subsurface ecology is therefore important.
Faunal activities in sediments of the deep-sea environment have been observed in the fossil record and in modern sediment retrieved using core samplers. In the former case, it is easy to observe deep-sea trace fossils in onland exposures: however, trace fossils are commonly flattened during diagenesis [4], and are generally overprinted by subsequent burrowing activities. In addition, trace fossils rarely provide clues to the nature of their producers, even though such information is an essential component of deep-sea biology. Hence, many researchers have tried to observe modern deep-sea burrows. The trails of such organisms on the sea floor and their burrows beneath the sea floor have been documented in core samples since the 1970s [5]. X-ray radiography has been used to observe sub-fossilized (sediment-filled) and active (open) burrows in deep-sea sediment cores [6]. In addition, axial tomodensitometry (computed tomography scanning) has been used to determine the three-dimensional morphology of burrows in marine sediment core samples [7].
However, burrows are commonly unobservable in core samples, because the soupy mud of the deep-water sea floor surface is deformed or disrupted during coring [8]. In addition, it is generally not possible to retrieve cores from unconsolidated sandy and gravelly sediments. Large organisms in the sediment often show escape movements, downward or upward, during coring, thereby disrupting the original burrow structures or life positions (H.N., personal observation). Furthermore, in the case of X-ray radiography and axial tomodensitometry, the observable volume is limited because of the size of the core and the device used for analysis; indeed, it is rarely possible to observe the entire structure of large-scale burrows in core samples.
We overcame these problems by in situ burrow casting—an extremely useful approach to understanding the autoecology of burrowers, the influence of burrows on the geochemistry of the host sediment [2], the nature of interspecific and conspecific biological interaction that occurs in burrows, and the palaeoecology of the organism that made the burrow [3].
Here we report the first burrow casting in the deep sea, to reveal the morphology of burrow structures in the substrate. Castings were made at a hydrocarbon seep and in an area of normal sea floor, away from any seep, both on the soft muddy sea floor in Sagami Bay, central Japan. In situ burrow casting, as described in this study, is expected to provide new insights into deep-sea ecology.
2. Material and methods
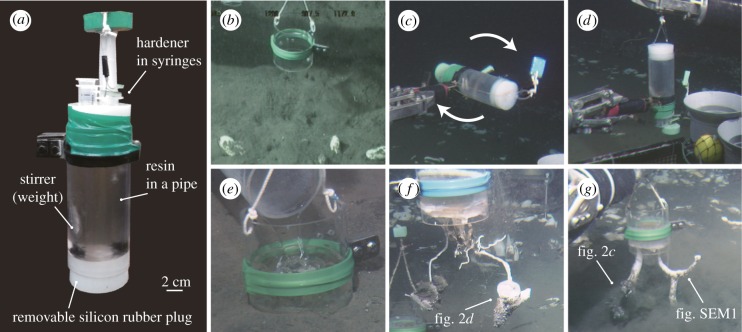
We observed the burrow morphology by employing an in situ burrow-casting device (named Anagatchinger) controlled by the remotely operated vehicle (ROV) Hyper-Dolphin (JAMSTEC). This method enables observations of the three-dimensional morphology of millimetre–decimetre-scale burrows in the deep-sea environment (figure 1). In situ burrow castings were made at two sites in Sagami Bay, central Japan: a hydrocarbon-seep (figure 2a: the Off Hatsushima seep, water depth 1173 m) and an area of normal sea floor away from any seep (figure 2b: OBB2 Station, water depth 1455 m). All of the field observations and burrow castings were made during cruise NT10-19 of the R/V Natsushima and cruise KY11-01 of the R/V Kaiyo. Polyester resin (Rigolac® 2004WM-2, Showa Denko K. K., Japan) and hardener (methyl ethyl ketone peroxide; Kayaku Akuzo Co. Ltd, Japan) were used to create casts of deep-sea burrows at a ratio of hardener to resin of 5 per cent by weight. The resin density is greater than that of water at the sea floor, meaning that it flows down burrows and creates a cast of the entire burrow lumen structure, sometimes capturing the burrow producer within the cast. The casting procedure (see the electronic supplementary material, video S1) was as follows. A plastic cylindrical frame (15 cm high×10.5 cm in diameter, covering an area of per 86 cm2) was partially buried in the sediment, surrounding several burrow openings. The resin was then mixed with hardener at the deep-sea floor, using the burrow-casting device (Anagatchinger), and was poured into the frame, covering the burrow openings and filling the burrows. After 2 days, the hardened casts were carefully retrieved from the sediment.
Figure 1.
Procedure of in situ burrow casting in the deep sea. (a) The casting device Anagatchinger. (b) Placement of the plastic cylindrical frame. (c) Mixing of resin and hardener. (d) Removal of the rubber plug to release the resin. (e) Pouring resin into burrows. (f) Recovering the casts of chambered burrows. (g) Recovering the casts of solemyid burrows. Underwater photos were taken by the ROV Hyper-Dolphin (JAMSTEC).
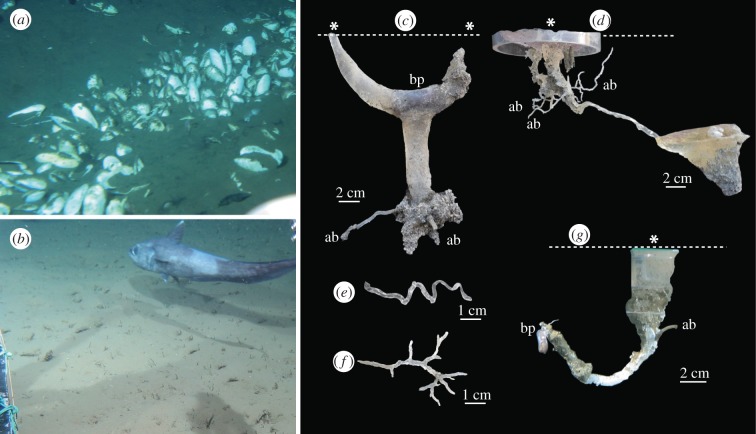
Figure 2.
Photographs of the sea floor study sites and burrow casts. (a) Sea floor at the Off Hatsushima hydrocarbon seep with dense aggregation of chemosymbiotic vesicomyid clam Calyptogena spp., from which the casts shown in (c–f) were recovered. (b) Sea floor at the OBB2 Station with a morid cod Coryphaenoides acrolepis, from which the cast shown in (g) was recovered. (c) Burrow of Acharax johnsoni. (d) Large burrow with a sub-hemispherical chamber. (e) Horizontal spiral burrow connected to the burrow shown in (d). (f) Ramified burrow. The original orientation (with respect to the sea floor) is unknown. (g) J-shaped burrow. Asterisks and dashed lines indicate burrow openings and the sea floor, respectively. bp, burrow producer; ab, associated burrow.
3. Results and discussion
We retrieved two plastic cylindrical frames at the Off Hatsushima site (a total of 11 burrow casts) and one at the OBB2 site (a total of two burrow casts).
At the seep site, two types of large vertical burrows were retrieved. First, a Y-shaped burrow with an elliptical cross section (2 cm in diameter and 21 cm deep) was recovered, reaching into a sandy layer below a 15 cm-thick surface mud layer (figure 2c). Its producer, the chemosymbiotic solemyid bivalve Acharax johnsoni, positioned itself at the junction of the Y or lower terminal of the burrow. The second burrow (figure 2d) has an inclined cylindrical shaft (6 mm in diameter and 15 cm deep) with a sub-hemispherical chambered terminus (8 cm in maximum diameter). Some ramified and spiral-shaped small burrows (1–3 mm in diameter; figure 2e,f) are connected to the large burrow. At the non-seep site, we recovered a J-shaped burrow (5 mm in diameter and 10 cm deep) with a small associated burrow (figure 2g). A polychaete worm was observed at the end of the J-shaped burrow.
The burrow casts show evidence of ecological interaction in the deep-sea sediment, as the burrow complex clearly demonstrates associations between large and small burrowers. The small burrower may use the special environment provided by the large burrow, which offers protection from predation and a supply of oxygen-rich sea water even in the decimetre-deep reducing sediment. Although faunal activity has been known to affect the oxygen dynamics of deep-sea sediments [9], the bioirrigation associated with a ramified burrow complex may promote a deeper and enhanced chemical flux across the sediment–water interface. Thus, burrows have a greater influence on benthic metabolism and nutrient fluxes on the deep-sea floor than previously thought.
Chemosynthetic biological communities associated with a deep-sea hydrocarbon seep are an important component of the marine ecosystem because of their high level of productivity and high biomass [10]. Such communities not only contain epibenthic invertebrates, such as Calyptogena bivalves, but also many burrowing bivalves such as solemyids, lucinids and thyasirids [10]. Because these burrows influence the chemical composition of sea water through the sediment–water interface, their density and ecology are expected to affect the benthic chemical flux of reduced components and to influence organisms in the habitat, ranging from microbes to megabenthos such as Calyptogena. Few studies have tried to recover infauna from seep sites, probably because the burrow producer lives within the sediment and is therefore invisible to ROVs and submersibles.
Through the in situ sampling method described herein, this study reveals the burrow morphology of the solemyid bivalve A. johnsoni, which has a shell length of up to 87 mm at the Off Hatsushima seep. On the basis of the ecology of shallow-water solemyid species [11], the morphology of the Y-shaped burrow indicates that A. johnsoni are agents of bidirectional bioirrigation, introducing oxygen-rich water into the reducing substrate and serving as a conduit for the extraction of sulphide-laden water out of the deep substrate via active pumping. The decimetre-scale burrows of this species occur throughout most of the site at the Off Hatsushima seep, raising the possibility that the burrows enhance cold-seep discharge. This irrigation may produce a complex geochemical zonation in the sediment and distribute sulphide-rich water to non-burrowing animals and microbes that live immediately below the deep-sea floor. The small burrows attached to Acharax burrows (figure 2c) may benefit from the bidirectional irrigation of the large burrow.
Acharax johnsoni occurs throughout the Pacific at water depths of 675–5379 m [12]. Other species of the genus grow to more than 190 mm in shell length, and inhabit the Pacific and Atlantic oceans [12,13], indicating that their ‘sulphide well’ enhances the discharge of cold seepage.
Chemosynthetic biological communities contain a diverse range of burrowing invertebrates. For example, thalassinidean crustaceans, which are well-known as an important bioturbator and bioirrigator in shallow-sea settings [1,2], have been reported from deep-sea chemoautotrophic communities in the Pacific and Atlantic oceans [14]. Many commensal animals (burrow associates and ectocommensals) including species of polychaete, echiuran, copepod, caridean, brachyuran, bryozoan, bivalve and fish, have been reported from the burrows of shrimps in shallow-water settings [1], and similar ecological relationships and geochemical impacts of burrows are expected to exist in the deep sea. In situ burrow casting, as described in this study, is expected to provide new insights into deep-sea ecology.
The in situ burrow casts also provide important new insight into the palaeoecology of deep-sea infauna. For example, the Acharax burrow can provide information on trace-producer and ethological interpretation of Y-shaped trace fossils such as Solemyatuba and similar burrows [11,15] in the deep-sea strata. Also, trace fossils in deep-sea strata (especially in mudstones without sand or gravel layers) occur mainly along bedding surfaces, and therefore have been considered to represent horizontal burrowing by the producer. However, vertical trace fossils may be flattened by sediment compaction during diagenesis [4]. Results from the present study confirm that vertical burrowing is also a major feature in the modern deep-sea environment.
Acknowledgements
We thank the crew of the R/V Natsushima and R/V Kaiyo, and the operation team of the ROV Hyper-Dolphin for assisting with the in situ burrow casting; E. Miyoshi and T. Urano for assistance in making the casting device; and A. A. Ekdale and Y. Kuriyama for comments on the manuscript. We appreciate the helpful comments of the editor and two anonymous reviewers, which improved the manuscript significantly. This work was financially supported by JSPS Fellowships awarded to K.S. (22-10734) and to R.G.J. (22-3819).
References
- 1.Atkinson R. J. A., Taylor A. C. 2005. Aspects of the physiology, biology and ecology of thalassinidean shrimps in relation to their burrow environment. Oceanogr. Mar. Biol. Annu. Rev. 43, 173–210 [Google Scholar]
- 2.Ziebis W., Forster S., Huettel M., Jørgensen B. B. 1996. Complex burrows of the mud shrimp Callianassa truncate and their geochemical impact in the sea bed. Nature 382, 619–622 10.1038/382619a0 (doi:10.1038/382619a0) [DOI] [Google Scholar]
- 3.Bromley R. G. 1996. Trace fossils: biology, taphonomy and applications. London, UK: Chapman & Hall [Google Scholar]
- 4.Naruse H., Nifuku K. 2008. Three-dimensional morphology of the ichnofossil Phycosiphon incertum and its implication for paleoslope inclination. Palaios 23, 270–279 10.2110/palo.2007.p07-020r (doi:10.2110/palo.2007.p07-020r) [DOI] [Google Scholar]
- 5.Ekdale A. A. 1980. Graphoglyptid burrows in modern deep-sea sediment. Science 207, 304–306 10.1126/science.207.4428.304 (doi:10.1126/science.207.4428.304) [DOI] [PubMed] [Google Scholar]
- 6.Wetzel A. 2008. Recent bioturbation in the Deep South China sea: a uniformitarian ichnologic approach. Palaios 23, 601–615 10.2110/palo.2007.p07-096r (doi:10.2110/palo.2007.p07-096r) [DOI] [Google Scholar]
- 7.Dufour S. C., Desrosiers G., Long B., Lajeunesse P., Gagnoud M., Labrie J., Archambault P., Stora G. 2005. A new method for three-dimensional visualization and quantification of biogenic structures in aquatic sediments using axial tomodensitometry. Limnol. Oceanogr.: Methods 3, 372–380 10.4319/lom.2005.3.372 (doi:10.4319/lom.2005.3.372) [DOI] [Google Scholar]
- 8.Gaillard C. 1991. Recent organism traces and ichnofacies on the deep-sea floor off New Caledonia southwestern Pacific. Palaios 6, 302–315 10.2307/3514910 (doi:10.2307/3514910) [DOI] [Google Scholar]
- 9.Glud R. N. 2008. Oxygen dynamics of marine sediments. Mar. Biol. Res. 4, 243–289 10.1080/17451000801888726 (doi:10.1080/17451000801888726) [DOI] [Google Scholar]
- 10.Levin L. A. 2005. Ecology of cold seep sediments: interactions of fauna with flow, chemistry and microbes. Oceanogr. Mar. Biol. Annu. Rev. 43, 1–46 10.1201/9781420037449.ch1 (doi:10.1201/9781420037449.ch1) [DOI] [Google Scholar]
- 11.Seilacher A. 1990. Aberrations in bivalve evolution related to photo- and chemosymbiosis. Hist. Biol. 3, 289–311 10.1080/08912969009386528 (doi:10.1080/08912969009386528) [DOI] [Google Scholar]
- 12.Okutani T., Fujikura K. 2002. Abyssal gastropods and bivalves collected by Shinkai 6500 on slope of the Japan Trench. Venus 60, 211–224 [Google Scholar]
- 13.Dall W. H. 1908. A gigantic Solemya and a new Vesicomya. Nautilus 22, 61–63 [Google Scholar]
- 14.Dworschak P. C., Cunha M. R. 2007. A new subfamily, Vulcanocalliacinae n.subfam., for Vulcanocalliax arutyunovi n. gen., n. sp. from a mud volcano in the Gulf of Cádiz (Crustacea, Decapoda, Callianassidae). Zootaxa 1450, 35–46 [Google Scholar]
- 15.Pervesler P., Uchman A. 2009. A new Y-shapaed trace fossil attributed to upogebiid crustaceans from Early Pleistocene of Italy. Acta Palaeontol. Pol. 54, 135–142 10.4202/app.2009.0114 (doi:10.4202/app.2009.0114) [DOI] [Google Scholar]