Abstract
Tikiguania estesi is widely accepted to be the earliest member of Squamata, the reptile group that includes lizards and snakes. It is based on a lower jaw from the Late Triassic of India, described as a primitive lizard related to agamids and chamaeleons. However, Tikiguania is almost indistinguishable from living agamids; a combined phylogenetic analysis of morphological and molecular data places it with draconines, a prominent component of the modern Asian herpetofauna. It is unlikely that living agamids have retained the Tikiguania morphotype unchanged for over 216 Myr; it is much more conceivable that Tikiguania is a Quaternary or Late Tertiary agamid that was preserved in sediments derived from the Triassic beds that have a broad superficial exposure. This removes the only fossil evidence for lizards in the Triassic. Studies that have employed Tikiguana for evolutionary, biogeographical and molecular dating inferences need to be reassessed.
Keywords: reptilia, agamidae, chamaeleonidae, palaeontology, phylogeny
1. Introduction
Lizards and snakes (Squamata) become increasingly common in the fossil record from the mid-Jurassic (170 Ma) [1], but early fossils of their sister lineage, Rhynchocephalia (the group that includes the living tuatara), are known from the Upper Triassic [2,3]. The squamate–rhynchocephalian divergence therefore probably occurred during the Triassic [4,5], but the origin of crown squamates remains poorly constrained. Confirmation of a Triassic crown squamate would demonstrate that living lineages of lizards and snakes diversified from each other almost immediately after squamates split from rhynchocephalians, elucidate the early evolution of key squamate characters [6,7] and provide a critical calibration for molecular divergence dating studies of reptiles in general.
Tikiguania estesi [8] is based on an isolated left dentary bone from the lower jaw (described as a ‘nearly complete left mandiblular ramus’ [8, p. 795]), recovered from screen-washing of bulk material excavated from the Late Triassic (Carnian, 216–229 Ma) Tiki Formation of north-central India (Madhya Pradesh). It was identified as an acrodontan lizard, and is currently the only recognized Triassic squamate. (To reduce ambiguity, in this paper, ‘acrodont’ refers to a type of tooth implantation, whereas ‘acrodontan’ refers to the clade consisting of Agamidae and Chamaeleonidae.) Tikiguania has consequently been cited in a wide variety of reptile studies spanning molecular divergence dating [9], evolutionary morphology [10] and palaeobiogeography [11].
The similarity of Tikiguania to living acrodontans (see figure 1) was frequently mentioned [8], but strangely no explicit comparisons were made with any living squamates. Instead, comparisons emphasized acrodont-toothed rhynchocephalians and Mesozoic squamates: Bharatagama [12], a possible acrodontan lizard from the Early Jurassic of India, and priscagamids from the early late Cretaceous of Mongolia, currently regarded as a fossil sister group of living acrodontans [10]. These comparisons demonstrated that Tikiguania was different from these ancient taxa, but what did not emerge was that the specimen is almost indistinguishable from certain living agamids, in particular the draconines. We here detail these similarities, and suggest a radical reinterpretation of Tikiguania which significantly impacts on all studies of reptile evolution involving this taxon.
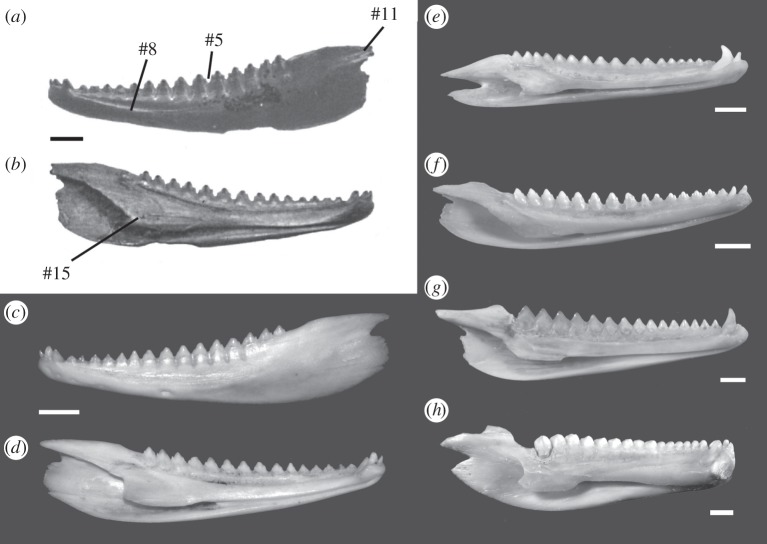
Figure 1.
Dentary of Tikiguania (a) labial and (b) lingual view, with the dentary of the living draconine agamid Calotes versicolor (ETVP 2900) in (c, d) for comparison. Numbers (#) refer to characters discussed in the main text and in table 1. (e–h) Dentaries of a representative selection of living acrodontan lizards, (e) Agama agama (SAMA R60184), (f) Bronchocoela marmorata (SAMA R03608), (g) Hypsilurus godefroyii (SAMA R05253 B) and (h) Uromastyx aegyptia (SAMA R48106). (a, b) Modified from [8]. Scale bars = 2 mm.
2. Material and methods
Parsimony [13] and Bayesian [14] phylogenetic analyses were employed on two datasets; data sources, matrices and analytical details are in the electronic supplementary material.
Analysis 1: Testing possible affinities with rhynchocephalians (acrodont-toothed reptiles also abundant in the Triassic). Tikiguania was included in a broad-scale morphological and molecular matrix for the major lineages of lepidosauromorphs, with archosauromorphs as the outgroup. The analysis included three nuclear genes, rag-1, c-mos and BDNF (brain-derived neurotrophic factor) (total 4224 aligned sites), widely sequenced across squamates [15], and 233 morphological traits drawn from previous studies [7,10].
Analysis 2: The affinities of Tikiguania within acrodontans. Tikiguania was inserted into a matrix of 22 mandibular characters scored across a range of acrodontans, along with approximately 1700 bp of mitochondrial data from ND2 and adjacent loci, the longest region currently broadly sampled across acrodontans [16].
Whereas combining the ‘lepidosauromorph’ and ‘acrodont’ matrices into a single comprehensive dataset is a laudable ideal, splitting the higher level terminal taxa in Analysis 1 into species-level terminals proved impractical, and many subtle characters that are informative and rigorously definable in restricted groups (e.g. agamids) are difficult to score across phylogenetically remote and morphologically divergent taxa (e.g. snakes, dibamids, amphisbaenians, archosauromorphs).
3. Results
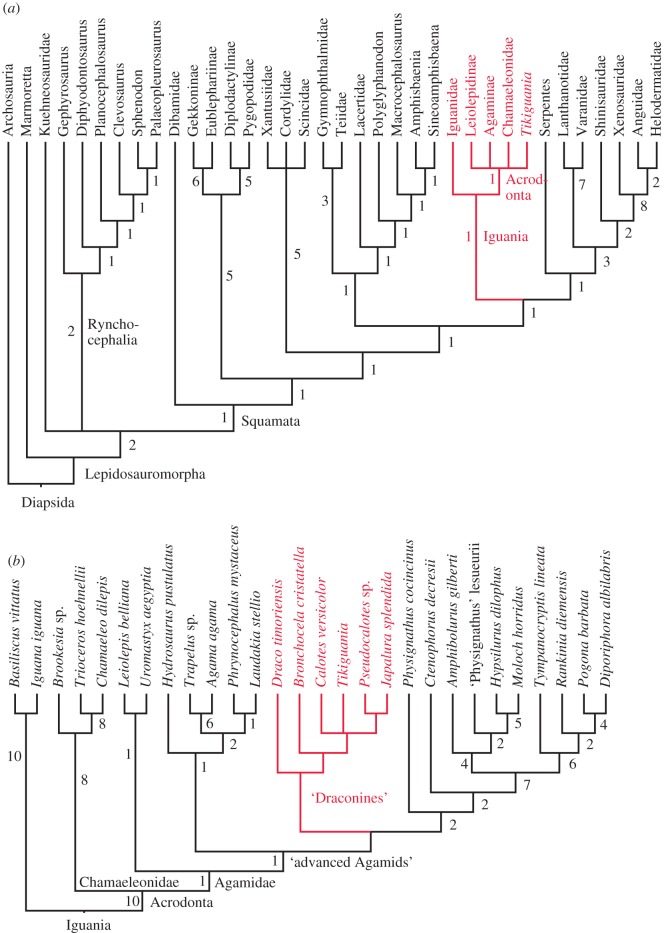
Analysis 1 groups Tikiguania with Acrodonta and thus within crown Squamata (figure 2a). These results should be robust to additional data: the tree is consistent with trees obtained from molecular analyses of lepidosauromorphs with more nuclear genes and/or denser taxon sampling [15,16]. Tikiguana is not related to rhynchocephalians; in particular, the narrow tapering dentary without a posterodorsal ‘coronoid’ extension, reduced and dorsal symphysial contact point, as well as tooth implantation (successively pleurodont/acrodont/pleuroacrodont anteroposteriorly) are typical of acrodontan lizards and not seen in early rhynchocephalians [12].
Figure 2.
(a) Phylogeny of lepidosauromorph reptiles, based on morphological and molecular data (analysis 1; strict consensus of 20 MPTs), showing that Tikiguania groups with acrodontan lizards, not rhynchocephalians. (b) Phylogeny of acrodontan lizards, based on morphological and molecular data (analysis 2; consensus of 8 MPTs) demonstrating that Tikiguania is nested within crown agamids. Majority-rule consensus; clades found in strict consensus indicated with branch supports >0. Clades without numbers (e.g. within ‘draconines’) are not present on strict consensus (i.e. have Bremer support of 0).
Analysis 2 nests Tikiguania deeply within living acrodontans (figure 2), grouping with advanced agamids (i.e. above Leiolepis and Uromastyx) in all maximum parsimony trees (MPTs) and with draconines in most MPTs. This conclusion is consistent with the striking (albeit phenetic) similarity between Tikiguana and living draconine agamids. In particular, Tikiguania has several traits (table 1) placing it deep within acrodontans, some uniquely acquired and unreversed (i.e. consistency index = 1) within the present phylogeny. Tikiguania was described as distinct from the other acrodontans in lacking a coronoid process of the dentary [8]. However, the only agamid explicitly mentioned was Uromastyx, which has a distinct crest rising vertically from the dentary that articulates with the labial surface of the coronoid bone. Other agamids, however, lack such a crest (see electronic supplementary material 2.3): they have at most a somewhat angular posterodorsal dentary margin that articulates with the base of the coronoid bone, not its ascending process.
Table 1.
Derived characters nesting Tikiguania within advanced acrodontan lizards.
| character # (see electronic supplementary material for list) [state change] and consistency index | description (see figure 1 for illustration) | clade (see figure 2b) |
|---|---|---|
| #11 [0 → 1], ci = 1 | long posterior extension of the dentary | acrodonta |
| #15 [0 → 1], ci = 0.33 | splenial facet smoothly continuous with the subdental lamina | acrodonta |
| #5 [0 → 1], ci = 1 | expanded tooth bases, in contact with each other | agamidae |
| #8 [0 → 1], ci = 1 | dentary tapering anteriorly in lateral view | ‘advanced agamids’ (Agamidae excluding Leiolepididae) |
4. Discussion
Phylogenetic analysis of morphological and molecular data confirms that T. estesi is a crown acrodontan lizard with affinities to advanced agamids (agamids excluding Leiolepis and Uromastyx). The presence of an acrodontan lizard in the Tiki formation was not previously seen as problematic, as iguanians (including acrodontans) were widely considered basal squamates [6,7], and mitochondrial DNA studies had indicated a very ancient origin of acrodontans [17]. However, although morphological evidence continues to robustly support a basal split between iguanians and other squamates [6,7,10], an increasing body of molecular and combined analyses [15,16,18,19] nest Iguania (including acrodontans) deeply within squamates. The acrodontan phylogeny of Macey et al. [17] has largely been supported by subsequent studies using nuclear genes, but this newer work suggests shallower (Cretaceous) branching times [19–22], consistent with a Triassic age for Squamata as a whole [18–22]. Any acrodontan—let alone an advanced agamid—in the Triassic is thus highly unexpected in the light of recent studies.
It is extremely unlikely that Tikiguania is an advanced agamid from the Triassic, and that the draconine jaw ‘morphotype’ has persisted largely unchanged for 216 Myr. Tikiguania came from a depth of 1.5 m within the Tiki Formation mudstone layers. As the specimen was screen washed from a load of five tonnes of excavated material, more precise depositional relationships are unknown. It shares with all of the fossil bones from this deposit a thin coating of haematite and calcite cementation, consistent with the specimen being interred in these sediments for some time, rather than a modern specimen or a reworked fossil [23]. However, a Triassic age for Tikiguania does not necessarily follow. Erosion or fissuring into the Tiki Formation at any time during the Neogene or Quaternary would have allowed more recent faunal remains to have been incorporated into the Triassic mudstones, long enough to develop the characteristic chemical patina. The Tiki Formation is widely exposed at or near the surface across more than 70 000 km2 near Tiki and Beohari (fig. 1 in [24]), suggesting a long period dating back to the Late Tertiary at least where its sediments would have been sufficiently superficial to capture more recent animal remains. Consistent with this, Tikiguania shows very little damage to fragile bone margins or tooth crowns, and is extremely similar to living draconine agamids that have occupied this region during the Tertiary and still do today.
Molecular studies [9,25,26] that have employed Tikiguania as a calibration point all interpreted Tikiguania as if it were the sister of all other squamates rather than an agamid, but this misreading of its phylogenetic position, ironically, has so far resulted in a calibration point for the origin of squamates in the Late Triassic, as was generally supposed prior to the discovery of Tikiguania [6]. We suggest avoiding this problematic fossil altogether. Morphological and palaeontological studies that have included Tikiguania also need to be revised. Tikiguania would have been evidence for an anomalously early (i.e. Triassic) age for what molecular studies suggest is a highly derived squamate clade (Acrodonta), implying that all major clades of squamates such as iguanians, anguimorphs, snakes, scincomorphs and gekkotans had diverged in the Triassic. However, none of these groups appear unequivocally in the fossil record until substantially later [5]. Indeed, some recent palaeontological and molecular studies of squamate divergence dates have not mentioned Tikiguania, presumably because of its problematic nature [19,20]. Recognizing Tikiguania as essentially modern removes any potential need to assume early diversification and long ghost lineages for all major squamate clades.
Acknowledgements
We thank the Australian Research Council for funding, Dr. J. I. Mead (East Tennessee State University) for access to specimens and Sam Morrison for access to the Australian Research Collaboration Service compute cloud.
References
- 1.Carroll R. 1975. Permo-Triassic ‘lizards’ from the Karroo. Paleont. Afr. 18, 17–87 [Google Scholar]
- 2.Fraser N. C., Benton M. J. 1989. The Triassic reptiles Brachyrhinodon and Polysphenodon and the relationships of the sphenodontids. Zool. J. Linn. Soc. 96, 413–445 10.1111/j.1096-3642.1989.tb02521.x (doi:10.1111/j.1096-3642.1989.tb02521.x) [DOI] [Google Scholar]
- 3.Heckert A. B., Lucas S. B., Frinehart L. F., Hunt A. P. 2008. A new genus and species of sphenodontian from the Ghost Ranch Coelophysis quarry (Upper Triassic: Apachean) Rock Point Formation, New Mexico, USA. Palaeontology 51, 827–845 10.1111/j.1475-4983.2008.00786.x (doi:10.1111/j.1475-4983.2008.00786.x) [DOI] [Google Scholar]
- 4.Evans S. E., Borsuk-Białynicka M. 2009. A small lepidosauromorph reptile from the Early Triassic of Poland. Palaeont. Polon. 65, 179–202 [Google Scholar]
- 5.Evans S. E., Jones M. E. H. 2010. The origin, early history and diversification of lepidosauromorph reptiles. In New aspects of mesozoic biodiversity (ed. Bandyopadhyay S.), pp. 27–44 Berlin, Germany: Springer; 10.1007/978-3-642-10311-7 (doi:10.1007/978-3-642-10311-7) [DOI] [Google Scholar]
- 6.Evans S. E. 2003. At the feet of the dinosaurs: the early history and radiation of lizards. Biol. Rev. 78, 513–551 10.1017/S1464793103006134 (doi:10.1017/S1464793103006134) [DOI] [PubMed] [Google Scholar]
- 7.Estes R., De Queiroz K., Gauthier J. 1988. Phylogenetic relationships within squamata. In Phylogenetic relationships of the lizard families: essays commemorating Charles L Camp (ed. Estes R., Pregill G.), pp. 119–281 Stanford, CA: Stanford University Press [Google Scholar]
- 8.Datta P. M., Ray S. 2006. Earliest lizard from the Late Triassic (Carnian) of India. J. Vert. Paleont. 26, 795–800 10.1671/0272-4634(2006)26[795:ELFTLT]2.0.CO;2 (doi:10.1671/0272-4634(2006)26[795:ELFTLT]2.0.CO;2) [DOI] [Google Scholar]
- 9.Gamble T., Bauer A. M., Colli G. R., Greenbaum E., Jackman T. R., Vitt L. J., Simons A. M. 2011. Coming to America: multiple origins of New World geckos. J. Evol. Biol. 24, 231–244 10.1111/j.1420-9101.2010.02184.x (doi:10.1111/j.1420-9101.2010.02184.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conrad J. L. 2008. Phylogeny and systematics of squamata (Reptilia) based on morphology. Bull. Amer. Mus. Nat. Hist. 310, 1–182 10.1206/310.1 (doi:10.1206/310.1) [DOI] [Google Scholar]
- 11.Prasad G. V. R., Bajpai S. 2008. Agamid lizards from the Early Eocene of western India: oldest Cenozoic lizards from South Asia. Palaeont. Elect. 11, 1–19 http://palaeo-electronica.org/2008_1/134/index.html [Google Scholar]
- 12.Evans S. E., Prasad G. V. R., Manhas B. K. 2002. Fossil lizards from the Jurassic Kota Formation of India. J. Vert. Paleont. 22, 299–312 10.1671/0272-4634(2002)022[0299:FLFTJK]2.0.CO;2 (doi:10.1671/0272-4634(2002)022[0299:FLFTJK]2.0.CO;2) [DOI] [Google Scholar]
- 13.Swofford D. L. 2002. PAUP*: phylogenetic analysis using parsimony (*and other methods). Version 4 . Sunderland, MA: Sinauer [Google Scholar]
- 14.Ronquist F., Huelsenbeck J. P. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 10.1093/bioinformatics/btg180 (doi:10.1093/bioinformatics/btg180) [DOI] [PubMed] [Google Scholar]
- 15.Townsend T., Larson A., Louis E., Macey J. R. 2004. Molecular phylogenetics of squamata: the position of snakes, amphisbaenians, and dibamids, and the root of the squamate tree. Syst. Biol. 53, 735–758 10.1080/10635150490522340 (doi:10.1080/10635150490522340) [DOI] [PubMed] [Google Scholar]
- 16.Wiens J. J., Kuczynski C. A., Townsend T., Reeder T. W., Mulcahy, D. G., Sites J. W., Jr 2010. Combining phylogenomics and fossils in higher level squamate reptile phylogeny: molecular data change the placement of fossil taxa. Syst. Biol. 59, 674–688 [DOI] [PubMed] [Google Scholar]
- 17.Macey J. R., Schulte J. A., Larson A., Ananjeva N. B., Wang Y., Pethiyagoda R., Rastegar-Pouyani N., Papenfuss T. J. 2000. Evaluating trans-Tethys migration: an example using acrodont lizard phylogenetics. Syst. Biol. 49, 233–256(doi:10.1093/sysbio/49.2.233) [DOI] [PubMed] [Google Scholar]
- 18.Vidal N., Hedges S. B. 2009. The molecular evolutionary tree of lizards, snakes and amphisbaenians. C. R. Biol. 332, 129–139 10.1016/j.crvi.2008.07.010 (doi:10.1016/j.crvi.2008.07.010) [DOI] [PubMed] [Google Scholar]
- 19.Müller J., Hipsley C.A., Head J.J., Kardjilov N., Hilger A., Wuttke M., Reisz R. R. 2011. Eocene lizard from Germany reveals amphisbaenian origins. Nature 473, 364–367 10.1038/nature09919 (doi:10.1038/nature09919) [DOI] [PubMed] [Google Scholar]
- 20.Townsend T., Mulcahy D. G., Sites J. W., Jr, Kuczynski C. A., Wiens J. J., Reeder T. W. 2011. Phylogeny of iguanian lizards inferred from 29 nuclear loci, and a comparison of concatenated and species-tree approaches for an ancient, rapid radiation. Mol. Phylogenet. Evol. 61, 363–380 10.1016/j.ympev.2011.07.008 (doi:10.1016/j.ympev.2011.07.008) [DOI] [PubMed] [Google Scholar]
- 21.Hugall A. F., Foster R., Lee M. S. Y. 2007. Calibration choice, rate smoothing, and the pattern of tetrapod diversification according to the long nuclear gene RAG-1. Syst. Biol. 56, 543–563 10.1080/10635150701477825 (doi:10.1080/10635150701477825) [DOI] [PubMed] [Google Scholar]
- 22.Hugall A. F., Lee M. S. Y. 2004. Molecular claims of Gondwanan age for Australian agamid lizards are untenable. Mol. Biol. Evol. 21, 2102–2110 10.1093/molbev/msh219 (doi:10.1093/molbev/msh219) [DOI] [PubMed] [Google Scholar]
- 23.Bao H., Koch P. L., Hepple R. P. 1998. Hematite and calcite coatings on fossil vertebrates . J. Sediment. Res. 68, 727–738 [Google Scholar]
- 24.Datta P. M. 2005. Earliest mammal with transversely expanded upper molar from the Late Triassic (Carnian) Tiki Formation, South Rewa Gondwana Basin, India. J. Vert. Paleont. 25, 200–207 10.1671/0272-4634(2005)025[0200:EMWTEU]2.0.CO;2 (doi:10.1671/0272-4634(2005)025[0200:EMWTEU]2.0.CO;2) [DOI] [Google Scholar]
- 25.Pyron R. A. 2010. A likelihood method for assessing molecular divergence time estimates and the placement of fossil calibrations. Syst. Biol. 59, 185–194 10.1093/sysbio/syp090 (doi:10.1093/sysbio/syp090) [DOI] [PubMed] [Google Scholar]
- 26.Hipsley C. A., Himmelmann L., Metzler D., Müller J. 2010. Integration of Bayesian molecular clock methods and fossil-based soft bounds reveals early Cenozoic origin of African lacertid lizards. BMC Evol. Biol. 9, 151–164 10.1186/1471-2148-9-151 (doi:10.1186/1471-2148-9-151) [DOI] [PMC free article] [PubMed] [Google Scholar]