Abstract
The northern wheatear (Oenanthe oenanthe) is a small (approx. 25 g), insectivorous migrant with one of the largest ranges of any songbird in the world, breeding from the eastern Canadian Arctic across Greenland, Eurasia and into Alaska (AK). However, there is no evidence that breeding populations in the New World have established overwintering sites in the Western Hemisphere. Using light-level geolocators, we demonstrate that individuals from these New World regions overwinter in northern sub-Sahara Africa, with Alaskan birds travelling approximately 14 500 km each way and an eastern Canadian Arctic bird crossing a wide stretch of the North Atlantic (approx. 3500 km). These remarkable journeys, particularly for a bird of this size, last between one to three months depending on breeding location and season (autumn/spring) and result in mean overall migration speeds of up to 290 km d−1. Stable-hydrogen isotope analysis of winter-grown feathers sampled from breeding birds generally support the notion that Alaskan birds overwinter primarily in eastern Africa and eastern Canadian Arctic birds overwinter mainly in western Africa. Our results provide the first evidence of a migratory songbird capable of linking African ecosystems of the Old World with Arctic regions of the New World.
Keywords: Africa, geolocator, northern wheatear, stable-hydrogen isotopes
1. Introduction
Tracking migratory animals between periods of the annual cycle is critical for understanding the evolution of range limits, life-history trade-offs and population dynamics [1]. The northern wheatear (Oenanthe oenanthe; wheatear hereafter) is a small (approx. 25 g), insectivorous migrant with one of the largest ranges of any songbird in the world, breeding from northeastern Canada (CN) across Europe and Asia into AK and extreme northwestern CN. Although there is abundant of tundra breeding habitat between the two Nearctic populations, they have not met. The eastern Canadian birds belong to the subspecies leucorhoa while the Alaskan birds belong to the nominate subspecies [2]. Despite sporadic reports in temperate North America [3], there is no direct evidence that wheatears have established permanent wintering sites in the Western Hemisphere, suggesting that both Nearctic populations overwinter with Eurasian populations in northern sub-Sahara Africa [2]. This would be remarkable because individuals from AK would have to travel about 14 500 km to their wintering grounds, while individuals from the eastern CN must cross the Atlantic Ocean. For individuals using these migration routes, it would be reasonable to expect that Alaskan birds overwinter in eastern Africa and eastern Canadian Arctic birds in western Africa.
We examined these hypothesized connections between the Western Hemisphere breeding grounds and African wintering range using miniaturized light-level geolocators [4] that were attached to individuals in AK and eastern Canadian Arctic. We also used stable-hydrogen isotopes in wing coverts to estimate the African wintering origin of individuals captured at these same breeding sites.
2. Material and methods
(a). Light-level geolocators
We attached 1.2 g light-level geolocators (model Mk10S with a 13 mm sensor stalk at a 30° angle to the horizontal, British Antarctic Survey) on 30 wheatears at Eagle Summit, AK, USA (65.5° N, 145.4° W) in June 2009, and 16 wheatears at Iqaluit, Baffin Island, Nunavut, CN (63.7° N, 68.5° W) in July 2010. Geolocators were attached using a Rappole-Tipton style harness [5] made from elastic silicone–rubber mixture (MVQ Arcus, Germany; http://www.arcus-shop.de/). Leg-loop length was adjusted based on body size [6] and the total attachment with harness weighed approximately 1.4 g. The mean body mass of AK birds was 24.7 g (±1.9 s.d., n = 30) and 28.3 g (±2.1, n = 16) for eastern CN birds. Tag mass represented less than or equal to 6.1% (5.7 ± 0.3%, mean ± s.d.) and less than or equal to 5.9% (4.9 ± 0.4%) for AK and CN birds, respectively, representing a relative load similar to the suggested upper permissible limit of 5 per cent [7].
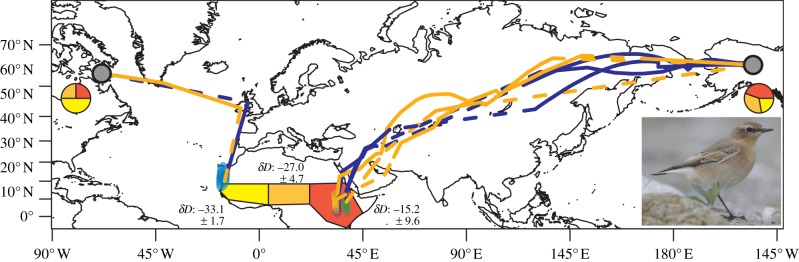
Data were downloaded from four devices (three from AK, one from CN). Light data were processed with programmes provided by the British Antarctic Survey (BASTrak suite). To identify the stationary wintering location during December, January and February (electronic supplementary material, figure S1), we used a total of 180 fixes per bird over 90 days. Wintering grounds were defined by kernel densities encompassing 50 per cent of the maximum density using the R package ‘adehabitat’ and the ad hoc method for the estimation of the smoothing parameter and the bivariate normal kernel (figure 1) [9]. Because we did not know whether the selection of sun elevation from the calibration (see the electronic supplementary material) applied equally well for the wintering grounds, we show 50 per cent kernel densities of the predicted location during the stationary wintering period for six different sun elevations (−2°, −2.5°, −3°, −3.5°, −4°, −4.5°; figure 1). Precision of fixes depends on season and topography (see the electronic supplementary material).
Figure 1.
Migration routes and wintering grounds of three northern wheatears breeding in Alaskan (AK) and one in the eastern Canadian Arctic (CN; grey dot, breeding area, blue, autumn migration, orange, spring migration, dashed lines indicate uncertainty in migration routes close to equinoxes). Fifty per cent kernel densities of winter fixes (beginning of December 2009–end of February; purple, bird AK-1; green, bird AK-2; orange, bird AK-3; blue, bird CN-1) are given depending on the sun elevation selected (with −2° for most southern and with −4.5° for most northern densities). Pie charts indicate the proportion of individuals (AK: n = 9, CN: n = 4) originating from one of the three pre-defined wintering regions (red, western; orange, central; yellow, eastern) [8] based on stable-hydrogen isotope (δD) values in winter grown feathers and the δD values within each wintering region (mean ± s.d. shown).
(b). Stable-hydrogen isotope analysis
Stable-hydrogen isotope values in precipitation (δDP) vary predictably according to large-scale variation in temperature, rainfall and evaporation [10], and these values are transferred up the food chain [11]. Because feathers are metabolically inert after growth, they retain the isotopic signature of their place of growth. Thus, feathers can be sampled during one period of the annual cycle to provide a rough estimate of the location of an individual during a previous period when that feather was grown [11,12]. We used this approach to estimate the wintering origin of wheatears captured at the AK and CN sites.
Approximately, one-third of wheatears undergo a partial moult of wing coverts in the wintering grounds [13]. Winter-grown coverts can be distinguished from breeding-ground coverts (grown after the previous year on the breeding grounds) by colour, shape and degree of wear [13]. At the AK site, we sampled feathers from the 29 of the 30 birds that were also affixed with geolocators. Of those, nine (31%) were identified as having winter-grown coverts. At the CN site, we sampled feathers from 87 birds, including those that were deployed with geolocators and only seven (8%) had clearly distinguishable winter-grown coverts. For all birds at both sites, we also sampled the second outmost tail feather grown the previous year on the breeding grounds [13], so we could (i) compare these values with δD values in winter-grown coverts and (ii) along with data from other breeding sites, estimate the discrimination factor between δD values in local precipitation and δD values in feathers (see the electronic supplementary material).
Stable hydrogen isotope ratios (2H/1H) are reported in delta (δ) notation in parts per thousand (‰) deviation from the Vienna standard mean ocean water-standard light Antarctic precipitation (VSMOW-SLAP) standard scale, where δD = [((ratiosample/ratiostandard)−1) × 1000]. To assign individuals to an African wintering region, we pre-defined three wintering regions (west, central and east) that together encompassed the reported wintering grounds of wheatears in Africa (figure 1) [8]. Within each of these wintering regions, we randomly selected 20–28 sites and used the Online Isotopes in Precipitation Calculator (OIPC) at http://wateriso.eas.purdue.edu/waterisotopes/ to derive a mean and s.d. of weighted mean growing-season δDP values (figure 1) [14]. Wing covert feathers were assigned a probability of origin for each region based on a likelihood function with a normal distribution [15]. We also incorporated analytical error and process error from the OPIC data (see the electronic supplementary material).
3. Results and discussion
Five of the 30 wheatears tagged in AK in 2009 returned in 2010. One could not be caught and another had lost its geolocator. Data from the remaining three geolocators revealed that the AK birds spent their winter in eastern Africa somewhere in Sudan or Uganda/Kenya (figure 1). During the autumn, birds travelled through northern Russia and Kazakhstan before crossing the Arabian Desert, a one-way trip that averaged 14 600 km (±360, mean ± s.d.) and took 91 (±11) days (approx. 160 km d−1). The same route was followed during the spring but the duration was approximately one month shorter (approx. 55 days; 250 km d−1; electronic supplementary material, figures S1 and S2).
Two of 16 wheatears tagged in the eastern Canadian Arctic in 2010 returned in 2011 but one lost its geolocator. Return rate was not significantly different from birds that were only colour-ringed at the same site (two out of 33 colour-ringed birds returned; 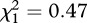 , p = 0.49). The CN bird appeared to cross about 3400 km from Baffin Island to the western British Isles in no more than four days (approx. 850 km d−1) possibly via Greenland (electronic supplementary material, figure S3) and continued south through Europe for another 4000 km to spend the winter in western Africa on the coast of Mauritania (figure 1). During spring migration, it took a similar route. In contrast to the AK birds, autumn migration for the CN bird was shorter (approx. 26 days, approx. 290 km d−1) than spring migration (approx. 55 days, approx. 130 km d−1).
, p = 0.49). The CN bird appeared to cross about 3400 km from Baffin Island to the western British Isles in no more than four days (approx. 850 km d−1) possibly via Greenland (electronic supplementary material, figure S3) and continued south through Europe for another 4000 km to spend the winter in western Africa on the coast of Mauritania (figure 1). During spring migration, it took a similar route. In contrast to the AK birds, autumn migration for the CN bird was shorter (approx. 26 days, approx. 290 km d−1) than spring migration (approx. 55 days, approx. 130 km d−1).
Results from stable-hydrogen isotopes also suggest that separation between these Western Hemisphere populations on the wintering grounds. Of the nine AK birds, four were assigned to eastern Africa with a likelihood of greater than or equal to 0.8, two birds to central Africa (greater than or equal to 0.9), one to western Africa (0.94), and two birds revealed no clear wintering origin as they had likelihoods of less than or equal to 0.5 for all of the three regions (figure 1). Of the seven Canadian birds with winter-grown coverts, three had negative δD values (between −68 and −71‰) that were outside the δD values of all three African wintering regions, suggesting that they probably moulted these feathers in western Europe during migration. Of the remaining four birds, two were assigned to western Africa (likelihood: greater than or equal to 0.7), one to central (0.97) and the other to eastern Africa (1.0).
The migratory journeys from both sides of the Western Hemisphere Arctic to sub-Saharan Africa could be a consequence of the Pleistocene expansion of the breeding range. The fact that Western Hemisphere wheatears have not colonized America during the winter suggests that selection has not acted against what appears to be a well-developed innate migratory programme [16]. Though our data from geolocators suggest population-specific winter regions and, on a broad scale, the results from stable isotope analysis generally support this, it is difficult to say with a high degree of confidence whether there is strong or weak connectivity between specific regions in Africa and breeding populations in the Arctic due to the low sample size and the poor spatial resolution of stable isotopes. Nevertheless, our results provide the first incontrovertible evidence that a migratory songbird regularly travels between Arctic regions of the Western Hemisphere and Africa. Scaled for body size, this is the one of the longest round-trip migratory journeys of any bird in the world and raises questions about how a bird of this size is able to successfully undertake such physically demanding journeys twice each year, particularly for inexperienced juveniles migrating on their own.
Acknowledgements
This work was supported by a Deutsche Forschungsgemeinschaft (BA 816/15-4) grant to F.B. and H.S. and a Canadian Foundation for Innovation grant to D.R.N. We thank B. Pattinson, R. Gill, E. Paul in AK and M. Mallory, M. E. Thomas, R. Armstrong in Nunavat for advice with permits and S. Sharbaugh, K. Winker, E. Dunn, J. Hussell, and D. Strickland for support and field work. E.D., J.H., D.S. and D.J.T.H. were supported by Bird Studies CN. A. Luckner assisted with stable-isotope analysis.
Reference
- 1.Newton I. 2007. The migration ecology of birds. London, UK: Elsevier [Google Scholar]
- 2.Cramp S. 1988. Handbook of the birds of Europe, the Middle East and North Africa. The birds of the Western Palearctic, vol. 5 Oxford, UK: Oxford University Press [Google Scholar]
- 3.Brunn B. 1980. The Greenland wheatear (Oenanthe oenanthe leucorrhoa) in North America. Am. Birds 34, 310–312 [Google Scholar]
- 4.Stutchbury B. J. M., Tarof S. A., Done T., Gow E., Kramer P. M., Tautin J., Fox J. W., Afanasyev V. 2009. Tracking long-distance songbird migration by using geolocators. Science 323, 896. 10.1126/science.1166664 (doi:10.1126/science.1166664) [DOI] [PubMed] [Google Scholar]
- 5.Rappole J. H., Tipton A. R. 1990. New harness design for attachment of radio transmitters to small passerines. J. Field Ornithol. 62, 335–337 [Google Scholar]
- 6.Naef-Daenzer B. 2007. An allometric function to fit leg-loop harnesses to terrestrial birds. J. Avian Biol. 38, 404–407 10.1111/j.2007.0908-8857.03863.x (doi:10.1111/j.2007.0908-8857.03863.x) [DOI] [Google Scholar]
- 7.Cochran W. W. 1980. Wildlife telemetry. In Wildlife management techniques manual (ed. Schemnitz S.), pp. 507–520 Washington, USA: The Wildlife Society [Google Scholar]
- 8.Walther B. A., et al. 2010. A database of Western Palearctic birds migrating within Africa to guide conservation decisions. In Proc. 12th Pan-African Ornithological Congress, 2008 (eds Harebottle D. M., Craig A. J. F. K., Anderson M. D., Rakotomanana H., Muchai M.), pp. 50–72 Cape Town, South Africa: Animal Demography Unit [Google Scholar]
- 9.R Development Core Team. 2010. R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org) [Google Scholar]
- 10.Bowen G. J. 2010. Isoscapes: spatial pattern in isotopic biogeochemistry. Annu. Rev. Earth Planet Sci. 38, 161–187 10.1146/annurev-earth-040809-152429 (doi:10.1146/annurev-earth-040809-152429) [DOI] [Google Scholar]
- 11.Hobson K. A., Wassenaar L. I. 2008. Tracking animal migration with stable isotopes. Amsterdam, The Netherlands: Academic Press [Google Scholar]
- 12.Oppel S., et al. 2011. High variation reduces the value of feather stable isotope ratios in identifying new wintering areas for aquatic warblers Acrocephalus paludicola in West Africa. J. Avian Biol. 42, 342–354 10.111/j.160-048X.2011.05252.x (doi:10.111/j.160-048X.2011.05252.x) [DOI] [Google Scholar]
- 13.Jenni L., Winkler R. 1994. Moult and ageing of European passerines. London, UK: Academic Press [Google Scholar]
- 14.Bowen G. J. 2010. The online isotopes in precipitation calculator, v. 2.2. See http://www.waterisotopes.org [Google Scholar]
- 15.Wunder M. B., Norris D. R. 2008. Improved estimates of certainty in stable-isotope-based methods for tracking migratory animals. Ecol. Appl. 18, 549–559 10.1890/07-0058.1 (doi:10.1890/07-0058.1) [DOI] [PubMed] [Google Scholar]
- 16.Maggini I., Bairlein F. 2010. Endogenous rhythms of seasonal migratory body mass changes and nocturnal restlessness in different populations of northern wheatear Oenanthe oenanthe. J. Biol. Rhythms 25, 268–276 10.1177/0748730410373442 (doi:10.1177/0748730410373442) [DOI] [PubMed] [Google Scholar]