Abstract
The impact of multiple invading species can be magnified owing to mutual facilitation—termed ‘invasional meltdown’—but invasive species can also be adversely affected by their interactions with other invaders. Using a unique reciprocal host–parasite relationship between a bitterling fish (Rhodeus amarus) and unionid mussels, we show that an invasive mussel reverses the roles in the relationship. Bitterling lay their eggs into mussel gills, and mussel larvae parasitize fish. Bitterling recently colonized Europe and parasitize all sympatric European mussels, but are unable to use a recently invasive mussel, Anodonta woodiana. The parasitic larvae of A. woodiana successfully develop on R. amarus, whereas larvae of European mussels are rejected by bitterling. This demonstrates that invading species may temporarily benefit from a coevolutionary lag by exploiting evolutionarily naive hosts, but the resulting relaxed selection may facilitate its exploitation by subsequent invading species, leading to unexpected consequences for established interspecific relationships.
Keywords: species interaction, coevolution, interspecific relationship, parasitism
1. Introduction
The impact of multiple invading species can be magnified owing to their positive feedback (‘invasional meltdown’) [1]. However, invasive species can also be adversely affected by interactions with other invaders. The effects of invasions may sometimes proceed via subtle processes, such as affecting coevolved relationships among native species (e.g. parasitism–mutualism) [2]. These are characterized by coevolutionary dynamics, when evolution of a trait in one partner is followed by counter-adaptation of the other partner [3]. Species translocations can affect host–parasite dynamics, with effects on hosts and parasites depending on their ability to cope with the new partners. A new species can become an alternative partner in mutualistic and antagonistic relationships, sometimes leading to a preference for the new partner over established coevolved partners [2,4]. This may have contrasting impacts; native species may benefit from adoption of a new partner, if the translocated species is suitable [4], but may also use unsuitable non-native partners maladaptively [5]. Then, demographic effects, including the risk of extinction, will depend on the extent of maladaptive preference and magnitude of any fitness cost.
We examined the effects of an invasive species on a coevolved host–parasite relationship between a freshwater fish—the European bitterling (Rhodeus amarus)—and unionid mussels. Bitterling (Acheilognathinae) are cyprinid fishes that oviposit into the gills of live mussels where their embryos complete development. Hosting bitterling embryos is costly to mussels [6] and they have evolved adaptations to eject bitterling eggs and embryos, mirrored by counter-adaptations in bitterling embryos to avoid ejection [6,7]. Bitterling are of east Asian origin, with more than 50 species distributed in east Asia and a single species in Europe [7]. European R. amarus has expanded relatively recently (centuries to millennia before present) from the Black Sea region into central and west Europe [8,9] where it exploits evolutionarily naive mussel populations [10].
Unionid mussels possess a larval stage (glochidium) that must attach to a fish host (bitterling or other species) to complete development. Female mussels brood their offspring internally and discharge ripe glochidia into the water. Glochidia attach to host fish and encyst, but may be rejected, indicating that glochidia infection is costly to the host [11]. Both bitterling and mussels can be host specialists or generalists [12,13] and the nature of their relationship varies from mutualism to one-sided or reciprocal parasitism [6]. Unionids are abundant throughout the Palearctic, but more diverse in east Asia [12].
We investigated the relationship between an invasive Asian mussel, Anodonta woodiana and R. amarus. Rhodeus amarus parasitize all sympatric European mussels [7,9], but avoid infection by their glochidia [6]. Anodonta woodiana is common and widely distributed in its natural range in east Asia and elsewhere where it has invaded [14]. Notably, A. woodiana is sympatric with numerous bitterling species over its native range and is used by many of them [15]. Populations of A. woodiana have established in Europe since the 1970s [14]. Preliminary observations suggested that a population of R. amarus in central Poland readily used A. woodiana for oviposition, although R. amarus embryos completely failed to survive embryonic development owing to ejection by the mussels [16]. This implies potentially severe consequences of A. woodiana invasion for R. amarus populations, although the precise impact depends on the relative preference of R. amarus for native European mussels and A. woodiana, and the competitive abilities of native and invasive mussels.
We experimentally addressed three key aspects of the bitterling–mussel relationship by testing (i) population consequences of A. woodiana establishment on R. amarus with access to a variable proportion of invasive A. woodiana and native Anodonta anatina; (ii) preference of R. amarus for native or A. woodiana hosts for oviposition; and (iii) the capacity of native mussels and A. woodiana to complete larval development on R. amarus and four control fish species.
2. Material and methods
Experiment 1 on population consequences of host availability was tested in outdoor pools, with six replicates for each of five treatments (0 : 4, 1 : 3, 2 : 2, 3 : 1, 4 : 0 of invasive A. woodiana: native A. anatina mussels) with five male and six female fish in each pool. Pools were monitored daily for emerging juvenile fish. For experiment 2, 16 replicates of oviposition tests (simultaneous choice between A. woodiana and A. anatina) were conducted in aquaria. Additionally, eight replicates were no-choice test, with fish having access only to A. woodiana. In experiment 3, infections by glochidia from A. woodiana and A. anatina were completed in aquaria on R. amarus and four control fish species (of European and Asian origin, all currently widespread Europe). For full details on experimental protocols, see the electronic supplementary material.
3. Results and discussion
(a). Impact of Anodonta woodiana on Rhodeus amarus
The presence of A. woodiana had strong negative effects on R. amarus recruitment. No bitterling emerged in pools when access was restricted to A. woodiana, in contrast to replicates with access to at least a single A. anatina. Fewer bitterling emerged in pools with a single A. anatina compared with pools with two or more A. anatina (general linear model (GLM): F4,25 = 8.06, p < 0.001; figure 1).
Figure 1.
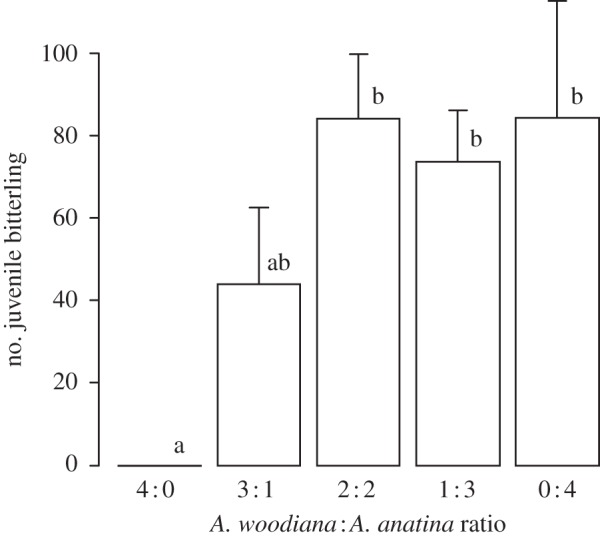
Bitterling reproductive success, estimated as the sum of the numbers of juveniles that departed from mussels in experimental pools across variable proportions of invasive Anodonta woodiana to native Anodonta anatina. Error bars represent 1 s.e. Different letters denote pairwise differences between treatment groups.
(b). Rhodeus amarus oviposition choice
Rhodeus amarus did not use A. woodiana for oviposition. In choice tests, male and female R. amarus readily inspected both A. woodiana and native mussels. No difference in pre-oviposition behaviour consisting of measures of interest (male and female siphon inspection) and male preference (ejaculation and leading rates) for each mussel species (general linear mixed model (GLMM): all p > 0.05). However, R. amarus never oviposited in A. woodiana and spawned only into native mussels (nine replicates); no oviposition occurred in either mussel in seven replicates (44%). The difference between oviposition into A. woodiana and native mussels was significant (exact binomial test: p = 0.004). Bitterling always failed to oviposit (eight replicates) during no-choice tests with only A. woodiana, although oviposition (followed by an immediate ejection of the eggs) was observed in outdoor pools with access only to A. woodiana (recorded as a casual observation during daily monitoring). Therefore, R. amarus failed to parasitize invasive A. woodiana, while it readily parasitized native mussels.
(c). Glochidial infections
Rhodeus amarus was an unsuitable host for native A. anatina (median of 4% of glochidia metamorphosed), but suitable for invasive A. woodiana (22%). A similar contrast in higher susceptibility to glochidia of A. woodiana was observed in the east Asian control cyprinids and, to a lesser extent, in European Leuciscus cephalus (table 1). Overall, invasive A. woodiana successfully developed on all fish hosts tested, despite minor differences between host species (Kruskal–Wallis: H4,44 = 13.8, p = 0.008, figure 2), whereas the development success of native A. anatina showed an apparent coevolutionary signal in host suitability (Kruskal–Wallis: H4,44 = 32.3, p < 0.001, figure 2), with low success in species of east Asian origin (present in Europe for several decades) [17] and R. amarus (itself of east Asian origin and descent, with its ancestor colonizing southern Europe 2–3 Ma [18], and most of continental Europe during the Holocene [8,9]). Notably, very low development success under experimental conditions indicated that R. amarus is a non-functional host of A. anatina. This confirms previous field observations that R. amarus is an unsuitable host for European unionids [6]. Hence, R. amarus is able to circumvent parasitism by native unionids, but is a suitable host of A. woodiana.
Table 1.
Glochidia development on Rhodeus amarus and control species. Initial number of glochidia attached to fish, total number of juvenile mussels that metamorphosed, number of fish in each treatment (n) and analysis of differences in the relative development success between Anodonta woodiana (AW) and Anodonta anatina (AA) for each fish species (GLMM, quasi-binomial error structure).
| fish species | mussel | initial glochidia load (median, range) | developed mussels (median, range) | n | F | d.f. | p |
|---|---|---|---|---|---|---|---|
| Rhodeus amarus | AW | 23 (4–34) | 6 (0–7) | 10 | 15.4 | 1,20 | 0.001 |
| AA | 19 (11–44) | 1 (0–3) | 12 | — | — | — | |
| European cyprinids | |||||||
| Leuciscus cephalus | AW | 167 (144–238) | 56 (27–84) | 6 | 8.3 | 1,12 | 0.014 |
| AA | 178 (93–458) | 28 (12–70) | 8 | — | — | — | |
| Barbus barbus | AW | 729 (472–804) | 399 (184–453) | 6 | 0.9 | 1,12 | 0.375 |
| AA | 728 (247–1294) | 360 (80–888) | 8 | — | — | — | |
| east Asian cyprinids | |||||||
| Pseudorasbora parva | AW | 159 (85–284) | 27 (4–127) | 10 | 26.2 | 1,16 | <0.001 |
| AA | 211 (115–312) | 0 (0–3) | 8 | — | — | — | |
| Carassius gibelio | AW | 800 (528–1176) | 240 (192–380) | 5 | 131.6 | 1,11 | <0.001 |
| AA | 474 (184–638) | 0 (0–3) | 8 | — | — | — | |
Figure 2.
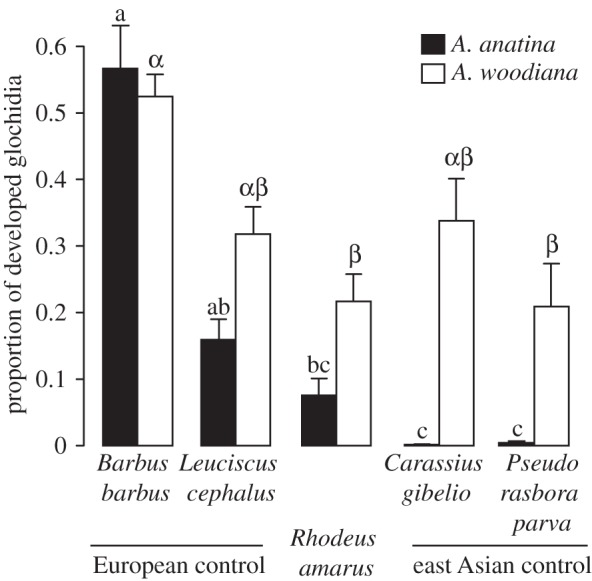
Relative development success (proportion that metamorphosed into juveniles) of glochidia of native Anodonta anatina and invasive Anodonta woodiana mussels on fish hosts. Error bars represent 1 s.e. Different letters denote pairwise differences between treatment groups for A. anatina (Latin) and A. woodiana (Greek).
4. Conclusions
Collectively, our data demonstrate that the host–parasite relationship between R. amarus and A. woodiana is a reversal of the relationship between R. amarus and native mussels in Europe. Rhodeus amarus did not parasitize A. woodiana, but was itself parasitized by A. woodiana. Anodonta woodiana has probably evolved a range of adaptations to counteract bitterling parasitism in Asia. In contrast, European R. amarus use mussels that are evolutionarily naive to bitterling parasitism and selection is probably relatively relaxed [7], comparable with cases of historical introductions of avian brood parasites and their hosts outside their native range [19,20]. After arriving in Europe, A. woodiana have come in contact with R. amarus at many locations throughout the latter's range. Interestingly, in our study R. amarus did not use A. woodiana for oviposition, whereas A. woodiana from Poland were readily used by R. amarus, followed by total ejections of all bitterling eggs [16]. Instead we observed that R. amarus avoided oviposition into A. woodiana and hence a strong negative impact on R. amarus population is predicted only if A. woodiana outcompetes and replace native unionids. While no decrease in the abundance of native unionids is currently observed at our study site (M. Vrtílek and M. Reichard 2011, personal observation), it is reported from other regions of Europe [21]. Importantly, A. woodiana populations in Europe represent multiple introductions from different source populations (originating from infected commercially imported Asian carp species) [14].
Coevolutionary arms races can emerge at different rates, even between spatially proximate populations, forming a geographical mosaic of coevolutionary hotspots and coldspots, often with contrasting outcomes across a mutualism–parasitism continuum [3]. We have demonstrated that an invading species may temporarily benefit from a coevolutionary lag by exploiting evolutionarily naive hosts, but the resulting relaxed selection may subsequently facilitate its exploitation by subsequent invading species.
Acknowledgements
Funding from the Czech Science Foundation (206/09/1163), Ministry of the Environment (0002071101) and ‘Czech University of Life Sciences Prague (CIGA 42110/1313/3104) is acknowledged. Data deposited in the Dryad Repository: http://dx.doi.org/10.5061/dryad.q31477f6.
References
- 1.Simberloff D., Von Holle M. 1999. Positive interactions of nonindigenous species: invasional meltdown? Biol. Inv. 1, 21–32 10.1023/A:1010086329619 (doi:10.1023/A:1010086329619) [DOI] [Google Scholar]
- 2.Kiers E. T., Palmer T. M., Ives A. R., Bruno J. F., Bronstein J. L. 2010. Mutualisms in a changing world: an evolutionary perspective. Ecol. Lett. 13, 1459–1474 10.1111/j.1461-0248.2010.01538.x (doi:10.1111/j.1461-0248.2010.01538.x) [DOI] [PubMed] [Google Scholar]
- 3.Thompson J. N., Cunningham B. C. 2002. Geographic structure and dynamics of coevolutionary selection. Nature 417, 735–738 10.1038/nature00810 (doi:10.1038/nature00810) [DOI] [PubMed] [Google Scholar]
- 4.Jahner J. P., Bonilla M. M., Badik K. J., Shapiro A. M., Forister M. L. 2011. Use of exotic hosts by Lepidoptera: widespread species colonize more novel hosts. Evolution 65, 2719–2724 10.1111/j.1558-5646.2011.01310.x (doi:10.1111/j.1558-5646.2011.01310.x) [DOI] [PubMed] [Google Scholar]
- 5.Chew F. S. 1977. Coevolution of pierid butterflies and their cruciferous foodplants. II. The distribution of eggs on potential foodplants. Evolution 31, 568–579 10.2307/2407522 (doi:10.2307/2407522) [DOI] [PubMed] [Google Scholar]
- 6.Reichard M., Ondračková M., Przybylski M., Liu H., Smith C. 2006. The costs and benefits in an unusual symbiosis: experimental evidence that bitterling fish (Rhodeus sericeus) are parasites of unionid mussels in Europe. J. Evol. Biol. 19, 788–796 10.1111/j.1420-9101.2005.01051.x (doi:10.1111/j.1420-9101.2005.01051.x) [DOI] [PubMed] [Google Scholar]
- 7.Smith C., Reichard M., Jurajda P., Przybylski M. 2004. The reproductive ecology of the European bitterling (Rhodeus sericeus). J. Zool. 262, 107–124 10.1017/S0952836903004497 (doi:10.1017/S0952836903004497) [DOI] [Google Scholar]
- 8.Van Damme D., Bogutskaya N., Hoffmann R., Smith C. 2007. The introduction of the European bitterling Rhodeus amarus to west and central Europe. Fish Fisher. 8, 79–106 10.1111/j.1467-2679.2007.00239.x (doi:10.1111/j.1467-2679.2007.00239.x) [DOI] [Google Scholar]
- 9.Bryja J., Smith C., Konečný A., Reichard M. 2010. Range-wide population genetic structure of the European bitterling (Rhodeus amarus) based on microsatellite and mitochondrial DNA analysis. Mol. Ecol. 19, 4708–4722 10.1111/j.1365-294X.2010.04844.x (doi:10.1111/j.1365-294X.2010.04844.x) [DOI] [PubMed] [Google Scholar]
- 10.Reichard M., Polačik M., Tarkan A. S., Spence R., Gaygusuz O., Ercan E., Ondračková M., Smith C. 2010. The bitterling mussel coevolutionary relationship in areas of recent and ancient sympatry. Evolution 64, 3047–3056 10.1111/j.1558-5646.2010.01032.x (doi:10.1111/j.1558-5646.2010.01032.x) [DOI] [PubMed] [Google Scholar]
- 11.Rogers-Lowery C. L., Dimock R. V., Jr, Kuhn R. E. 2007. Antibody response of bluegill sunfish during development of acquired resistance against the larvae of the freshwater mussel Utterbackia imbecillis. Dev. Comp. Immunol. 31, 143–155 10.1016/j.dci.2006.05.011 (doi:10.1016/j.dci.2006.05.011) [DOI] [PubMed] [Google Scholar]
- 12.Bauer G., Wächtler K. 2001. Ecology and evolution of the freshwater mussels unionoida. Berlin, Germany: Springer [Google Scholar]
- 13.Reichard M., Liu H., Smith C. 2007. The co-evolutionary relationship between bitterling fishes and freshwater mussels: insights from interspecific comparisons. Evol. Ecol. Res. 9, 239–259 [Google Scholar]
- 14.Watters G. T. 1997. A synthesis and review of the expanding range of the Asian freshwater mussel Anodonta woodiana (Lea, 1834) (Bivalvia: Unionidae). Veliger 40, 152–156 [Google Scholar]
- 15.Kondo T., Yamashita J., Kano M. 1984. Breeding ecology of five species of bitterling (Pisces: Cyprinidae) in a small creek. Physiol. Ecol. Jpn. 21, 53–62 [Google Scholar]
- 16.Reichard M., Przybylski M., Kaniewska P., Liu H., Smith C. 2007. A possible evolutionary lag in the relationship between freshwater mussels and European bitterling. J. Fish Biol. 70, 709–725 10.1111/j.1095-8649.2007.01333.x (doi:10.1111/j.1095-8649.2007.01333.x) [DOI] [Google Scholar]
- 17.Kottelat M., Freyhof J. 2007. Handbook of European freshwater fishes. Cornol, Switzerland: Kottelat [Google Scholar]
- 18.Bohlen J., Šlechtová V., Bogutskaya N., Freyhof J. 2006. Across Siberia and over Europe: phylogenetic relationships of the freshwater fish genus Rhodeus in Europe and the phylogenetic position of R. sericeus from the River Amur. Mol. Phyl. Evol. 40, 856–865 10.1016/j.ympev.2006.04.020 (doi:10.1016/j.ympev.2006.04.020) [DOI] [PubMed] [Google Scholar]
- 19.Lahti D. C. 2005. Evolution of bird eggs in the absence of cuckoo parasitism. Proc. Natl Acad. Sci. USA 102, 18 057–18 062 10.1073/pnas.0508930102 (doi:10.1073/pnas.0508930102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothstein S. I. 1990. A model system for coevolution: avian brood parasitism. Annu. Rev. Ecol. Syst. 21, 481–508 10.1146/annurev.es.21.110190.002405 (doi:10.1146/annurev.es.21.110190.002405) [DOI] [Google Scholar]
- 21.Cianfanelli S., Lori E., Bodon M. 2007. Non-indigenous freshwater molluscs and their distribution in Italy. In Biological invaders in inland waters: profiles, distribution, and threats (ed. Gherardi F.), pp. 103–121 Berlin, Germany: Springer [Google Scholar]


