Abstract
We investigate the optimal behaviour of an organism that is unable to obtain a reliable estimate of its mortality risk. In this case, natural selection will shape behaviour to be approximately optimal given the probability distribution of mortality risks in possible environments that the organism and its ancestors encountered. The mean of this distribution is the average mortality risk experienced by a randomly selected member of the species. We show that if an organism does not know the exact mortality risk, it should act as if the risk is less than the mean risk. This can be viewed as being optimistic. We argue that this effect is likely to be general.
Keywords: natural selection, optimism, predation
1. Introduction
Organisms face many sources of mortality, including disease and predation risk. The risk of mortality experienced by members of a species will vary in space and time. In some cases, an organism may be able to obtain a reliable estimate of the local mortality risk, but in other cases, this will not be possible. This paper addresses the latter case, where the risk of mortality is not accurately known. As explained below (§4), our approach is distinct from previous work such as Bouskila & Blumstein [1], which is essentially concerned with the possible asymmetry of the fitness function.
In the absence of reliable information, natural selection will shape the characteristics of a species (i.e. traits including behaviour) to be approximately optimal given the probability distribution of mortality risks in possible environments that the organism and its ancestors encountered. The mean of this distribution is the average mortality risk experienced by a randomly selected member of the species. We show that if an organism does not know the exact local mortality risk, its characteristics should correspond to a risk that is less than the mean risk (if it were not otherwise taking variability into account). Because animals should behave as if their risk of dying is less than the mean risk, the action of natural selection results in behaviour which can be viewed as optimistic [2,3].
2. The model
Our model incorporates two types of mortality. One type is independent of the organism's behaviour (for some organisms, death from disease or bad weather might be essentially independent of behaviour). The other source depends on behaviour; for example, many animals can increase their access to resources such as food or mates at the cost of an increase in their probability of being killed by a predator [4–9]. θ is a ‘background’ rate of mortality that is independent of the organism's behaviour (cf. [10,11]) and μ represents the density of predators in the local environment.
We assume that natural selection acts on a trait, u. In our main example, u mediates the trade-off between two mortality risks: the rate of predation, μb(u), and the death rate from other sources (such as starvation), a(u). In this context, u could be the organism's level of energy reserves [12]. If we consider a fixed period (e.g. winter), the expected future reproductive success at the end of this period is
where T is the duration of the period and V(u) denotes the value of having survived the period. This value might increase with u if, for example, u represented energy reserves at the end of winter, or might just be a positive constant.
3. Results
When μ is known, differentiating with respect to u and setting the equation to zero gives the optimal behaviour, uf, as:  , or
, or
 |
3.1 |
Now we assume that μ is not known, but that each winter each population member experiences a density of predators that is chosen independently from some distribution. Then the value of behaviour becomes an expectation across all possible μ values:
Differentiating with respect to u, setting the derivative to zero, and dividing through by common factors, we find that the optimal behaviour, u*, is given by
where S = exp{−(a(u*)+θ+μb(u*))T} is the probability of survival and
 |
Using E(SY) = Cov(S,Y) + E(S)E(Y), we obtain Cov(S,Y)/E(S) + E(Y) = 0. Noting that Cov(S, Y) = Cov(S, μ), we obtain:
 |
3.2 |
The motivation for defining 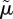 in this way comes from contrasting the right-hand side of equation (3.2) with that of equation (3.1):
in this way comes from contrasting the right-hand side of equation (3.2) with that of equation (3.1): 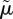 represents the known density of predators under which optimal behaviour is the same as the optimal behaviour when the density of predators is not known. We refer to
represents the known density of predators under which optimal behaviour is the same as the optimal behaviour when the density of predators is not known. We refer to  as the effective density of predators.
as the effective density of predators.
Since S decreases with μ, the covariance is negative, and  . This establishes our main result: the effective density of predators is less than its mean value.
. This establishes our main result: the effective density of predators is less than its mean value.
Figure 1 shows how optimal behaviour, effective predator density and survival alter with increasing variance (i.e. uncertainty) in predator density for a given mean predator density (see electronic supplementary material, appendix 1). As can be seen, it is optimal to become more optimistic as uncertainty increases.
Figure 1.
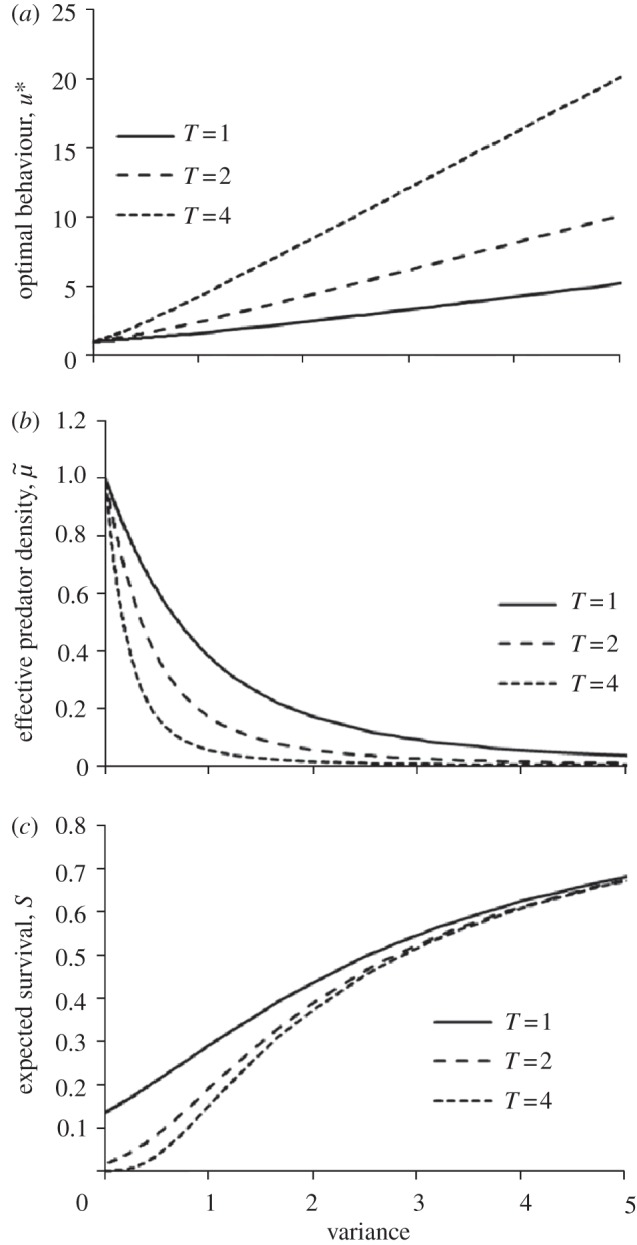
(a) Optimal behaviour, (b) effective predator density and (c) expected overwinter survival as a function of variance in predator density. The mean predator density is equal to 1 throughout. Predator density is assumed to have a gamma distribution (see electronic supplementary material, appendix 1 for details). V(u) = constant for all u so that optimal behaviour maximizes the probability of overwinter survival, starvation rate a(u) = 1/u, predation rate function b(u) = u. θ = 0 (only relevant for figure (c)).
The effect of uncertainty on behaviour also increases with the length of time which the animal needs to stay alive, T. This is because as T increases (and survival chances decrease), the animal should put more and more weight on the possibility that the environment is good if it is to survive.
In our model of overwinter survival, the background mortality rate θ has no effect on optimal behaviour.
In the electronic supplementary material, appendix 2, we show that optimism about mortality can also apply over an animal's lifetime. We take the control variable u to represent the animal's rate of reproduction. As this variable increases, the animal's rate of mortality also increases, so there is a trade-off between survival and current reproduction. The animal's expected lifespan L(u) depends on this rate of reproduction, the background mortality rate θ and the density of predators μ. The animal's lifetime reproductive success is thus uL(u). We take optimal behaviour to maximize the expected value of this quantity. We show that if an organism does not know the exact local mortality risk, it should act as if the risk is less than the mean risk. This applies to uncertainty about θ or μ. In other words, the animal should be optimistic with regard to both sources of mortality.
In the face of uncertainty, optimism about mortality also applies (and can even be enhanced) when the risk of mortality can be learned, as shown in the electronic supplementary material, appendix 3.
Our analysis so far has assumed that stochasticity affects each member of the population independently. This is known as demographic stochasticity. At the other extreme, stochasticity might affect all population members in the same way. This is known as environmental stochasticity. For example, if predator density varies on a local spatial scale, then different population members are affected largely independently by this variation in predators. In contrast, if variation in predator density is strongly correlated over the whole of the population's range, then all population members experience a similar predator density in a given year, and stochasticity is environmental. In our model of overwinter survival, because stochasticity is demographic, the appropriate fitness currency is expected future reproductive success, where the expectation is across possible predator densities. If, instead, stochasticity is environmental, the appropriate currency is geometric mean future reproductive success [13,14]. As McNamara [15] shows, maximizing geometric mean future reproductive success is equivalent to maximizing arithmetic mean future reproductive success, but with a modified weighting of the probabilities of possible environmental conditions. This weighting increases the probability of bad conditions and decreases the probability of good conditions. As a consequence, environmental stochasticity reduces optimism compared with demographic stochasticity [2]. In the case of our overwinter survival model, the optimal behaviour depends on just the mean of θ and the mean of μ. In other words, it is not optimal to be either optimistic or pessimistic with respect to these variables.
4. Discussion
Bouskila & Blumstein [1] claim that organisms should over-estimate predation risk. There are two problems with this conclusion. One is that it depends on particular assumptions about fitness [16,17]. More fundamentally, the approach of Bouskila & Blumstein does not include any measure of uncertainty. It simply compares the loss in reproductive success associated with either increasing or decreasing the estimated predation risk by a given amount [18]. In other words, their conclusion rests on the asymmetry of the fitness function. If the predation risk is unknown, it is not really meaningful to talk about over- or underestimating it. To determine what the best estimate should be, it is necessary to specify the possible values of the predation risk and the probabilities that these values occur. Once this is done, it is possible to compare the optimal behaviour under the unknown risk with the optimal behaviour were the risk known and equal to the mean of the probability distribution.
We have made this comparison in two particular cases and have shown that the effective predation risk is less than its mean value. In other words, it is optimal to be optimistic about predator density. Such a bias is only applicable when behaviour is compared with that based on the expected mean predation risk as the true risk, without having taken into account the variability (i.e. the distribution of possible values; cf. [3]).
The analysis in the electronic supplementary material, appendix 2, also considers the consequences of uncertainty in background mortality risk θ. Again it is optimal to be optimistic about this risk. Although the model in the electronic supplementary material, appendix 2, assumes that an animal can make a trade-off between reproduction and mortality, we believe that our qualitative conclusions will hold in other contexts. To justify this view, we note that if the mortality risk is high then the animal will obtain very little reproductive success whatever it does. In contrast when mortality is low, there is the potential for considerable reproductive success and it is important to exploit this potential. Thus, behaviour should be dominated by the optimum under the favourable conditions. That is, optimism should be favoured. For a given mean mortality risk, mean lifetime reproductive success increases as variability (uncertainty) increases (electronic supplementary material, figure A2.1c of appendix 2). This arises because the lifetime reproductive success is a nonlinear (convex) function of the mortality risk (cf. [1]). Again, we expect this finding to be general.
Our model assumes that the organism does not learn about the predation risk over time, but our qualitative conclusions can also apply when learning occurs, so long as some uncertainty remains (see electronic supplementary material, appendix 3). When learning is possible, Welton et al. [19] identify another reason to be optimistic; by taking risks, an organism can gain better information about its environment that it can later exploit should it survive.
When different members of a population experience different conditions, but the distribution of experienced conditions is roughly the same in each generation, natural selection maximizes mean lifetime reproductive success. This is the scenario considered in the electronic supplementary material, appendix 2. In contrast, when population members all experience the same local conditions within a generation, but there is variation across generations, natural selection maximizes geometric mean fitness. In these circumstances, environmental stochasticity reduces optimism compared with demographic stochasticity and it can be optimal to be pessimistic [2].
Acknowledgements
This work forms part of European Research Council Advanced Grant 250209.
References
- 1.Bouskila A., Blumstein D. T. 1992. Rules of thumb for predation hazard assessment: predictions from a dynamic model. Am. Nat. 139, 161–176 10.1086/285318 (doi:10.1086/285318) [DOI] [Google Scholar]
- 2.McNamara J. M., Trimmer P. C., Eriksson A., Marshall J. A. R., Houston A. I. 2011. Environmental variability can select for optimism or pessimism. Ecol. Lett. 14, 58–62 10.1111/j.1461-0248.2010.01556.x (doi:10.1111/j.1461-0248.2010.01556.x) [DOI] [PubMed] [Google Scholar]
- 3.Houston A. I., Trimmer P. C., Fawcett T. W., Higginson A. D., Marshall J. A. R., McNamara J. M. 2012. Is optimism optimal? Functional causes of apparent behavioural biases. Behav. Proc. 89, 172–178 [DOI] [PubMed] [Google Scholar]
- 4.Lima S. L., Dill L. M. 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640 10.1139/z90-092 (doi:10.1139/z90-092) [DOI] [Google Scholar]
- 5.Lima S. L. 1998. Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Adv. Study Behav. 27, 215–290 10.1016/S0065-3454(08)60366-6 (doi:10.1016/S0065-3454(08)60366-6) [DOI] [Google Scholar]
- 6.Verdolin J. L. 2006. Meta-analysis of foraging and predation risk trade-offs in terrestrial systems. Behav. Ecol. Sociobiol. 60, 457–464 10.1007/s00265-006-0172-6 (doi:10.1007/s00265-006-0172-6) [DOI] [Google Scholar]
- 7.Magnhagen C. 1991. Predation risk as a cost of reproduction. Trends Ecol. Evol. 6, 183–186 10.1016/0169-5347(91)90210-O (doi:10.1016/0169-5347(91)90210-O) [DOI] [PubMed] [Google Scholar]
- 8.Elgar M. A., Schneider J. M. 2004. Evolutionary significance of sexual cannibalism. Adv. Study Behav. 34, 135–163 10.1016/S0065-3454(04)34004-0 (doi:10.1016/S0065-3454(04)34004-0) [DOI] [Google Scholar]
- 9.Herberstein M. E., Schneider J. M., Elgar M. A. 2002. Costs of courtship and mating in a sexually cannibalistic orb-web spider: female mating strategies and their consequences for males. Behav. Ecol. Sociobiol. 51, 440–446 10.1007/s00265-002-0460-8 (doi:10.1007/s00265-002-0460-8) [DOI] [Google Scholar]
- 10.McNamara J. M., Houston A. I. 1994. The effect of a change in foraging options on intake rate and predation rate. Am. Nat. 144, 978–1000 10.1086/285721 (doi:10.1086/285721) [DOI] [Google Scholar]
- 11.Werner E. E., Anholt B. R. 1993. Ecological consequences of the trade-off between growth and mortality rates mediated by foraging activity. Am. Nat. 142, 242–272 10.1086/285537 (doi:10.1086/285537) [DOI] [PubMed] [Google Scholar]
- 12.McNamara J. M., Houston A. I. 1990. The value of fat reserves and the tradeoff between starvation and predation. Acta Biotheoretica 38, 37–61 10.1007/BF00047272 (doi:10.1007/BF00047272) [DOI] [PubMed] [Google Scholar]
- 13.Houston A. I., McNamara J. M. 1999. Models of adaptive behaviour. Cambridge, UK: Cambridge University Press [Google Scholar]
- 14.Tuljapurkar S. D., Orzack S. H. 1980. Population dynamics in variable environments. 1. Long-run growth rates and extinction. Theor. Popul. Biol. 18, 314–342 10.1016/0040-5809(80)90057-X (doi:10.1016/0040-5809(80)90057-X) [DOI] [Google Scholar]
- 15.McNamara J. M. 1995. Implicit frequency dependence and kin selection in fluctuating environments. Evol. Ecol. 9, 185–203 10.1007/BF01237756 (doi:10.1007/BF01237756) [DOI] [Google Scholar]
- 16.Abrams P. A. 1994. Should prey overestimate the risk of predation. Am. Nat. 144, 317–328 10.1086/285677 (doi:10.1086/285677) [DOI] [Google Scholar]
- 17.Koops M. A., Abrahams M. V. 1998. Life history and the fitness consequences of imperfect information. Evol. Ecol. 12, 601–613 10.1023/A:1006512927409 (doi:10.1023/A:1006512927409) [DOI] [Google Scholar]
- 18.Trimmer P. C., Houston A. I., Marshall J. A. R., Mendl M. T., Paul E. S., McNamara J. M. 2011. Decision-making under uncertainty: biases and Bayesians. Anim. Cogn. 14, 465–476 10.1007/s10071-011-0387-4 (doi:10.1007/s10071-011-0387-4) [DOI] [PubMed] [Google Scholar]
- 19.Welton N. J., McNamara J. M., Houston A. I. 2003. Assessing predation risk: optimal behaviour and rules of thumb. Theor. Popul. Biol. 64, 417–430 10.1016/S0040-5809(03)00097-2 (doi:10.1016/S0040-5809(03)00097-2) [DOI] [PubMed] [Google Scholar]


