Abstract
In otherwise mutualistic relationships between aphids and ants, attendance by ants often has negative impacts on aphids. For example, in a previous study using traps in the field, the aphid Tuberculatus quercicola, which exhibits mutualistic interactions with ants, showed extremely low dispersal rates, despite having long wings. This study investigates whether components of the flight apparatus (mesonotum length, flight muscle and wings) differ between aphids attended by ants and not attended by ants. Randomized block analysis of variance, using body length as a covariate, showed that ant attendance has a negative influence on aphid flight apparatus. This result indicates that aphids produce honeydew at the expense of resource investment in flight apparatus. Since the dispersal of T. quercicola is limited under ant attendance, the reduction in flight apparatus could precede a decrease in body size. This study also showed that flight apparatus was more developed in aphids under ant-exclusion conditions. This may imply that T. quercicola fly when ants are not available. The maintenance of flight apparatus in T. quercicola might therefore be partly explained by gene flow on the rare occasions that this aphid species disperses.
Keywords: mutualism, flight apparatus, Quercus dentata
1. Introduction
In otherwise mutualistic relationships between aphids and ants, attendance by ants often has negative impacts on aphids. For instance, aphid body size or embryo number may decline owing to the increased cost of honeydew production [1,2]. Alternatively, colony development may be suppressed [3]. Moreover, attending ants act as inhibitory agents of the dispersal of aphids [4]. Ant mandibular secretions may inhibit alate development [5], while ant semiochemicals may reduce the walking activity of apterous aphids [6]. To date, studies of how ants influence aphid dispersal have been based on observational studies, where the number of dispersing aphids was compared against the presence and the absence of ants. However, it remains unknown whether ant attendance directly influences the development of flight apparatus (e.g. wings and flight muscle) in aphids.
The aphid Tuberculatus quercicola feeds on Quercus dentata, and exhibits mutualistic interactions with ants. During the summer, regardless of colony density or ant attendance, all nymphs develop into alate viviparous females that produce parthenogenetic offspring. The lack of wing dimorphism in T. quercicola provides a simple system to evaluate the extent of dispersal ability. In a previous study using traps and weekly observations, T. quercicola exhibited low dispersal rates, in which the total numbers of winged individuals trapped and observed in trees across all seasons were 8/1342 (trapped/observed) [4]. This suggests that, despite possessing wings, the dispersal of T. quercicola is limited by attendance of ants, leading to the hypothesis that attending ants have negative impacts on the flight apparatus of T. quercicola.
This study investigates whether the flight apparatus of aphids differs between those attended by ants and not attended by ants. The following parameters were evaluated for each condition: (i) differences in mesonotum length and wing areas and (ii) differences in flight muscle development and whole thorax section. All parameters were corrected against body length. Based on the findings, the implications of ant attendance on resource investment to flight apparatus by aphids are discussed.
2. Material and methods
(a). Rearing aphids
Individuals of T. quercicola were reared on the host plant Q. dentata at the Ishikari Coast of Hokkaido, North Japan (43° N, 141° E). At the field site, colonies of T. quercicola were attended by the red wood ant Formica yessensis. All experiments were conducted in a predator-free environment using four Q. dentata trees that were, on average, 3 m in height. In early June 2011, five to eight branches with a pair of shoots were randomly selected from each tree for the experiments. All of the leaves were removed from each shoot except for one, so that only two leaves remained on each branch. Each pair of leaves was located at the apex of the branch, forming a Y-shaped twig. On one leaf, two plastic tubes (diameter 4 mm, length 6 cm) were attached with plastic tape along the petiole to allow the approach of ants. To remove the effects of genetic differences among the aphids on the results, prior to the experiment one aphid clone was reared on each of the study trees. One fourth-instar nymph was collected from each study tree, and transferred to the leaf connected the tubes by using a small brush. After transfer, the leaf was bagged with a nylon net (33 × 22 cm) to propagate the clone with ant attendance (ant-attended treatment) for about two weeks. In late June, 7–10 second- or third-instar individuals of ant-attended colonies were transferred onto the other leaf, which was then bagged with a nylon net (ant-excluded treatment). To maintain a low density of 8–15 individuals per leaf, and prevent overcrowding, several individuals were removed during the course of the experiment. Two to three weeks after transfer, the collection of winged aphids from both treatments was started. Because winged aphids of more than two days old start to produce the first instar, analyses are required to quantify resource allocation for both winged adults and the first-instar nymphs. Therefore, to calculate the entire resource investment of an aphid, the aphids used to quantify flight muscle were collected within 2 days after the emergence of winged adults. To avoid potential variation in aphid nutritional status, which might be influenced by the nutritional quality of Q. dentata, the collection interval for a pair of ant-attended and ant-excluded treatments was arranged to be less than one week. A total of 21 branches was established from the four trees between 7 July and 21 September 2011. Four to 14 aphids were collected per bagged leaf and fixed in 99.5 per cent ethanol. The average number of aphids for each treatment per branch and per tree (mean ± s.d.) was as follows: 8.5 ± 2.2 for the ant-attended treatment and 8.8 ± 2.2 for the ant-excluded treatment.
(b). Measurements of aphids
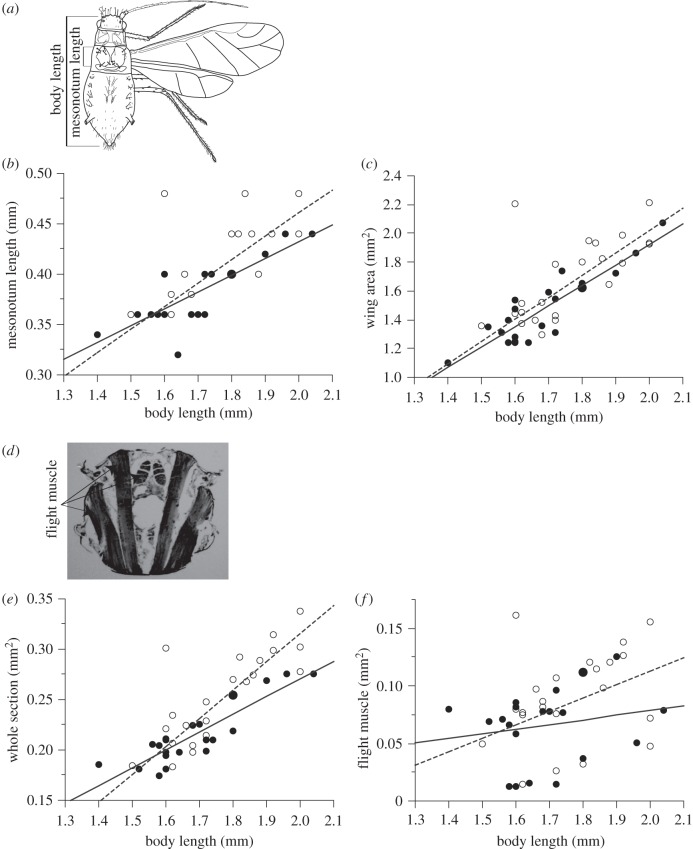
Mesonotum length (figure 1a) was measured as indices of the development of flight apparatus because forewing is connected to flight muscle in mesonotum. Body length was measured as a covariate in statistical analyses. The body part dimensions were measured using an eyepiece micrometre installed in a binocular microscope (magnification 1000×). The measurements of aphids were ordered for each colony (branch), and the medians were used for statistical analyses.
Figure 1.
Measurement parts are indicated in: (a) a picture of a typical Tuberculatus species after Quednau [7] and (d) a photograph of flight muscle. Comparison of ant-attended and ant-excluded aphids for (b) mesonotum length, (c) wing area, (e) whole section, and (f) flight muscle. The linear regression lines shown as solid lines fitted to the closed circles and the broken lines fitted to the open circles indicate the relationships between body length and each parameter for ant-attended and ant-excluded aphids, respectively. Regression equation, regression coefficient and statistical significance for the regression coefficient of solid and broken lines are as follows: (b) y = 0.167x + 0.098, r2 = 0.637, p < 0.0001 and y = 0.231x − 0.002, r2 = 0.705, p < 0.0001, (c) y = 1.427x − 0.93, r2 = 0.795, p < 0.0001 and y = 1.546x − 1.072, r2 = 0.738, p < 0.0001, (e) y = 0.177x − 0.083, r2 = 0.784, p < 0.0001 and y = 0.279x − 0.243, r2 = 0.827, p < 0.0001, and (f) y = 0.04x − 0.001, r2 = 0.036, p = 0.407 and y = 0.116x − 0.12, r2 = 0.212, p = 0.036.
(c). Image analysis of wings and flight muscle
Only aphids within the two day period after emergence as adults were used in this study, to exclude the effects of autolysis on flight muscle development. Either the left or the right forewing and hindwing were cut from a body, and the areas were captured using the eyepiece of a digital microscope AM-423X (Bigc.com, Torrance, CA, USA). The areas were measured using Image J [8]. After measuring body size and wing area, the aphids were dehydrated in xylene and embedded in paraffin wax. The embedded paraffin blocks were cut to a series of sections of flight muscle (6–8 μm) using a microtome, and the sections were stained by using a haematoxylin-eosin method. For each aphid, one to six pieces of the sections were captured with the eyepiece of a digital microscope. The whole thorax section and flight muscle area (figure 1d) were measured using Image J, and the sections that had the maximum whole thorax section and flight muscle area were selected as representative values. The measurements of wings, the whole thorax section and flight muscle area were ordered for each colony (branch), and the medians were used for statistical analyses.
(d). Statistical analysis
To control for individual variation in the host plant, randomized block analysis of variance (ANOVA) was used for all measurement scores. This strategy should minimize any effects peculiar to single leaves. In this randomized block design, the study trees and branches were assigned to blocks. Body length was included as a covariate in the ANOVA model. The ANOVA model contained ‘tree’, ‘branch nested within tree’, ‘ant’, ‘body length’ and an interaction term ‘ant × body length’. ANOVA was performed twice. It was first performed to include an interaction term. Then, if no significant difference was found in the first ANOVA, it was performed without an interaction term. P-values were adjusted with Bonferroni multiple corrections. Besides ANOVA, the regression between body length and each parameter were analysed. The same analyses were also applied to the data of all individuals collected from both treatments.
3. Results
No significant difference was found in any of the interaction terms, but it was found for the whole thorax section (F1,18 = 10.98, p = 0.004) in the first ANOVAs. In the second ANOVA without interaction terms, all traits, except for the whole thorax section, were significantly larger in individuals from the ant-excluded treatment when compared with the ant-attended treatment (table 1). The means and standard deviations for the traits were as follows: the mean mesonotum length was 0.38 ± 0.03 mm in the ant-attended treatment and 0.41 ± 0.04 mm in the ant-excluded treatment (figure 1b). The mean total wing area was 1.47 ± 0.24 mm2 in the ant-attended treatment and 1.66 ± 0.27 mm2 in the ant-excluded treatment (figure 1c). The mean whole thorax section was 0.21 ± 0.03 mm2 in the ant-attended treatment and 0.25 ± 0.05 mm2 in the ant-excluded treatment (figure 1e). The mean flight muscle area was 0.07 ± 0.03 mm2 in the ant-attended treatment and 0.09 ± 0.04 mm2 in ant-excluded treatment (figure 1f). In ANOVAs of all individuals, no significant difference was found in any of the interaction terms in the first ANOVAs. In the second ANOVA without interaction terms, all traits were significantly larger in individuals from the ant-excluded treatment when compared with the ant-attended treatment (electronic supplementary material, table S1 and figure S1).
Table 1.
ANOVA for (a) mesonotum length, (b) total wing area, (c) whole thorax selection, and (d) flight muscle area.
| source of variation | d.f. | SS | F-value | p-value |
|---|---|---|---|---|
| (a) mesonotum length | ||||
| tree | 3 | 0.0034 | 4.5687 | 0.0143 |
| branch (tree) | 17 | 0.0109 | 2.5515 | 0.0257 |
| ant | 1 | 0.0024 | 9.5221 | 0.0061 |
| body length | 1 | 0.0035 | 13.8505 | 0.0014 |
| error | 19 | 0.0048 | ||
| (b) total wing area | ||||
| tree | 3 | 0.0512 | 2.9058 | 0.0614 |
| branch (tree) | 17 | 0.4697 | 4.7048 | 0.0008 |
| ant | 1 | 0.0621 | 10.5760 | 0.0042 |
| body length | 1 | 0.3108 | 52.9181 | <0.0001 |
| error | 19 | 2.9973 | ||
| (c) whole thorax section | ||||
| tree | 3 | 0.0023 | 5.4298 | 0.0077 |
| branch (tree) | 17 | 0.0062 | 2.5318 | 0.0291 |
| ant | 1 | 0.0017 | 11.9138 | 0.0028 |
| body length | 1 | 0.0135 | 93.5903 | <0.0001 |
| ant × body length | 1 | 0.0016 | 10.9799 | 0.0039 |
| error | 18 | 0.0026 | ||
| (d) flight muscle area | ||||
| tree | 3 | 0.0057 | 9.6288 | 0.0004 |
| branch (tree) | 17 | 0.0322 | 9.6586 | <0.0001 |
| ant | 1 | 0.0010 | 5.2005 | 0.0343 |
| body length | 1 | 0.0020 | 10.2307 | 0.0047 |
| error | 19 | 0.0037 | ||
4. Discussion
This study demonstrated that ant attendance has a direct negative influence on the development of flight apparatus in aphids. Existing research has shown that when T. quercicola is under ant attendance, honeydew excretions are increased by up to twofold compared with aphids under ant-exclusion conditions, causing the amino acids to increase in the honeydew [2]. Therefore, it is possible that a shortage of amino acids causes lower protein synthesis, and hence a reduction in the flight muscle of T. quercicola. Since T. quercicola dispersal is limited under ant attendance conditions, it is possible that a reduction in flight apparatus precedes a decrease in body size.
The results also showed that the flight apparatus of aphids under ant-exclusion conditions was significantly more developed. This may then imply that T. quercicola fly when the ants are not available. Indeed, almost all T. quercicola caught in the field traps were observed from early July to mid-July, when lowest number of ants were attending aphid colonies, perhaps because honeydew-foraging ants were being recruited into nest budding (I. Yao 2011, unpublished data). Hence, aphids may have an opportunity to fly and colonize new host plants of high nutritional quality. The maintenance of flight apparatus in T. quercicola, which exhibits low dispersal, may therefore be partly explained by continued gene flow on the rare occasions that winged aphids disperse.
The flexibility in the development of flight apparatus in aphids suggests that mutualistic interactions with ants might be subjected to fluctuations in host plant quality or the availability of ants. Unstable mutualisms have been reported by molecular phylogenetic studies, where gains or losses of ant attendance have occurred at least five times in the course of the evolution of Chaitophorus aphids [9] and Tuberculatus aphids [10]. Further studies on other aphid species are needed to clarify whether flexibility in the development of flight apparatus is associated with labile aphid–ant mutualisms.
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research (C) (no. 21570012 to I.Y.) financed by the Japan Society for the Promotion of Science (JSPS).
References
- 1.Yao I., Shibao H., Akimoto S. 2000. Costs and benefits of ant attendance to the drepanosiphid aphid Tuberculatus quercicola. Oikos 89, 3–10 10.1034/j.1600-0706.2000.890101.x (doi:10.1034/j.1600-0706.2000.890101.x) [DOI] [Google Scholar]
- 2.Yao I., Akimoto S. 2002. Flexibility in the composition and concentration of amino acids in honeydew of the drepanosiphid aphid Tuberculatus quercicola. Ecol. Entomol. 27, 745–752 10.1046/j.1365-2311.2002.00455.x (doi:10.1046/j.1365-2311.2002.00455.x) [DOI] [Google Scholar]
- 3.Katayama N., Suzuki N. 2002. Cost and benefit of ant attendance for Aphis craccivora (Hemiptera: Aphididae) with reference to aphid colony size. Can. Entomol. 134, 241–249 10.4039/Ent134241-2 (doi:10.4039/Ent134241-2) [DOI] [Google Scholar]
- 4.Yao I. 2010. Contrasting patterns of genetic structure and dispersal ability in ant-attended and non-attended Tuberculatus aphids. Biol. Lett. 6, 282–286 10.1098/rsbl.2009.0781 (doi:10.1098/rsbl.2009.0781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleinjan J. E., Mittler T. E. 1975. A chemical influence of ants in wing development in aphids. Entomol. Exp. Appl. 18, 384–388 10.1007/BF00628368 (doi:10.1007/BF00628368) [DOI] [Google Scholar]
- 6.Oliver T. H., Mashanova A., Leather S. R., Cook J. M., Jansen V. A. A. 2007. Ant semiochemicals limit apterous aphid dispersal. Proc. R. Soc. B 274, 3127–3131 10.1098/rspb.2007.1251 (doi:10.1098/rspb.2007.1251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quednau F. W. 1999. Atlas of the drepanosiphine aphids of the world. Part I: Panaphidini Oestlund, 1922-Myzocallidina Börner, 1942 (1930) (Hemiptera: Aphididae: Calaphidinae), vol. 31 Gainesville, FL: Contributions of the American Entomological Institute [Google Scholar]
- 8.Abramoff M. D., Magelhaes P. J., Ram S. J. 2004. Image processing with ImageJ. Biophotonics Int. 11, 36–42 [Google Scholar]
- 9.Shingleton A. W., Stern D. L. 2003. Molecular phylogenetic evidence for multiple gains or losses of ant mutualism within the aphid genus Chaitophorus. Mol. Phylogenet. Evol. 26, 26–35 10.1016/S1055-7903(02)00328-7 (doi:10.1016/S1055-7903(02)00328-7) [DOI] [PubMed] [Google Scholar]
- 10.Yao I. 2011. Phylogenetic comparative methods reveal higher wing loading in ant-attended Tuberculatus aphids (Hemiptera: Aphididae). Can. Entomol. 143, 34–43 10.4039/n10-050 (doi:10.4039/n10-050) [DOI] [Google Scholar]