Abstract
Ambient noise can mask acoustic cues, making their detection and discrimination difficult for receivers. This can result in two types of error: missed detections, when receivers fail to respond to the appropriate cues, and false alarms, when they respond to inappropriate cues. Nestling birds are error-prone, sometimes failing to beg when parents arrive with food (committing missed detections) or begging in response to stimuli other than a parent's arrival (committing false alarms). Here, we ask whether the frequency of these errors by nestling tree swallows (Tachycineta bicolor) increases in the presence of noise. We found that nestlings exposed to noise had more missed detections than their unexposed counterparts. We also found that false alarms remained low overall and did not differ significantly between noise and quiet treatments. Our results suggest that nestlings living in noisy environments may be less responsive to their parents than nestlings in quieter environments.
Keywords: ambient noise, begging, missed detections, false alarms, error, nesting birds
1. Introduction
Across the globe, anthropogenic noise is increasing in intensity, duration and extent. This has implications for the conservation of many species, as ambient noise can mask acoustic cues used to avoid predators, find food and communicate. If animals living in noisy environments fail to respond to salient cues, such as alarm calls or sounds indicating the presence of prey or potential mates, these so-called missed detections could ultimately reduce survival or reproductive success [1].
Individuals can reduce the risk of missed detections by lowering their threshold for response and becoming less selective about the cues to which they respond. This strategy should reduce missed detections, but it will inevitably increase the risk of false alarms, i.e. responding to incorrect or inappropriate cues [2]. Thus, ambient noise could force animals into a trade-off between accepting more missed detections or risking more false alarms. This trade-off can be a strong selective factor shaping animal signalling systems [2].
Nestling birds are susceptible to both missed detections and false alarms. The frequency of missed detections (i.e. failing to beg when parents arrive) is relatively low, occurring on less than 20 per cent of parental visits [3]. This is presumably because the first nestling to beg when parents arrive at the nest is often most likely to be fed [4,5]. Thus, a rapid response to the first sign of a parent's arrival, such as contact calls [6] and sounds made when it lands [7] increases the chance of a feeding. The ‘hair trigger’ response needed to ensure a feeding, however, also brings with it an increase in false alarms (i.e. begging in response to stimuli other than parents with food), with up to 50 per cent of begging responses to stimuli such as noises in the environment or the sounds of parents leaving the nest [3,7–9].
This susceptibility of nestlings to errors might make them particularly vulnerable to the masking effects of noise. Indeed, masking of parent–offspring communication is one proposed explanation for the decreased reproductive success of songbirds nesting near noise sources [10,11]. Yet, the effect of noise on errors in parent–offspring communication has not been examined.
The goal of our study, therefore, was to determine whether the frequency of begging errors by nestling tree swallows (Tachycineta bicolor) increased in the presence of noise. We measured nestling begging, in the presence and absence of added noise, in response to the playback of: (i) a parent landing at the nest while giving a contact call known to stimulate begging (‘parent-with-call’), (ii) the same sound of the parent landing, but without the call (‘parent-without-call’), and (iii) a common grackle (Quiscalis versicolor), a nest predator [12], landing on a nest-box (‘predator’). We considered failing to beg in response to the parental landing sounds as a missed detection and begging in response to the predator landing sound as a false alarm [13]. We played the parent landing with and without the call because the latter should be a more ambiguous cue of a parent's arrival [3,8]. Thus, an increase in response to this stimulus in the presence of noise would suggest that nestlings had lowered their threshold for response, which might help explain increases in false alarm rates, should they occur.
2. Material and methods
This study was conducted between May and July 2009 in the Gaspereau Valley of Nova Scotia, Canada, on a population of box-nesting tree swallows. Detailed methods are provided in the electronic supplementary material.
When broods were 10–12 days old (mean ± s.d.: 11 ± 0.1), four nestlings from each of 24 broods were randomly divided into pairs, assigned to either a noise treatment (playback of white noise with a frequency range of 0–22 kHz and an amplitude of 65 dB SPL, C weighting), which raised sound levels surrounding the nestlings by 10 dB above background (see the electronic supplementary material), or a quiet treatment (no added noise) and placed in one of two unoccupied nest-boxes in the field. The nest-boxes were identical apart from the presence or absence of noise, which was swapped between the boxes with each successive trial. Noise ran continuously, beginning an hour before the trial and continuing until the trial was complete.
After the hour, one of the three stimulus sounds (see above) was played back every minute for 20 min, with all three stimuli presented, in random order, within every 3 min period. This yielded seven presentations of each stimulus sound per trial.
We ultimately excluded the first 3 min of each trial because nestlings did not respond to the parent-without-call or predator stimuli during that time. We arbitrarily divided the remaining time into two deprivation periods that reflected shorter (4–12 min; hereafter ‘shorter deprivation’) and longer (13–21 min; ‘longer deprivation’) periods of time without food and hence different hunger levels. We included deprivation period in our analyses because the frequency of both correct detections (responding to the parent's arrival) and false alarms increase with hunger [13].
An observer viewed the video tapes and noted, after each sound was played, the number of nestlings that begged in each treatment (range 0–2), as a measure of the brood's strength of response. To test whether this response rate varied between noise and quiet treatments, and in relation to time without food, we used generalized estimating equations [14]. We first analysed responses to the parental stimuli, using a model that included stimulus (parent-with-call, parent-without-call), treatment (noise, quiet), deprivation period (shorter, longer) and their interaction as main effects, and trial (i.e. brood) as a repeated-measures subject. The second model was identical except that it tested responses to the predator stimulus only.
3. Results
The parent-with-call stimulus elicited begging responses in 96 per cent of trials, while the parent-without-call stimulus did so in 54 per cent of trials. Response rate was significantly higher for the parent-with-call stimulus (χ2 = 62.76, p < 0.0001; figure 1a,b) and increased with deprivation time (χ2 = 7.13, p = 0.008; figure 1a,b), with no significant interaction between stimulus and deprivation period (χ2 = 1.25, p = 0.26).
Figure 1.
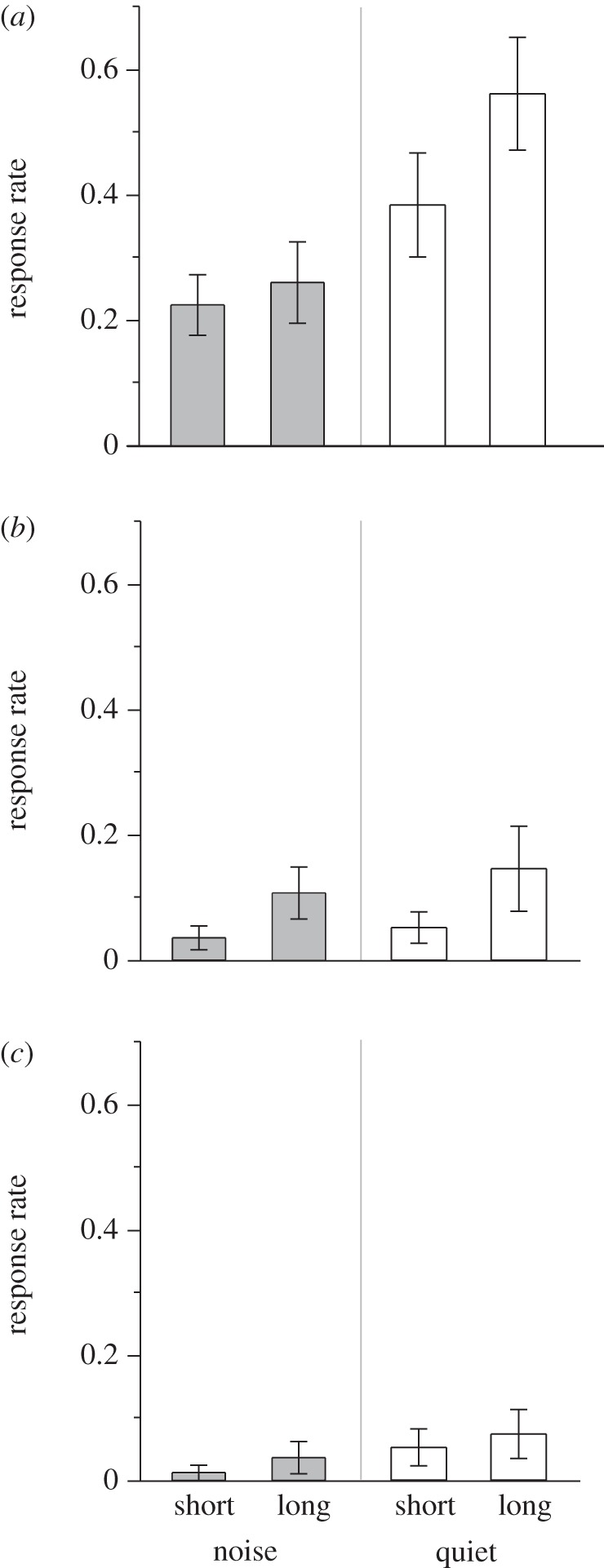
Mean (±s.e.) begging response rate by nestling tree swallows in noise and quiet treatments during short (4–12 min into trial) and long (13–21 min into trial) deprivation periods for: (a) parent-with-call, (b) parent-without-call, and (c) predator stimulus sounds. Response rate is the proportion of playbacks that stimulated begging (out of the six possible for each stimulus within each deprivation period; 3 playbacks/stimulus × 2 nestlings).
Response rate to the parental stimuli was significantly lower in the noise treatment than in the quiet treatment (χ2 = 7.23, p = 0.007; figure 1a,b), with no significant interaction between treatment and stimulus (χ2 = 1.01, p = 0.31) or treatment and deprivation period (χ2 = 2.36, p = 0.13).
The predator stimulus elicited responses in 35 per cent of trials. Response rate did not vary with treatment (χ2 = 0.69, p = 0.41), food deprivation (χ2 = 0.42, p = 0.52) or their interaction (χ2 = 0.62, p = 0.43; figure 1c).
4. Discussion
The results of our study add to mounting evidence that the perception of acoustic signals is compromised by noise. In particular, our demonstration of an effect on young animals adds to evidence for similar effects in adults across a variety of taxa [1]. Nestlings exposed to noise were less likely to beg to playbacks of parents arriving at the nest than their unexposed counterparts. Nestlings in noise did not increase their response to ambiguous signals, such as parents arriving without calling, nor did they have higher frequencies of false alarms than nestlings in the quiet treatment.
The most obvious explanation for the increase in missed detections is that noise masks the sounds of the parent's arrival, making it difficult for nestlings to discriminate sounds produced by the parent from other irrelevant stimuli. Noise might also have alarmed or distracted nestlings [15–17], and so decreased overall readiness to respond, but if that were the case, then nestlings in noise should have shown a decrease in response to all stimuli, and not only to the parental stimuli.
Noise did not increase response rate to relatively ambiguous signals of a parent's arrival or increase the frequency of false alarms. The low false alarm rate in the noise treatment is consistent with the failure of nestlings to compensate for missed detections by increasing their overall responsiveness. Nestlings might avoid increasing responsiveness because the cost of missing the parent's arrival may be small when compared with the cost of begging to a predator, even if the risk of doing so is relatively low. Conversely, in nature, nestlings might not need to increase their responsiveness, because various mitigating factors reduce the chance of missing feedings. For example, when nestlings fail to respond, parents might call to stimulate begging, as they do for unresponsive younger nestlings [6]. Also, visual or tactile cues could also signal parental arrivals.
The results of our study suggest that nestling birds in noisier environments could miss the arrival of their parents with food more often than nestlings in quieter environments. The consequences of this are not known, but could range from missed feedings to extended feeding visits, as parents attempt to stimulate unresponsive nestlings. In turn, longer feeding visits could reduce overall feeding rates or increase effort as parents try to compensate for lost feedings or time. Noise might also change sibling interactions, if larger nestlings that typically respond sooner either fail to beg or are slower to beg in noise, potentially allowing later begging nestlings a chance for a feeding.
In the only study of the long-term impacts of noise on nestling birds, neither feeding rates nor growth rates differed between tree swallow broods raised in noise and control nests without noise [18]. Thus, if missed detections did increase (which was not measured in the study of Leonard & Horn [18]), they did not appear to affect growth rates. Instead, it may have been the parents, rather than the nestlings, that incurred costs, such as more calling and longer feeding visits, that may not be reflected in feeding rate per se.
Whatever long-term impact noise has on nestling passerines, it is likely to vary with age, being most severe for young nestlings, which must rely more on acoustic cues until their eyes open at about day five. Also, while we used white noise for the present experiment because it is easily controlled and characterized, the impact of noise is likely to vary with the structure and levels of noise exposure, particularly within the frequency range of relevant acoustic cues. We used relatively low noise levels in the current experiment (65 dB). Higher noise levels, such as those associated with average city traffic, would be expected to have a greater impact than documented here. Further study is clearly needed to determine how begging errors vary with different levels and types of noise, and on whether young animals and their parents can adapt to the potential impacts on their communication system.
Acknowledgements
We thank Claire Horn for fieldwork, the Coldwell, Hynes and Minor families for use of their land, and Tonya Haff for comments on an earlier draft. This study was supported by an NSERC Discovery Grant awarded to M.L.L.
References
- 1.Barber J. R., Crooks K. R., Fristrup K. M. 2010. The costs of chronic noise exposure for terrestrial organisms. Trends Ecol. Evol. 25, 180–189 10.1016/j.tree.2009.08.002 (doi:10.1016/j.tree.2009.08.002) [DOI] [PubMed] [Google Scholar]
- 2.Wiley R. H. 2006. Signal detection and animal communication. Adv. Stud. Behav. 36, 217–248 10.1016/S0065-3454(06)36005-6 (doi:10.1016/S0065-3454(06)36005-6) [DOI] [Google Scholar]
- 3.Dor R., Kedar H., Winkler D. W., Lotem A. 2007. Begging in the absence of parents: a ‘quick on the trigger’ strategy to minimize costly misses. Behav. Ecol. 18, 97–102 10.1093/beheco/arl056 (doi:10.1093/beheco/arl056) [DOI] [Google Scholar]
- 4.Leonard M. L., Horn A. G. 1996. Provisioning rules in tree swallows. Behav. Ecol. Sociobiol. 38, 341–347 10.1007/s002650050250 (doi:10.1007/s002650050250) [DOI] [Google Scholar]
- 5.Budden A. E., Wright J. 2001. Falling on deaf ears: the adaptive significance of begging in the absence of a parent. Behav. Ecol. Sociobiol. 49, 474–481 10.1007/s002650100323 (doi:10.1007/s002650100323) [DOI] [Google Scholar]
- 6.Leonard M. L., Horn A. G., Brown C. R., Fernandez N. 1997. Parent–offspring recognition in tree swallows, Tachycineta bicolor. Anim. Behav. 54, 1107–1116 10.1006/anbe.1997.0559 (doi:10.1006/anbe.1997.0559) [DOI] [PubMed] [Google Scholar]
- 7.Leonard M. L., Horn A. G. 2001. Begging in the absence of parents by nestling tree swallows. Behav. Ecol. 12, 501–505 10.1093/beheco/12.4.501 (doi:10.1093/beheco/12.4.501) [DOI] [Google Scholar]
- 8.Dickens M., Hartley I. R. 2007. Stimuli for nestling begging in blue tits Cyanistes caeruleus: hungry nestlings are less discriminating. J. Avian Biol. 38, 421–426 10.1111/j.0908-8857.2007.04074.x (doi:10.1111/j.0908-8857.2007.04074.x) [DOI] [Google Scholar]
- 9.Blumer E., Celis P., Gil D. 2008. Parent-absent begging: evidence for sibling honesty and cooperation in the spotless starling (Sturnus unicolor). Behav. Ecol. 19, 279–284 10.1093/beheco/arm134 (doi:10.1093/beheco/arm134) [DOI] [Google Scholar]
- 10.Halfwerk W., Holleman L. J. M., Lessells C. M., Slabbekoorn H. 2011. Negative impact of traffic noise on avian reproductive success. J. Appl. Ecol. 48, 210–219 10.1111/j.1365-2664.2010.01914.x (doi:10.1111/j.1365-2664.2010.01914.x) [DOI] [Google Scholar]
- 11.Kociolek A. V., Clevenger A. P., Clair C. C., St, Proppe D. S. 2011. Effects of road networks on bird populations. Conserv. Biol. 25, 241–249 10.1111/j.1523-1739.2010.01635.x (doi:10.1111/j.1523-1739.2010.01635.x) [DOI] [PubMed] [Google Scholar]
- 12.Robertson R. J., Stutchbury B. J., Cohen R. R. 1992. Tree swallow. In The birds of North America, no. 11 (eds Poole A., Stettenheim P., Gill F.). Washington, DC: The Academy of Natural Sciences, Philadelphia and the American Ornithologists Union [Google Scholar]
- 13.Leonard M. L., Horn A. G. 2005. False alarms and begging in nestling birds. Anim. Behav. 69, 701–708 10.1016/j.anbehav.2004.05.022 (doi:10.1016/j.anbehav.2004.05.022) [DOI] [Google Scholar]
- 14.Ziegler A. 2011. Generalized estimating equations. New York, NY: Springer [Google Scholar]
- 15.Chan A. A. Y.-H., Giraldo-Perez P., Smith S., Blumstein D. T. 2010. Anthropogenic noise affects risk assessment and attention: the distracted prey hypothesis. Biol. Lett. 6, 458–461 10.1098/rsbl.2009.1081 (doi:10.1098/rsbl.2009.1081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson P. A., Berzins I. K., Fogarty F., Hamlin H. J., Guillette L. J., Jr 2011. Sound, stress, and seahorses: the consequences of a noisy environment to animal health. Aquaculture 311, 129–138 10.1016/j.aquaculture.2010.11.013 (doi:10.1016/j.aquaculture.2010.11.013) [DOI] [Google Scholar]
- 17.Purser J., Radford A. N. 2011. Acoustic noise induces attention shifts and reduces foraging performance in three-spined sticklebacks (Gasterosteus aculeatus). PLoS ONE 6, e17478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonard M. L., Horn A. G. 2008. Does ambient noise affect growth and begging call structure in nestlings birds? Behav. Ecol. 19, 502–507 10.1093/beheco/arm161 (doi:10.1093/beheco/arm161) [DOI] [Google Scholar]


