Abstract
Spatial reorientation by layout geometry occurs in numerous species, but its underlying mechanisms are debated. While some argue that navigating animals' sense of place is based on geometric computations over three-dimensional representations, others claim it depends on panoramic image-matching processes. Because children reorient by subtle three-dimensional perturbations of the terrain and not by salient two-dimensional brightness contours on surfaces or freestanding columns, children's sense of place cannot be explained by image matching. To test image-matching theories in a different species, the present experiment investigates the reorientation performance of domestic chicks (Gallus gallus) in environments similar to those used with children. Chicks, like children, spontaneously reoriented by geometric relationships of subtle three-dimensional terrains, and not by salient two-dimensional brightness contours on surfaces or columns. These findings add to the evidence for homologous navigation systems in humans and other vertebrates, and they cast doubt on image-matching theories of reorientation in these species.
Keywords: navigation, reorientation, geometry, image matching
1. Introduction
Maintaining or re-establishing one's position and heading is critical for any navigating animal. Research reveals that the distances and directions of three-dimensional surfaces provide the primary basis for the sense of place in diverse animals [1–3]. In Cheng's [4] groundbreaking experiments, rats that were disoriented in a rectangular arena subsequently reoriented in accord with the arena's shape, ignoring odour, colour and pattern cues that distinguished its symmetrical locations. Similar behaviour has been observed in many species, including ants, chicks and human children [5–7]. Although all tested species use featural cues in some tasks and environments [4,8,9], all reliably navigate by the shape of the testing arena.
The explanation for this effect is debated. Some argue that the sense of place depends on a geometric analysis of the three-dimensional terrain [1,10–12], but others claim that two-dimensional image-matching processes account for the priority of large-scale surfaces in the rectangular environments in which most animals have been tested [7,13–15]. Because different three-dimensional layouts are always accompanied by distinguishable two-dimensional images [13], and these images can alter the perception of three-dimensional layouts [16], this debate is best resolved by experiments that test these features against each other. If reorientation depends on image matching, navigation performance should be impaired by reducing the size and contrast of brightness contours in images of the layout. In contrast, if reorientation depends on representations of three-dimensional extended surfaces, performance should be impaired by reducing the presence of extended surfaces at distinctive distances.
Such studies have been conducted with human children. Lee & Spelke [17–19] showed that children spontaneously reorient using the geometric shape of surface layouts, under conditions that minimize two-dimensional brightness contours in images from the child's perspective. In contrast, children failed to reorient in arrays composed of freestanding columns or of two-dimensional patterns that created prominent brightness contours in such images. Further studies showed that the geometric analysis of the environment is specific to distance relationships between extended surfaces [16,20], in accord with the findings of studies of oriented, navigating rats and human adults [1,21]. In the study on which the current experiments are based [19], three-year-old children reoriented successfully in a circular arena containing either a short, white three-dimensional rectangular frame or two smooth, parallel bumps that protruded from a floor of similar brightness, despite the subtle brightness changes in images of these two arrays. In contrast, children failed to reorient by the shape of a salient, black, two-dimensional rectangular form of the same size and location on the array's floor or by a rectangular array of four tall, dark columns, despite the large and prominent brightness contours in visual images of these arrays. These findings provide evidence that children regain their sense of place by computing over representations of three-dimensional extended surfaces, contrary to image-matching theories and in accord with theories of navigation by terrain geometry.
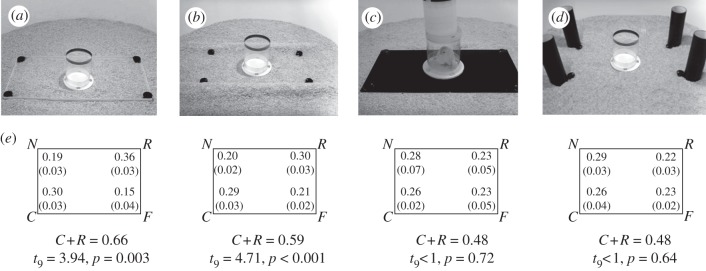
Do the processes guiding navigation in humans differ from those of other animals, who reorient by image matching? Here, we explore this hypothesis, and the rival hypothesis that diverse animals navigate by representing three-dimensional extended surfaces, by testing the navigation performance of domestic chicks in recreations of the environments of Lee & Spelke (figure 1). Chicks are of especial interest in this case, because there is evidence that chicks have an innate capacity to reorient using environmental shape [6,22], and that chicks can be trained to locate food in a rectangular array of separated columns through local view-matching processes [23,24]. Nevertheless, training paradigms encourage attention to and use of landmarks through processes distinct from those that establish one's sense of location [21]. By developing a working memory task with chicks that involves no training on geometry, the present study not only allows for more valid comparisons between navigation mechanisms across species, but also provides some perspective as to the differences between navigation by mechanisms that encode one's own position by surface layout geometry and mechanisms that encode the positions of objects with respect to landmarks.
Figure 1.
Photographs of test environments: (a) 2 cm borders, (b) curved bumps, (c) two-dimensional form (chick shown inside transparent cylinder), and (d) column array. (e) Proportions of searches (s.e.m. values in parentheses) at the correct (C), geometrically rotational (R), and incorrect ‘near’ (N) and ‘far’ (F) corners. Two-tailed t-tests show total geometrically correct searches (C + R) compared against a chance value of 0.5. Because hiding places varied across trials, all data have been rotated into alignment.
2. Material and methods
(a). Subjects
Subjects were 40 laboratory-hatched domestic chicks (Gallus gallus). Chicks were reared individually in aluminium cages (22 × 30 × 40 cm) within an isolated, temperature-controlled room. Food and water were provided ad libitum.
(b). Apparatus
Subjects were tested individually in a white circular arena (130 cm wide and 50 cm deep) with a sawdust-covered floor (10 cm deep) within a quiet testing room. A one-way screen covered the top of the arena to block external visual cues; a central lamp illuminated the arena from above. A video camera mounted to the ceiling was used to record the test sessions.
In all experimental conditions, a rectangular array (30 × 60 cm2) of feeders (3 cm wide) was buried inside the sawdust such that only the covers were visible. In one condition, a 2 cm high rectangular frame surrounded the feeders such that each feeder was located directly at a corner of the rectangle (figure 1a). In another condition, parallel bumps, made by pipes covered in sawdust, bordered each long side of the rectangular array (figure 1b). Two further conditions presented a black two-dimensional rectangle (flat aluminium covered with contact paper) at the level of the sawdust (figure 1c) or four cylinders (7.5 cm wide and 26 cm tall) fixed directly behind each feeder such that there was a rectangular array of large freestanding columns (figure 1d).
At the centre of the arena was a flat, circular (30 cm wide) rotating surface, with both a transparent and an opaque cylinder attached to a pulley above. The transparent cylinder served as the observation chamber, and the opaque cylinder served as the disorientation chamber.
(c). Experimental procedures
Training—day 1 after hatching, chicks were shown a mealworm (Tenebrio molitor) being placed inside one feeder in an empty circular arena and then covered. The cover had a small, yellow dot on one side to encourage pecking responses that caused the cover to fall off and reveal the mealworm. On day 2, chicks were placed inside the transparent chamber within an otherwise empty circular arena (except for one feeder), shown the hiding event, and released to approach the feeder, peck the cover off and access the mealworm. On day 3, chicks were presented with four identical feeders, arranged in a square. Chicks were required to attend to and approach the one correct feeder to be rewarded. On day 4, chicks were presented with the same array from day 3, but were disoriented (by lowering the opaque chamber and rotating the platform clockwise and counter-clockwise) following the hiding event and then released to search. This procedure served both to familiarize chicks with the opaque chamber and the rotation procedure, and to assess whether the testing arena was free of other distinguishing cues. On day 5, chicks were given the same oriented search task as on day 3. Each training phase consisted of 10 trials; the goal location was varied on each trial. Only chicks that successfully passed 70 per cent of the trials on each day (except Day 4) went on to the next phase.
Testing—on day 6, chicks were presented for the first time with one of the four environments described above. Twelve unrewarded disoriented test trials were administered—three trials at each corner with randomized orders. First choices were recorded; after chicks made their first pecking attempt, they were placed back into the central chamber.
3. Results
Chicks searched according to geometry significantly more than predicted by chance with the 2 cm border and curved bumps, but they searched randomly with the two-dimensional form and column array (figure 1e). A repeated-measures ANOVA with the search location as the dependent measure and sex and presence of an informative three-dimensional surface layout as independent measures showed a main effect of search location, F3,108 = 2.872, p = 0.04, and an interaction between search location × surface layout, F3,108 = 4.572, p = 0.005. No other significant effects or interactions were found. Chicks' search performance in each environment is shown in figure 1. Searches at the correct location did not differ from searches at its geometric twin in any condition (t's < 1, n.s.), suggesting successful disorientation. Reorientation by geometry was more reliable in environments with subtle surface layouts (2 cm border, curved bumps) than in environments with visually highly salient non-terrain cues (two-dimensional form, columns), t38 = 3.955, p < 0.001.
4. Discussion
Like children, chicks reoriented by perturbations to the three-dimensional terrain that produced only subtle image contrast borders, and they failed to reorient by visually salient two-dimensional forms or object arrays that produced more prominent contrast borders. This cue specificity for three-dimensional surface layouts provides evidence in accord with geometry-based navigation theories and against image-matching theories. Although retinal image-like representations are formed and used by trained animals, especially insects [7,25,26], to navigate in familiar environments, the findings observed in spontaneous tasks with children and in the present study, with six-day-old chicks with strictly limited experience, suggest that spontaneous reorientation may involve evolutionarily ancient, early and widely available, geometric computations of the three-dimensional terrain. These behavioural results are consistent with numerous neurobiological findings of cells in the rat hippocampus and surrounding areas that are selectively responsive to the three-dimensional borders of the environment [27,28], even in rats blinded at birth and deprived of all visual image-matching experience [29]. Converging studies of both spontaneous and learned navigation behaviour across a wide range of species, including insects, will help solidify this claim.
Acknowledgements
All experiments comply with Italian guidelines for the ethical treatment of animals in behavioural research.
We thank Tommaso Pecchia and Cinzia Chiandetti for their help in preparing these experiments. S.A.L. was funded by post-doctoral Fellowships from NIH-grant HD 23103 to E.S.S. and from the Center for Mind/Brain Sciences at University of Trento.
References
- 1.O'Keefe J., Burgess N. 1996. Geometric determinants of the place fields of hippocampal neurons. Nature 381, 425–428 10.1038/381425a0 (doi:10.1038/381425a0) [DOI] [PubMed] [Google Scholar]
- 2.Cheng K., Newcombe N. S. 2005. Is there a geometric module for spatial reorientation? Squaring theory and evidence. Psychon. Bull. Rev. 12, 1–23 10.3758/BF03196346 (doi:10.3758/BF03196346) [DOI] [PubMed] [Google Scholar]
- 3.Burgess N. 2008. Spatial cognition and the brain. Ann. NY Acad. Sci. 1124, 77–97 10.1196/annals.1440.002 (doi:10.1196/annals.1440.002) [DOI] [PubMed] [Google Scholar]
- 4.Cheng K. 1986. A purely geometric module in the rats' spatial representation. Cognition 23, 149–178 10.1016/0010-0277(86)90041-7 (doi:10.1016/0010-0277(86)90041-7) [DOI] [PubMed] [Google Scholar]
- 5.Hermer L., Spelke E. 1994. A geometric process for spatial reorientation in young children. Nature 370, 57–59 10.1038/370057a0 (doi:10.1038/370057a0) [DOI] [PubMed] [Google Scholar]
- 6.Chiandetti C., Vallortigara G. 2008. Is there an innate geometric module? Effects of experience with angular geometric cues on spatial re-orientation based on the shape of the environment. Anim. Cogn. 11, 139–146 10.1007/s10071-007-0099-y (doi:10.1007/s10071-007-0099-y) [DOI] [PubMed] [Google Scholar]
- 7.Wystrach A., Beugnon G. 2009. Ants learn geometry and features. Curr. Biol. 19, 61–66 10.1016/j.cub.2008.11.054 (doi:10.1016/j.cub.2008.11.054) [DOI] [PubMed] [Google Scholar]
- 8.Learmonth A. E., Newcombe N. S., Huttenlocher J. 2001. Toddlers' use of metric information and landmarks to reorient. J. Exp. Child Psychol. 80, 225–244 10.1006/jecp.2001.2635 (doi:10.1006/jecp.2001.2635) [DOI] [PubMed] [Google Scholar]
- 9.Sovrano V. A., Bisazza A., Vallortigara G. 2002. Modularity and spatial reorientation in a simple mind: encoding of geometric and nongeometric properties of a spatial environment by fish. Cognition 85, 51–59 10.1016/S0010-0277(02)00110-5 (doi:10.1016/S0010-0277(02)00110-5) [DOI] [PubMed] [Google Scholar]
- 10.Gallistel C. R. 1990. The organization of learning. Cambridge, MA: MIT Press [Google Scholar]
- 11.Vallortigara G. 2009. Animals as natural geometers. In Cognitive biology: evolutionary and developmental perspectives on mind, brain and behavior (eds Tommasi L., Nadel L., Peterson M.), pp. 83–104 Cambridge, MA: MIT Press [Google Scholar]
- 12.Lee S. A., Spelke E. S. 2010. Two systems of spatial representation underlying navigation. Exp. Brain Res. 206, 179–188 10.1007/s00221-010-2349-5 (doi:10.1007/s00221-010-2349-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng K. 2008. Whither geometry? Troubles of the geometric module. Trends Cogn. Sci. 12, 355–361 10.1016/j.tics.2008.06.004 (doi:10.1016/j.tics.2008.06.004) [DOI] [PubMed] [Google Scholar]
- 14.Stürzl W., Cheung A., Cheng K., Zeil J. 2008. The information content of panoramic images I: the rotational errors and the similarity of views in rectangular experimental arenas. J. Exp. Psychol. Anim. Behav. Process. 34, 1–14 10.1037/0097-7403.34.1.1 (doi:10.1037/0097-7403.34.1.1) [DOI] [PubMed] [Google Scholar]
- 15.Sheynikhovich D., Chavarriaga R., Strösslin T., Arleo A., Gerstner W. 2009. Is there a geometric module for spatial orientation? Insights from a rodent navigation model. Psychol. Rev. 116, 540–566 10.1037/a0016170 (doi:10.1037/a0016170) [DOI] [PubMed] [Google Scholar]
- 16.Lee S. A., Winkler-Rhoades N., Spelke E. S. Submitted. Reorientation is guided by perceived surface distance, not by image matching or comparison. [DOI] [PMC free article] [PubMed]
- 17.Lee S.A., Spelke E. S. 2008. Children's use of geometry for reorientation. Dev. Sci. 11, 743–749 10.1111/j.1467-7687.2008.00724.x (doi:10.1111/j.1467-7687.2008.00724.x) [DOI] [PubMed] [Google Scholar]
- 18.Lee S. A., Spelke E. S. 2010. A modular geometric mechanism for reorientation in children. Cogn. Psychol. 61, 152–176 10.1016/j.cogpsych.2010.04.002 (doi:10.1016/j.cogpsych.2010.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S. A., Spelke E. S. 2011. Young children reorient by computing layout geometry, not by matching images of the environment. Psychon. Bull. Rev. 18, 192–198 10.3758/s13423-010-0035-z (doi:10.3758/s13423-010-0035-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S. A., Sovrano V. A., Spelke E. S. 2012. Navigation as a source of geometric knowledge: young children's use of length, angle, distance, and direction in a reorientation task. Cognition 123, 144–161 10.1016/j.cognition.2011.12.015 (doi:10.1016/j.cognition.2011.12.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doeller C. F., Burgess N. 2008. Distinct error-correcting and incidental learning of location relative to landmarks and boundaries. Proc. Natl. Acad. Sci. USA 105, 5909–5914 10.1073/pnas.0711433105 (doi:10.1073/pnas.0711433105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vallortigara G., Sovrano V. A., Chiandetti C. 2009. Doing Socrates' experiment right: controlled-rearing studies of geometrical knowledge in animals. Curr. Opin. Neurobiol. 19, 20–26 10.1016/j.conb.2009.02.002 (doi:10.1016/j.conb.2009.02.002) [DOI] [PubMed] [Google Scholar]
- 23.Pecchia T., Vallortigara G. 2010. Re-orienting strategies in a rectangular array of landmarks by domestic chicks (Gallus gallus). J. Comp. Psychol. 124, 147–158 10.1037/a0019145 (doi:10.1037/a0019145) [DOI] [PubMed] [Google Scholar]
- 24.Pecchia T., Vallortigara G. 2010. View-based strategy for reorientation by geometry. J. Exp. Biol. 213, 2987–2996 10.1242/jeb.043315 (doi:10.1242/jeb.043315) [DOI] [PubMed] [Google Scholar]
- 25.Cartwright B. A., Collett T.S. 1982. How honey bees use landmarks to guide their return to a food source. Nature 295, 560–564 10.1038/295560a0 (doi:10.1038/295560a0) [DOI] [Google Scholar]
- 26.Wystrach A., Cheng K., Sosa S., Beugnon G. 2011. Geometry, features, and panoramic views: ants in rectangular arenas. J. Exp. Psychol. Anim. Behav. Process. 37, 420–435 10.1037/a0023886 (doi:10.1037/a0023886) [DOI] [PubMed] [Google Scholar]
- 27.Lever C., Wills T., Cacucci F., Burgess N., O'Keefe J. 2002. Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature 416, 90–94 10.1038/416090a (doi:10.1038/416090a) [DOI] [PubMed] [Google Scholar]
- 28.Solstad T., Boccara C. N., Kropff E., Moser M., Moser E. I. 2008. Representation of geometric borders in the entorhinal cortex. Science 322, 1865–1868 10.1126/science.1166466 (doi:10.1126/science.1166466) [DOI] [PubMed] [Google Scholar]
- 29.Save E., Cressant A., Thinus-Blanc C., Poucet B. 1998. Spatial firing of hippocampal place cells in blind rats. J. Neurosci. 18, 1818–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]