Abstract
Different vegetation types can generate variation in microclimates at local scales, potentially buffering species from adverse climates. To determine if species could respond to such microclimates under climatic warming, we evaluated whether ectothermic species (butterflies) can exploit favourable microclimates and alter their use of different habitats in response to year-to-year variation in climate. In both relatively cold (Britain) and warm (Catalonia) regions of their geographical ranges, most species shifted into cooler, closed habitats (e.g. woodland) in hot years, and into warmer, open habitats (e.g. grassland) in cooler years. Additionally, three-quarters of species occurred in closed habitats more frequently in the warm region than in the cool region. Thus, species shift their local distributions and alter their habitat associations to exploit favourable microclimates, although the magnitude of the shift (approx. 1.3% of individuals from open to shade, per degree Celsius) is unlikely to buffer species from impacts of regional climate warming.
Keywords: bioclimate modelling, biodiversity, fine-scale
1. Introduction
Climate change has driven a variety of responses in plants and animals that have highlighted the ecological importance of temperature [1]. Studies report both geographical-scale range shifts in response to climate change over kilometres to hundreds of kilometres [2], and local behavioural changes driven by fine-scale microclimates over centimetres to tens of metres [3]. Different as these spatial scales of response may be, they are unlikely to be entirely independent of each other, forming two components of a species’ thermal niche. The potential, therefore, exists for fine-scale microclimates to buffer species from climate change, if both (i) the species are able to move between habitats to cooler conditions and (ii) their other ecological requirements are met by the new habitat. However, the degree to which species can execute such switching in response to climate is unknown.
Here, we analyse two well-resolved, independent datasets to examine habitat switching in response to climate. We analysed the habitat associations of a set of European butterfly species that occur in Britain in northern Europe, and in Catalonia (Spain) in southern Europe, representing both the cooler, leading edge of the distributions and warmer, trailing edge populations, respectively. The regions were selected as they are far enough apart to be climatically distinct, yet close enough together to share common species. We use abundance data to test if species associate with cooler, more ‘closed’ habitats (e.g. woodland) in warmer years; and with warmer, more ‘open’ habitats (e.g. grassland) in relatively cool years. We also test whether species are more strongly associated with shadier habitats in the warmer region.
2. Material and methods
(a). Study sites and datasets
Britain (latitude 50–60° N) is cool and wet, with warm summers and mild winters, whereas Catalonia, in Spain (40.5–43° N) has hotter, drier summers and warmer winters (table 1). Butterfly abundance data were obtained from the UK and Catalan Butterfly Monitoring Schemes. Recorders make weekly visits to sites throughout the flight season, walking transects of 2–4 km and recording all butterflies seen within a 5 m corridor [4]: 36 species were analysed.
Table 1.
Average annual climatologies of Britain and Catalonia.
| region | latitude (° N) | average annual climate 1971–2000 |
||
|---|---|---|---|---|
| max temperature (°C) | mean temperature (°C) | min temperature (°C) | ||
| Britain | 50–60 | 13.38 | 9.74 | 6.09 |
| Catalonia | 40.5–43 | 19.05 | 14.10 | 9.15 |
(b). Quantifying habitat types
For historical reasons, open and closed habitats were quantified differently in Britain and Catalonia. In Britain, the dominant habitat type of each section of transect was recorded using 40 standardized habitat classifications. In Catalonia, habitat types were assessed according to the CORINE (coordination of information on the environment) Biotopes Manual, and the cover of different plant communities estimated to the nearest 10 per cent. For Britain, we categorized habitat information into two main groupings: closed habitats, corresponding to shady, wooded areas (coniferous and deciduous woodland) or dense shrub; and open habitats, corresponding to all other habitats. For Catalonia, transect sections with 60 per cent or more closed vegetation were considered closed (otherwise open), to match the British classification as closely as possible. Only transects that included both open and closed habitats were included in subsequent analyses (n = 85 transects in Britain; n = 81 in Catalonia), as populations from ‘open-only’ or ‘closed-only’ transects may (or may not) have exchanged individuals with nearby (unsurveyed) habitats.
(c). Quantifying butterfly habitat associations
The density of each butterfly species in open and closed habitats was calculated for each transect, for each year from 1994 to 2009, the period over which monitoring schemes operated simultaneously. Density values were used to calculate an index (Ihab) with values between –1 and +1, with –1 representing transects on which the species was found exclusively in closed habitats, whereas +1 for transects on which the species was found only in open habitats. A value of 0 represented a neutral habitat association. The calculation took the following form:
| 2.1 |
where Dopen denotes density in open habitats, and Dclosed denotes density in closed habitats.
(d). Measuring changes in habitat association with year-to-year climate variation
For both regions, we calculated the annual mean habitat index Ihab across all sites for each species. Species were excluded from these analyses if they occurred at fewer than 10 transect sites, or for fewer than 10 years. Because microclimatic differences change with the temperature variable selected [5], yearly index values were regressed against indices of annual maximum, mean and minimum temperature for each region (Britain, Central England Temperature data; Catalonia, Agencia Española de Meteorología data). We tested for phylogenetic autocorrelation in the response variables by conducting Moran's I-test with Geary randomizations [6], extracting phylogenetic relatedness from the published phylogeny in Cowley et al. [7] and following the exact method described in Oliver et al. [8].
(e). Measuring habitat association differences between Britain and Catalonia
Habitat indices were compared between regions, deriving a single habitat association value per species, in each region. The mean of the annual index values was taken for each species for 1994–2009. Each species was then represented by one habitat index value for Britain, and another for Catalonia. Species appearing on fewer than 10 transects were again omitted. The average difference in index values between the regions was then compared with that predicted from relationships to annual climate (see §4).
3. Results
(a). Habitat association differences with year-to-year climate variation
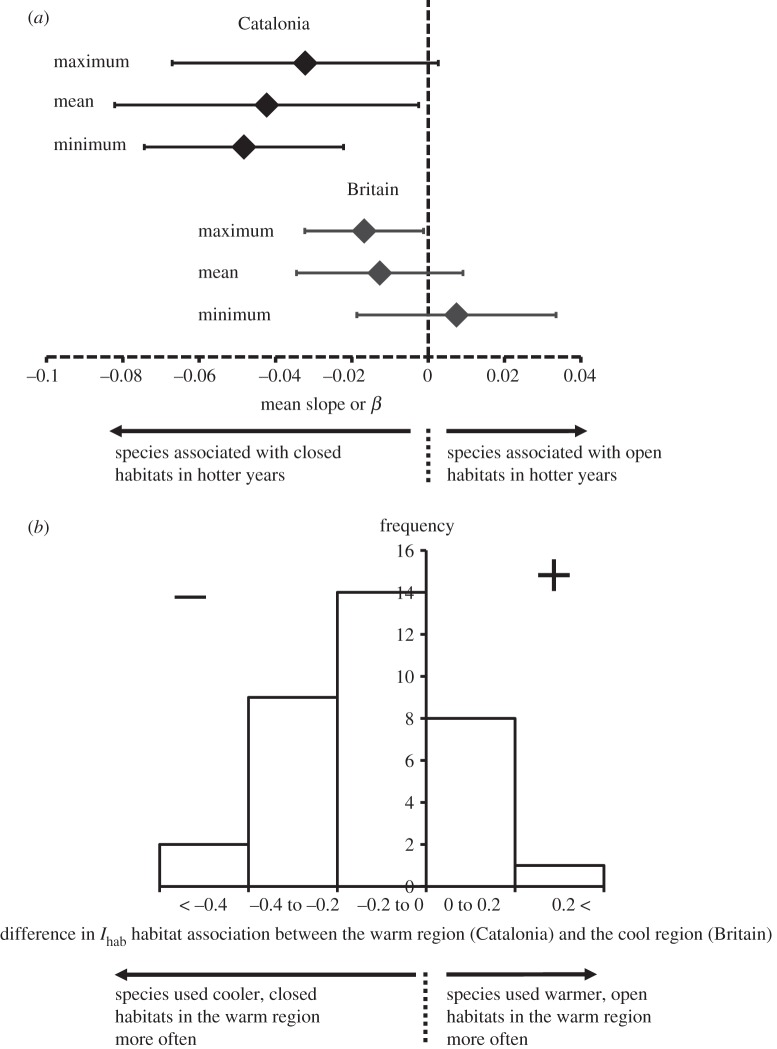
Higher fractions of individuals used cooler, closed habitats more frequently in years with higher maximum temperatures (figure 1a). Generalized linear model (GLM) slopes of Ihab against maximum temperature in each year were mainly negative and different from zero (one sample t-test: Britain t = 2.22, d.f. = 25, p = 0.04; Catalonia t = 1.91, d.f. = 23, p = 0.07; electronic supplementary material, tables S1 and S2). Similar results were obtained for analyses of the mean and minimum temperature in Catalonia, confirming greater use of closed habitats in warmer years (GLM slopes were mainly negative and significantly different from zero, analyses with mean temperature, t = 2.28, d.f. = 23, p = 0.03; minimum temperature, t = 2.28, d.f. = 23, p = 0.03; electronic supplementary material, table S2). However, there was no significant relationship between minimum or mean temperature and Ihab in Britain (p > 0.2 in both cases).
Figure 1.
Changes in butterfly habitat association with (a) year-to-year and (b) geographical variation in climate. (a) Generalized-linear model slopes of annualized habitat associations against annual climate for maximum, mean and minimum temperatures. Error bars denote 95% CIs of the means across species. (b) Difference in the open or closed habitat association of butterflies in Britain and Catalonia. Positive values denote more frequent association with warmer, open habitats (e.g. grassland) in the warmer region, whereas negative values denote species more associated with cooler, closed habitats (e.g. woodland) in the warmer region.
For all models, Shapiro–Wilk tests for non-normality were performed on the residuals. Of 200 models tested, 13 (6.5%) returned a positive result at the 0.05 level, consistent with a typical false positive rate. Tests for phylogenetic effects in the response variables also came out non-significant (Britain: max temperature I = −0.03, p = 0.41; mean temperature I = −0.05, p = 0.65; min temperature I = −0.06, p = 0.85. Catalonia: max temperature I = −0.08, p = 0.93; mean temperature I = −0.10, p = 0.97; min temperature I = −0.11, p = 0.98).
(b). Habitat association differences between Britain and Catalonia
Butterflies were associated with closed habitats more frequently in the warm region (Catalonia) than in the cool region (Britain, figure 1b). Ihab indices were on average 0.12 lower in Catalonia (paired t-test: t = 3.87, d.f. = 33, p < 0.0005). Approximately, three-quarters (25 of 34) of species were associated with closed habitats more frequently in the warmer region (Catalonia) than in the cooler region (Britain).
Differences in index between the regions were not significantly different from a normal distribution (Kolmogorov–Smirnov test: Z = 0.676, n = 34, p = 0.75), suggesting that use of a paired t-test is valid. No evidence of an effect of phylogeny on values of Ihab was found for either region (Britain: I = −0.08, p = 0.99; Catalonia: I = −0.04, p = 0.78).
The average difference in shadiness index values between Britain and Catalonia (0.12) can be compared with the difference expected between the two regions, calculated from year-to-year thermal habitat sensitivity of the species within each region. Slopes of species relationships to annual climate were input into GLMs as dependent variables, with region as a factor, to test for differences in species’ response to climate between Britain and Catalonia, which, if significant for a particular climate variable, would mean it could not be robustly used to derive an expected regional difference. Region was non-significant for species relationships to maximum (GLM: f = 0.72, d.f. = 49, p = 0.40) and mean (GLM: f = 1.90, d.f. = 49, p = 0.18) temperature, but was significant in the case of relationships to minima (GLM: f = 5.28, d.f. = 49, p = 0.03), so we proceeded with predicted differences only between the regions for maximum and mean temperatures.
For thermal maxima, species shifted habitat by an average Ihab value of 0.017 and 0.032 per degree Celsius in Britain and Catalonia, respectively (figure 1a). The average Ihab of the two regions, 0.025, combined with the fact that Catalonian maxima are 5.67°C hotter than those in Britain, would predict a mean index difference of 0.025 × 5.67 = 0.14 between Britain and Catalonia (table 2). This is similar to the observed 0.12. For mean temperatures, species shifted habitat by an average index of 0.013 and 0.042 per degree Celsius in the two study regions (figure 1a), i.e. a mean shift of 0.028. Given a mean temperature difference of 4.36°C between the regions (table 1), this would predict a mean index difference of 0.028 × 4.36 = 0.12 (table 2), the same as that observed.
Table 2.
Predicted index differences between Britain and Catalonia, and the corresponding shifts in relative habitat density implied.
| source | predicted Ihab index difference (Britain versus Catalonia) | corresponding shift in relative density from closed to open habitat (Dopen – Dclosed) |
|---|---|---|
| interannual relationships to mean temperature | 0.12 | 0.06 |
| interannual relationships to maximum temperature | 0.14 | 0.07 |
| actual index comparison between the two regions | 0.12 | 0.06 |
4. Discussion
We found a consistent effect of microclimate on species’ habitat associations, with higher fractions of individuals being found in cooler, closed habitats in hotter years and in the hotter region. In Britain, butterflies used closed habitats in years with higher maximum temperatures. In Catalonia, species associated more frequently with closed habitats when any temperature variable increased. In conjunction with previous studies, mainly of individual species or static geographical patterns of habitat associations [3,9], our multi-species analysis suggests that thermal habitat shifts in space and time are likely to be widespread.
The between-year results within each region are robust, with species shifting into shadier habitats in hotter years. However, some of the between-region differences in habitat associations may be attributable to differences in habitat measurement techniques (see §2). In addition, different mechanisms may be responsible for the responses of species to annual and regional climatic differences. Nonetheless, despite the contrasting scales (spatial versus temporal), we find a consistent habitat shifting response to climate, and therefore potentially a consistent mechanism. This consistency also suggests that our reported regional difference is likely to be genuine, rather than an artefact of differences in habitat measurement. The reported differences in regional habitat associations could be due to more than just a direct impact of climate on the butterflies. Some regional effects could arise from varying associations with host plants, local adaptations to the region or the presence of natural enemies. Note that these effects may themselves be influenced by climate.
Although we found thermal habitat sensitivity in most species, its magnitude is surprisingly small. Table 2 provides an indication of what the index differences mean in terms of movement of individuals between habitats, with shifts in relative density calculated by solving equation (2.1). Predictions indicate that the differences in climate between Britain and Catalonia cause an average 6–7% of individuals to shift from open to closed habitat. Dividing this by the temperature difference between the regions, roughly 1.3 per cent of individuals shift habitat per degree Celsius change in temperature. This shift could take the form of changes in the proportion of time spent in each habitat by each individual, changes in the fraction of individuals selecting each habitat, changes to activity schedules, changes to birth and death schedules or some combination of the above. The small magnitude of the shift does however indicate that most species that mainly occupy open habitats in Britain are also predominantly associated with open habitats in Catalonia, implying that other ecological constraints or resources (e.g. host plants, nectar sources and mating behaviours) are more important determinants of broad vegetation associations of species (see Dennis et al. [10]).
Hence, although we have determined some plasticity in species habitat association with respect to climate, this plasticity is unlikely to be sufficient to buffer populations from future climate change. Few species can be expected to shift fully into shady habitats, and hence avoid new, higher temperatures. However, species that are constrained to one broad vegetation category may still shift within that vegetation type, such as from shorter into taller grass [11] and from Equator-facing onto pole-facing hillsides [12].
We conclude that the effects of microclimate on butterfly habitat association are consistent, that these effects result in a shift to cooler habitats in warmer years (and vice versa) but that the absolute size of the shift (approx. 1.3% of individuals per degree Celsius) means that major changes in vegetation associations are unlikely to buffer many species from climate change.
Acknowledgements
We thank the Catalonia BMS, the UK BMS, the Met Office Hadley Centre and Agencia Española de Meteorología for data access. The Catalonia BMS is funded by the Departament de Territori i Sostenibilitat de la Generalitat de Catalunya. The UK BMS is funded by a consortium of UK government agencies. Both schemes are indebted to volunteers who submit data. A.J.S. was funded by NERC studentship grant no. NE/F009836/1.
References
- 1.IPCC 2007. Climate change 2007: impacts, adaptation and vulnerability. Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Hitch A. T., Leberg P. L. 2007. Breeding distributions of North American bird species moving north as a result of climate change. Conserv. Biol. 21, 534–539 10.1111/j.1523-1739.2006.00609.x (doi:10.1111/j.1523-1739.2006.00609.x) [DOI] [PubMed] [Google Scholar]
- 3.Ashton S., Gutiérrez D., Wilson R. J. 2009. Effects of temperature and elevation on habitat use by a rare mountain butterfly: implications for species responses to climate change. Ecol. Entomol. 34, 437–446 10.1111/j.1365-2311.2008.01068.x (doi:10.1111/j.1365-2311.2008.01068.x) [DOI] [Google Scholar]
- 4.Pollard E., Yates T. S. 1993. Monitoring butterflies for ecology and conservation: the British butterfly monitoring scheme. London, UK: Chapman and Hall [Google Scholar]
- 5.Suggitt A. J., Gillingham P. K., Hill J. K., Huntley B., Kunin W. E., Roy D. B., Thomas C. D. 2011. Habitat microclimates drive fine-scale variation in extreme temperatures. Oikos 120, 1–8 10.1111/j.1600-0706.2010.18270.x (doi:10.1111/j.1600-0706.2010.18270.x) [DOI] [Google Scholar]
- 6.Gittleman J. L., Kot M. 1990. Statistics and a null model for estimating phylogenetic effects. Syst. Zool. 39, 227–241 10.2307/2992183 (doi:10.2307/2992183) [DOI] [Google Scholar]
- 7.Cowley M. J. R., et al. 2001. Density-distribution relationships in British butterflies. I. The effect of mobility and spatial scale. J. Anim. Ecol. 70, 410–425 10.1046/j.1365-2656.2001.00508.x (doi:10.1046/j.1365-2656.2001.00508.x) [DOI] [Google Scholar]
- 8.Oliver T., Hill J. K., Thomas C. D., Brereton T., Roy D. B. 2009. Changes of habitat specificity of species at their climatic range boundaries. Ecol. Lett. 12, 1091–1102 10.1111/j.1461-0248.2009.01367.x (doi:10.1111/j.1461-0248.2009.01367.x) [DOI] [PubMed] [Google Scholar]
- 9.Weiss S. B., Murphy D. D., White R. R. 1988. Sun, slope, and butterflies: topographic determinants of habitat quality for Euphydryas Editha. Ecology 69, 1486–1496 10.2307/1941646 (doi:10.2307/1941646) [DOI] [Google Scholar]
- 10.Dennis R. L. H., Shreeve T. G., Van Dyck H. 2006. Habitats and resources: the need for a resource-based definition to conserve butterflies. Biodivers. Conserv. 15, 1943–1966 10.1007/s10531-005-4314-3 (doi:10.1007/s10531-005-4314-3) [DOI] [Google Scholar]
- 11.Roy D. B., Thomas J. A. 2003. Seasonal variation in the niche, habitat availability and population fluctuations of a bivoltine thermophilous insect near its range margin. Oecologia 134, 439–444 [DOI] [PubMed] [Google Scholar]
- 12.Thomas C. D., Bodsworth E. J., Wilson R. J., Simmons A. D., Davies Z. G., Musche M., Conradt L. J. 2001. Ecological and evolutionary processes at expanding range margins. Nature 411, 577–581 10.1038/35079066 (doi:10.1038/35079066) [DOI] [PubMed] [Google Scholar]