Abstract
The acquisition of linguistic competency from more experienced social partners is a fundamental aspect of human language. However, there is little evidence that non-human primates learn to use their vocalizations from social partners. Captive chimpanzees (Pan troglodytes) produce idiosyncratic vocal signals that are used intentionally to capture the attention of a human experimenter. Interestingly, not all apes produce these sounds, and it is unclear what factors explain this difference. We tested the hypothesis that these attention-getting (AG) sounds are socially learned via transmission between mothers and their offspring. We assessed 158 chimpanzees to determine if they produced AG sounds. A significant association was found between mother and offspring sound production. This association was attributable to individuals who were raised by their biological mother—as opposed to those raised by humans in a nursery environment. These data support the hypothesis that social learning plays a role in the acquisition and use of communicative vocal signals in chimpanzees.
Keywords: chimpanzee, vocal learning, language evolution
1. Introduction
The acquisition of linguistic competency from more skilled and experienced social partners is a fundamental aspect of human language. Although vocal learning is found in some bird species, there is little evidence that any non-human primates, including chimpanzees (Pan troglodytes), the closest living relative to humans, learn to use their vocalizations from social partners [1]. Indeed, much of the data available on the production of non-human primate vocalizations suggest that they are relatively fixed in both form and usage [2]. However, recent studies have shown that captive chimpanzees and orangutans produce idiosyncratic vocal signals that are collectively (pongo pygmaeus and pongo abelii) referred to as ‘attention-getting’ (AG) sounds [3,4]. As their name implies, AG sounds are used intentionally to capture the attention of an otherwise inattentive human experimenter [3,5,6], and are often produced in tight temporal synchrony with the production of manual gestures [7]. Specifically, chimpanzees are more likely to produce AG sounds when a human is present in conjunction with a preferred food item than when either (the human or the food item) are present alone [3]. In addition, AG sounds are also produced by chimpanzees to capture the attention of an inattentive human experimenter, before requesting a tool needed to complete a problem-solving task [8].
Interestingly, however, not all apes in captivity produce AG sounds, despite being raised in relatively comparable physical environments [9,10]. Thus, the factors that explain these individual differences are not yet clear. In fact, relatively little is known regarding the development and use of AG sounds by captive apes at the individual and group level.
In the current study, we tested the hypothesis that AG sounds are socially learned via transmission between mothers and their offspring. If social learning accounts for individual differences in the production of AG sounds, then whether or not an individual produces AG sounds should be significantly associated with their use (or lack thereof) by their biological mother. Moreover, if social learning is in fact the mediating factor determining the use of AG sounds, the concordance rates in the use of AG sounds would be significantly higher in offspring raised by their biological mothers (mother-reared) compared with offspring separated at birth from their mother and raised by humans (nursery-reared) [11].
2. Material and methods
To test this hypothesis, we assessed 158 captive chimpanzees to determine if they produced AG sounds to get the attention of a human experimenter (AG+) or not (AG–) [3]. Subjects included 93 females and 65 males living at the University of Texas MD Anderson Cancer Center. Subjects ranged in age from 9 to 49 years, 90 were mother-reared, 29 were nursery-reared, 36 were wild-born and three had unknown rearing histories.
All tests were virtually identical to those used in previous studies assessing communication skills in captive chimpanzees [3,6,12]. The procedure included two conditions: the ‘toward’ condition and the ‘away’ condition. Each subject participated in three trials consecutively per condition to determine if the subject produced AG sounds directed to humans. The order of the conditions was counterbalanced across subjects. Subjects were tested within their undisturbed social groups in the outdoor area of their enclosure.
For the toward condition, the experimenter stood approximately 1 m from the front of the subject's enclosure with their body and eyes directed toward the subject, while holding a small, clear container of grapes that was plainly visible to the focal animal. For the away condition, the experimenter stood approximately 1 m from the front of the subject's enclosure with their body oriented to the side, facing away from the focal subject and directed towards the chimpanzee(s) in the neighbouring enclosure, while holding a small, clear container of grapes that was plainly visible to the focal animal. Each trial lasted 30 s. At the end of each trial, the focal subject, and all individuals within immediate view of the experimenter, were given a single grape, regardless of the focal subject's behaviour during the trial. A 30 s (minimum) inter-trial interval was observed, during which the experimenter walked away from, or out of the immediate view of, the subject.
Test sessions were distributed throughout the day (between 07.30 and 17.00) and all trials were recorded on a Sony Handycam Digital Video Camera Recorder. If the subject walked away during a trial, the trial continued. If the subject did not return, or appeared to show no interest in returning, the trial was terminated and attempted again at a later time.
AG sounds were defined as clearly audible sounds that are produced with the mouth, occur within the context of requesting food or attention from a human, and are accompanied by overt visual monitoring of the experimenter. For each chimpanzee, the total number of AG sounds produced was summed across the six test trials. Subjects who made at least one AG sound were classified as AG+, and those who failed to produce any AG sounds were classified as AG–.
We also characterized the AG+ sounds to determine if the type of AG sounds produced were concordant between mothers and their offspring. To accomplish this, the experimenter classified each AG sound produced by the subject from the audio/video recordings of each trial, according to the descriptions included in the ethogram depicted in table 1. The experimenter (L.R.) was blind to the hypothesis of the study and was relatively unfamiliar with the pedigree of the animals in the facility. This was done to remove any potential subjective bias in the characterization of the number as well as the type of AG sound produced by the chimpanzees. To confirm objective classification of the AG sound types, a second observer independently scored a subset of the trials (one trial from each of 29 subjects classified as AG+) using the same AG sound type ethogram (table 1). There was 100 per cent agreement between this second observer and the experimenter. On one trial, however, the second observer noted two different types of AG sounds produced by the subject (Raspberries (RS) and Kisses), whereas the experimenter had noted only one AG sound type (RS).
Table 1.
AG sound type ethogram.
| AG sound | description |
|---|---|
| extended grunts (EG) | voiced, atonal sounds produced by the chimpanzees with an open mouth |
| kisses (KI) | produced by inhaling air through pursed lips. |
| lip smacks (LS) | produced by placing upper and lower lips tightly together then pulling them apart quickly making an audible ‘pop’ sound |
| pants (PA) | audible, rapid, rhythmic sequence of inhaling and exhaling |
| raspberries (RS) | produced by blowing air out through pursed lips |
| teeth chomps (TC) | produced by clacking teeth together so that the hitting together of upper and lower jaws is audible |
3. Results
Of the 158 chimpanzees tested, 85 were classified as AG– and 73 as AG+, a distribution that is not significantly different from chance. We then examined the association of AG sound production between mother and offspring. Data were available for 71 mother–offspring dyads; of these dyads, 59 of the offspring were mother-reared, whereas 12 were nursery-reared. Overall, a significant association was found between mother and offspring AG sound production χ2 (1, n = 71) = 6.66, p = 0.010. This association was almost entirely attributable to offspring who were raised by their biological mother. Indeed, the proportion of mother-reared dyads who were concordant for the use of AG sounds was significantly higher (42/59 dyads) compared with nursery-reared individuals (4/12 dyads) χ2 (1, n = 71) = 6.26, p = 0.012, supporting the hypothesis that chimpanzees are acquiring the use of these signals via social learning (figure 1). There was no significant association between rearing and AG sound production (χ2 (1, n = 71) = 1.23, p = 0.268).
Figure 1.
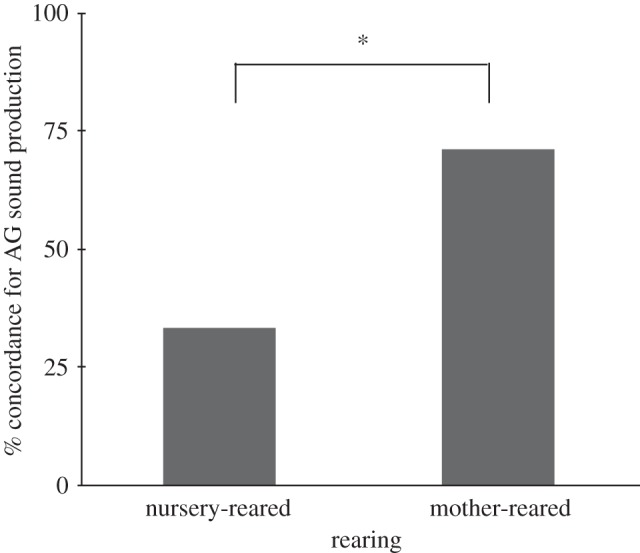
Concordance between mother and offspring for AG sound production (*p = 0.012).
In addition, a binomial test revealed that chimpanzee offspring are significantly more likely to produce the same type of AG sound as their mother (p = 0.033) than any other type of AG sound. Of the 19 AG+ mother-reared offspring whose mother's are also AG+, 14 (74%) produce the same type of sound as their mother (figure 2).
Figure 2.
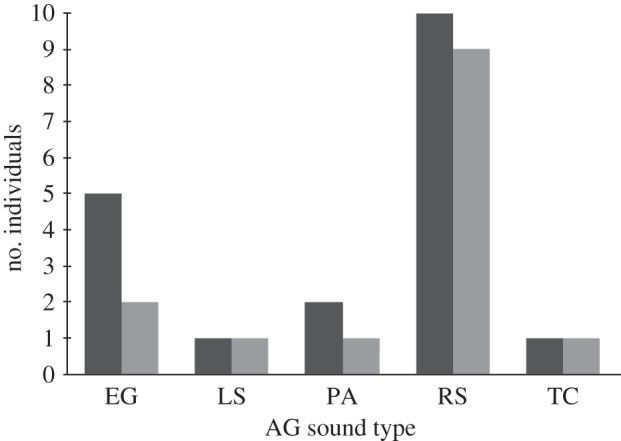
AG sound types for mother-reared subjects whose mothers are also AG+ (n = 19). AG sounds are classified based on the descriptions in table 1. Note that two subjects (offspring) produced two types of AG during their vocal assessments, RS and EG. The mothers of both subjects produced RS. Both subjects were included in our analysis as concordant for vocal type with their mother. Light grey bars denote AG+ offspring with mothers that produce same AG sound type and dark grey bars denote total offspring (AG+) that produce each AG sound type.
4. Discussion
The data presented above suggest that AG sounds are socially learned via transmission between mothers and their offspring. We found that those chimpanzees who were reared by their biological mothers are more likely to be concordant for the use of AG sounds with their mother than are chimpanzees raised by humans in a nursery environment. Moreover, mother-reared chimpanzees are significantly more likely to produce the same type of AG sound as their mother than any other type of AG sound.
These data support the hypothesis that social learning plays a role in the acquisition of communicative vocal signals in chimpanzees. Previously, group-level structural variation has been reported in the calls of chimpanzees in the wild as well as in captivity [13–15]. For example, Crockford et al. [14] report structural differences in the pant hoot vocalizations of male chimpanzees living in neighbouring communities, but not between groups from a distant community. These results could not be accounted for by genetic or habitat differences, suggesting that the chimpanzees may be actively modifying the structure of their calls to facilitate group identification [14]. The data presented here are consistent with these observations and provide direct evidence that social learning may be an important factor in the acquisition and use of communicative signals by chimpanzees.
The most parsimonious explanation for the results reported here is that those offspring who were reared by their biological mother viewed their biological mothers producing AG sounds—a behaviour that probably resulted in some form of reinforcement from human carers (e.g. food), or did not observe their mothers producing AG sounds. Subsequently, these mother-reared offspring learned (or did not learn) to produce AG sounds themselves within the same contexts. In contrast, those offspring who were reared in a nursery setting did not view their mother's behaviour, and therefore were less likely to be concordant with them for AG sound production compared with their mother-reared counterparts. It is important to note that the number of nursery-reared individuals in which data from their mother were also available is relatively small (n = 12) compared with mother-reared chimpanzees (n = 59). Therefore, some caution is warranted in interpreting the results for the nursery-reared individuals. In addition, the mechanism by which nursery-reared chimpanzees classified as AG+ acquired the use of AG sounds is not clear, and is beyond the scope of this study. However, social learning (e.g. from peers) may have played a role in the acquisition and use of AG sounds by these subjects as well.
In summary, and consistent with findings on tool use in wild and captive chimpanzees [16,17], social learning appears to play an important role in the acquisition and use of vocal signals in chimpanzees. Previous data indicate that the production of AG sounds selectively activate the Broca's area homologue in chimpanzees, suggesting that this critical language region was involved in vocal signalling in the common ancestor of both modern humans and chimpanzees [18,19]. We propose that over the course of evolution, there was increased selection for expansion in both the form and function of vocal communication in early hominids. Concurrently, selection for increasing motor control needed for speech resulted in the nearly sevenfold increase in cortical representation of Broca's area in humans compared with chimpanzees [20], eventually resulting in unprecedented vocal flexibility, and full blown spoken language in modern humans.
Acknowledgements
The authors thank the veterinary and animal care staff at the Michale E. Keeling Center for Comparative Medicine and Research, and Jamie L. Russell for her assistance with AG sound type classification. This research was supported by Award nos. R15DC011005 from the National Institute on Deafness and Other Communication Disorders to J.P.T., and NS42867 and NS36605 from the National Institute Of Neurological Disorders and Stroke and HD56232 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development to W.D.H. Chimpanzee maintenance at the Keeling Center is funded by NIH/NCRR U42-RR15090.
References
- 1.Janik V. M., Slater P. J. B. 1997. Vocal learning in mammals. Adv. Study Behav. 26, 59–99 10.1016/S0065-3454(08)60377-0 (doi:10.1016/S0065-3454(08)60377-0) [DOI] [Google Scholar]
- 2.Seyfarth R. M., Cheney D. L. 2010. Production, usage, and comprehension in animal vocalisations. Brain Lang. 115, 92–100 10.1016/j.bandl.2009.10.003 (doi:10.1016/j.bandl.2009.10.003) [DOI] [PubMed] [Google Scholar]
- 3.Hopkins W. D., Taglialatela J. P., Leavens D. A. 2007. Chimpanzees differentially produce novel vocalisations to capture the attention of a human. Anim. Behav. 73, 281–286 10.1016/j.anbehav.2006.08.004 (doi:10.1016/j.anbehav.2006.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cartmill E., Byrne R. W. 2007. Orangutans modify their gestural signaling according to their audience's comprehension. Curr. Biol. 17, 1–14 10.1016/j.cub.2006.10.053 (doi:10.1016/j.cub.2006.10.053) [DOI] [PubMed] [Google Scholar]
- 5.Hostetter A. B., Russell J. L., Freeman H., Hopkins W. D. 2007. Now you see me, now you don't: evidence that chimpanzees understand the role of the eyes in attention. Anim. Cogn. 10, 55–62 10.1007/s10071-006-0031-x (doi:10.1007/s10071-006-0031-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leavens D. A., Hostetter A. B., Wesley M. J., Hopkins W. D. 2004. Tactical use of unimodal and bimodal communication by chimpanzees, Pan troglodytes. Anim. Behav. 67, 467–476 10.1016/j.anbehav.2003.04.007 (doi:10.1016/j.anbehav.2003.04.007) [DOI] [Google Scholar]
- 7.Hopkins W. D., Cantero M. 2003. From hand to mouth in the evolution of language: the influence of vocal behaviour on lateralized hand use in manual gestures by chimpanzees (Pan troglodytes). Dev. Sci. 6, 55–61 10.1111/1467-7687.00254 (doi:10.1111/1467-7687.00254) [DOI] [Google Scholar]
- 8.Russell J. L., Braccini S., Buehler N., Kachin M. J., Schapiro S. J., Hopkins W. D. 2005. Chimpanzees (Pan troglodytes) intentional communication is not contingent upon food. Anim. Cogn. 8, 263–272 10.1007/s10071-005-0253-3 (doi:10.1007/s10071-005-0253-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hopkins W. D., Taglialatela J., Leavens D. A., Russell J. L., Schapiro S. J. 2010. Behavioural and brain asymmetries in chimpanzees. In The mind of the chimpanzee (eds Lonsdorf E. V., Ross S. R., Matsuzawa T.), pp. 60–74 Chicago, IL: University of Chicago Press [Google Scholar]
- 10.Hopkins W. D., Taglialatela J. P., Leavens D. A. 2011. Do chimpanzees have voluntary control of their facial expressions and vocalisations? In Primate communication and human language: vocalisation, gestures, imitation and deixis in humans and non-humans (eds Vilain A., Schwartz J.-L., Abry C., Vauclair J.), pp. 71–90 Amsterdam, The Netherlands: John Benjamins Publishing Company [Google Scholar]
- 11.Bard K. A., Gardner K. H. 1996. Influences on development in infant chimpanzees: enculturation, temperament, and cognition. In Reaching into thought: the minds of the great apes (eds Russon A. E., Bard K. A., Parker S. T.), pp. 235–256 New York, NY: Cambridge University Press [Google Scholar]
- 12.Hostetter A. B., Cantero M., Hopkins W. D. 2001. Differential use of vocal and gestural communication by chimpanzees (Pan troglodytes) in response to the attentional status of a human (Homo sapiens). J. Comp. Psychol. 115, 337–343 10.1037/0735-7036.115.4.337 (doi:10.1037/0735-7036.115.4.337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arcadi A. C. 1996. Phrase structure of wild chimpanzee pant hoots: patterns of production and interpopulation variability. Am. J. Primatol. 39, 159–178 (doi:10.1002/(SICI)1098-2345(1996)39:3<159::AID-AJP2>3.0.CO;2-Y) [DOI] [PubMed] [Google Scholar]
- 14.Crockford C., Herbinger I., Vigilant L., Boesch C. 2004. Wild chimpanzees produce group-specific calls: a case for vocal learning? Ethology 110, 221–243 10.1111/j.1439-0310.2004.00968.x (doi:10.1111/j.1439-0310.2004.00968.x) [DOI] [Google Scholar]
- 15.Marshall A. J., Wrangham R. W., Arcadi A. C. 1999. Does learning affect the structure of vocalisations in chimpanzees? Anim. Behav. 58, 825–830 10.1006/anbe.1999.1219 (doi:10.1006/anbe.1999.1219) [DOI] [PubMed] [Google Scholar]
- 16.Whiten A., Goodall J., McGrew W. C., Nishida T., Reynolds V., Sugiyama Y., Tutin C. E. G., Wrangham R. W., Boesch C. 1999. Cultures in chimpanzees. Nature 399, 682–685 10.1038/21415 (doi:10.1038/21415) [DOI] [PubMed] [Google Scholar]
- 17.Lonsdorf E. V., Eberly L. E., Pusey A. E. 2004. Sex differences in learning in chimpanzees. Nature 428, 715–716 10.1038/428715a (doi:10.1038/428715a) [DOI] [PubMed] [Google Scholar]
- 18.Taglialatela J. P., Russell J. L., Schaeffer J. A., Hopkins W. D. 2008. Communicative signaling activates ‘Broca's’ homologue in chimpanzees. Curr. Biol. 18, 343–348 10.1016/j.cub.2008.01.049 (doi:10.1016/j.cub.2008.01.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taglialatela J. P., Russell J. L., Schaeffer J. A., Hopkins W. D. 2011. Chimpanzee vocal signaling points to a multimodal origin of human language. PLoS ONE 6, e18852. 10.1371/journal.pone.0018852 (doi:10.1371/journal.pone.0018852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schenker N. M., Hopkins W. D., Spocter M. A., Garrison A., Stimpson C. D., Erwin J. M., Hof P. R., Sherwood C. C. 2010. Broca's area homologue in chimpanzees (Pan troglodytes): probabilistic mapping, asymmetry and comparison to humans. Cereb. Cortex 20, 730–742 10.1093/cercor/bhp138 (doi:10.1093/cercor/bhp138) [DOI] [PMC free article] [PubMed] [Google Scholar]


