Abstract
It has been hypothesized that radiation-induced oxidative stress is the mechanism for a wide range of negative impacts on biota living in radioactively contaminated areas around Chernobyl. The present study tests this hypothesis mechanistically, for the first time, by modelling the impacts of radiolysis products within the cell resulting from radiations (low linear energy transfer β and γ), and dose rates appropriate to current contamination types and densities in the Chernobyl exclusion zone and at Fukushima. At 417 µGy h−1 (illustrative of the most contaminated areas at Chernobyl), generation of radiolysis products did not significantly impact cellular concentrations of reactive oxygen species, or cellular redox potential. This study does not support the hypothesis that direct oxidizing stress is a mechanism for damage to organisms exposed to chronic radiation at dose rates typical of contaminated environments.
Keywords: Chernobyl, Fukushima, radiation, oxidative stress, biota, cell
1. Introduction
Oxidative stress results from ‘a mismatch between the production of damaging reactive oxygen species (ROS) and the organisms’ capacity to mitigate their damaging effects’ [1, p. 75]. At high dose rates, cellular oxidizing stress plays an important role in cell damage from ionizing radiation, and antioxidants may have a protective effect at such dose rates. For example, injection of vitamin E (α-tocopherol) increased 30-day survival rates of mice exposed to acute high dose rate (1.2 × 107 µGy h−1) of low linear energy transfer (LET) radiation [2].
Significant radiation-induced oxidative stress of flora and fauna at lower dose rates (here defined as up to ca 400 µGy h−1 from internal and external sources) has also been hypothesized. Plant responses have been linked to oxidizing stress and antioxidant capacity in field and experimental studies [3]. Significantly, lower antioxidant concentrations have been observed in birds (barn swallow, Hirundo rustica; great tit, Parus major) inhabiting areas contaminated by Chernobyl [4]—an effect attributed to radiation-induced oxidative stress. This hypothesis is supported by observations of decreased levels of the antioxidants retinol, α-tocopherol and carotenoids in blood plasma, liver and egg yolk of barn swallows living near Chernobyl [4].
However, the relationship between antioxidant concentrations and oxidative stress is complex [1]. For example, studies in plants have found increased concentrations of antioxidant enzymes with radiation exposure [5] at low dose rates. Other low dose rate studies have found no changes in antioxidant concentrations either in plants [6] or birds [7], although the latter did observe a significant difference in metabolites produced by reactive oxygen (ROM). Recently, Bonisoli-Alquati et al. [8] found that ‘oxidative damage of sperm was negatively related to sperm motility’ in birds exposed to radiation at Chernobyl, but that ‘the highest values [of high sperm motility] were associated with relatively high radiation levels’ (p. 105).
The low radiation dose rate oxidizing stress hypothesis has not, to our knowledge, yet been tested at a mechanistic level. The present study tests this hypothesis (using previously published data on oxidizing stress in birds at Chernobyl) by modelling, for the first time, the capacity of selected antioxidants to reduce radiolysis products at radiation dose rates appropriate to current contamination densities pertaining at Chernobyl and Fukushima.
2. Material and methods
For a given dose rate, D (Gy s−1), we can calculate the rate of production of ion pairs per unit mass of an organism by considering the radiolysis products of water when exposed to ionizing radiation. These include H2; H2O2; 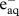 ; H·; OH·; HO2· (see electronic supplementary material). For low LET radiation, G-values giving radiolysis products per Joule of absorbed radiation energy are given in electronic supplementary material, table S1 (from Choppin et al. [9]).
; H·; OH·; HO2· (see electronic supplementary material). For low LET radiation, G-values giving radiolysis products per Joule of absorbed radiation energy are given in electronic supplementary material, table S1 (from Choppin et al. [9]).
The rate of production of each radiolysis product, rp, per unit mass of tissue (mol kg−1 s−1) is
| 2.1 |
where θ is the fractional water content of the tissue.
Here, we investigate the potential impact of radiolysis products on cellular antioxidant concentrations. Assuming that the rate of replenishment of antioxidant molecules occurs at a rate proportional to the difference between the current concentration, CT (mol l−1) and a ‘target’ equilibrium concentration, CTE (mol l−1), the following equation describes the rate of change of CT:
| 2.2 |
where rf is the fractional replenishment rate of antioxidant molecules (s−1). For boundary condition CT = CTE at t = 0, this has solution:
| 2.3 |
The impact of radiolysis on the redox status of the cell can be quantified by considering the impact on redox potential, Eh (volts) of decreased glutathione (GSH) owing to oxidation to oxidized glutathione (GSSG) concentrations [10,11]:
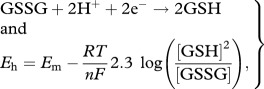 |
2.4 |
where Em is the mid-point potential (−0.240 V at pH 7; [11]), R is the gas constant (8.314 J K−1 mol−1) , T is temperature (Kelvin), F is the Faraday constant (9.6485 × 104 C mol−1), n is the number of electrons involved in the redox of the couple, in this case, two.
3. Results
(a). Effect of radiolysis on cellular antioxidant concentrations
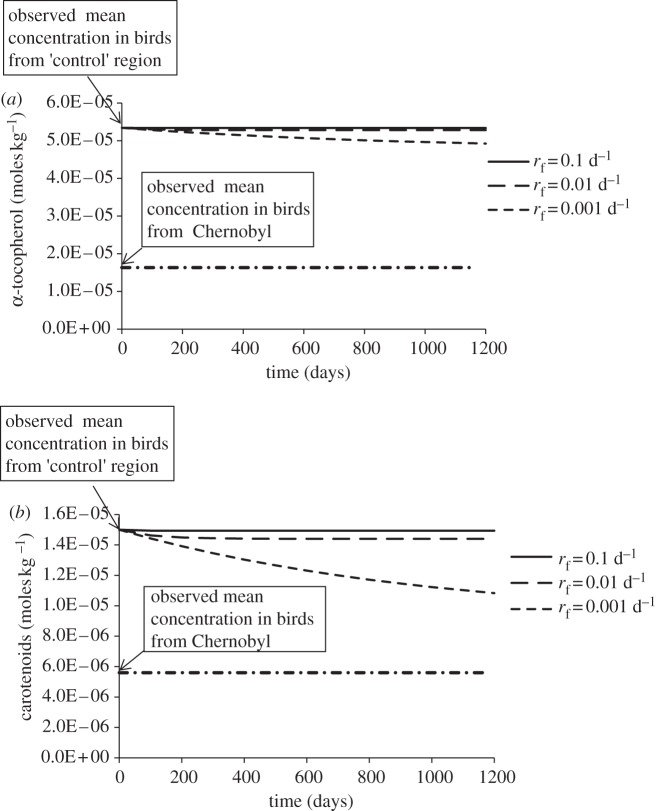
A study by Møller et al. [4] observed that barn swallow liver cell α-tocopherol and carotenoid concentrations decreased at sites near Chernobyl (contaminated with approx. 3.9 µGy h−1 external dose) compared with a control site. Equation (2.3) was used to determine whether radiolysis could account for these changes. Setting the equilibrium antioxidant concentration CTE to that of the control site, and assuming that the cell has minimal antioxidant capacity (i.e. no other enzymatic or non-enzymatic antioxidants operate in the cell), the change in concentration, CT, can be calculated. Figure 1 shows the effect of ionizing radiation of 417 µGy h−1 (much higher than the birds were exposed to; see electronic supplementary material) on cellular α-tocopherol and carotenoid concentrations for three different fractional rates of replenishment: 0.001, 0.01 and 0.1 d−1.
Figure 1.
Changes in (a) α-tocopherol and (b) carotenoids in birds' liver as a result of 417 µGy h−1 ionizing radiation (many times higher than the mean at the Chernobyl study sites; see electronic supplementary material). (Anti-oxidant concentrations were estimated from Møller et al. [4].)
(b). Effect of radiolysis on cellular redox potential
The effect of radiolysis on cellular redox potential was investigated by again assuming the cell has minimal antioxidant capacity, i.e. just GSH and no other enzymatic or non-enzymatic antioxidants. Equation (2.3) was used to determine reduction in GSH concentration as a function of time following chronic exposure to 417 µGy h−1 at low LET radiation. Equation (2.4) was used to calculate the impact of this change in the GSH–GSSG balance on the redox potential of the cell. We have here assumed a hypothetical GSH concentration of 1 mM and have calculated the GSH/GSSG concentration starting with 1 per cent conversion of GSH to GSSG. Glutathione concentrations in cells typically range from 1 to 11 mM [11–13]. Figure 2 shows the change in GSH concentration and cellular redox potential for 1200 days exposure to 417 µGy h−1 low LET radiation assuming an unrealistically low (0.001 d−1) replenishment rate of GSH.
Figure 2.
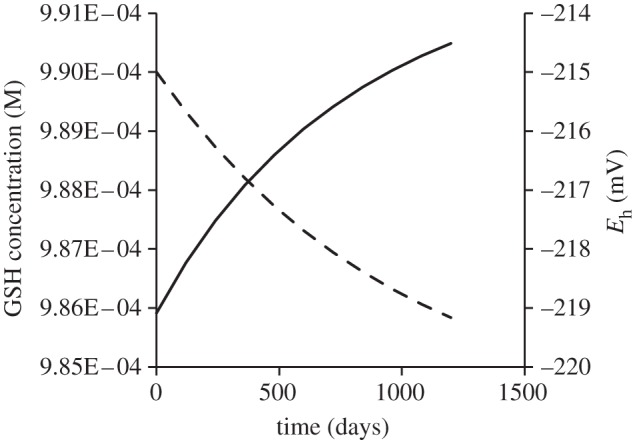
Predicted changes in glutathione (GSH) concentration (dashed line) and cellular redox potential (Eh) following 1200 day exposure to 10 mGy d−1 (417 µGy h−1) ionizing radiation assuming initial 1 mM total (GSH + GSSG) and an unrealistically slow glutathione replenishment rate of 0.001 d−1. Solid line, Eh (mV).
(c). Comparison of rate of production of reactive oxygen species with reactive oxygen metabolite concentrations
The rate of production of ROS from radiolysis (table 1) is compared with measurements from barn swallows at a contaminated (up to 2.9 µGy h−1) site at Chernobyl using data presented in Bonisoli-Alquati et al. [7]. Table 2 compares the daily rate of production of ROS by 417 µGy h−1 radiation with the difference in ROM between a contaminated and a control site. It can be seen (table 2) that daily rate of production of ROS by ionizing radiation represents a minuscule fraction (ca 10−5) of the difference in ROM between contaminated (external dose rate of 2.9 µGy h−1) and control sites observed by Bonisoli-Alquati et al. [7]. As this study [7] measured only hydroperoxides, the difference between radiation-induced ROS production and ROM would in reality be greater.
Table 1.
Rate of production (mol kg−1 s−1) of radiolysis products of water at different exposures to γ- and high-energy β-radiation. (A typical cellular water content of θ = 0.8 is assumed.)
| dose rate (mGy d−1) | dose rate (µGy h−1) | H2 | H2O2 | 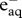 |
H· | OH· | HO2· | ΣROS |
|---|---|---|---|---|---|---|---|---|
| 1 | 41.7 | 4.35E–16 | 6.76E–16 | 2.59E–15 | 5.74E–16 | 2.59E–15 | 2.5E–17 | 6.9E–15 |
| 10 | 417 | 4.35E–15 | 6.76E–15 | 2.59E–14 | 5.74E–15 | 2.59E–14 | 2.5E–16 | 6.9E–14 |
| 100 | 4170 | 4.35E–14 | 6.76E–14 | 2.59E–13 | 5.74E–14 | 2.59E–13 | 2.5E–15 | 6.9E–13 |
Table 2.
Daily production of ROS by radiolysis at 10 mGy d−1 (417 µGy h−1) compared to differences in ROM (concentration of hydroperoxides) in the plasma of barn swallows between contaminated and control sites (data from [7]).
| sex | ROM mM H2O2 equivalents |
||||
|---|---|---|---|---|---|
| contaminated (ca 3 µGy h−1) | control | difference (ΔROM) | rate of production of ROS (mM d−1) | rate of production of ROS | |
| ΔROM (d−1) | |||||
| males | 2.45 | 2.03 | 0.42 | 5.96 × 10−6 | 1.42 × 10−5 |
| females | 2.92 | 1.97 | 0.95 | 5.96 × 10−6 | 6.27 × 10−6 |
4. Discussion
We calculated the direct effects of radiolysis on antioxidant concentrations at a total (external + internal) radiation dose rate of 417 µGy h−1 low LET radiation, representative of the highest doses to organisms in the Chernobyl zone (see electronic supplementary material) and also relevant to current contamination densities and dose rates at Fukushima [14]. No significant changes in antioxidant concentrations or cellular redox potential were calculated. Assuming that only single antioxidants were used to reduce radiolysis products, and that fractional replenishment rates were as (unrealistically) low as 0.001 d−1, antioxidant concentrations observed in ‘control’ birds are not reduced to those of exposed birds over 1200 days (figure 1). Differences in ROM between contaminated and control sites [7] cannot be explained by direct effects of radiolysis because the observed differences are orders of magnitude larger than the rate of production of ROS by radiolysis. The functional replenishment rate of glutathione in a range of animal tissues under different dietary conditions ranges from 10 per cent to 100 per cent per day [15] highlighting how conservative our assumptions are. Furthermore, Schafer & Buettner [11] suggest that changes in redox potential that cause cell changes including, sequentially, proliferation, differentiation, apoptosis and necrosis need to be of order of 60 mV, whereas changes calculated here are less than 5 mV over a 1200 day period.
We note that despite the minor direct impact of radiation on redox status of the cell and on antioxidant concentrations, it is well known that even low dose ionizing radiation can cause negative effects via DNA damage. Such damage is direct—caused by strand breaks and deletions—or indirect, from the free-radical products of water radiolysis in the immediate vicinity of nucleotides. At dose rates of order of 417 µGy h−1 (representing the most contaminated parts of the Chernobyl exclusion zone), radiation effects on organisms would be expected, and have indeed been observed [16,17]. The present study shows that observed effects are unlikely to be due to radiolysis products directly causing oxidative stress, significantly clarifying discussions about low-level radiation and oxidative stress. Thus, while some radiation effects on organisms are likely (although see, for example, Wickliffe et al. [18]) at dose rates pertaining in the most contaminated sites at Chernobyl, the results of our study do not support the hypothesis [4,7,8] that direct oxidizing stress is the damage mechanism. It may also help us to explain the variety and inconsistency of radiation-induced antioxidant responses apparently observed at low doses. Although not directly tested against data from organisms other than birds, these results are also likely to apply to other organisms: direct generation of radiolysis products is not nearly high enough to affect oxidative stress even though there is variation in antioxidative capacity between different organisms. Some of the studies on birds take account of habitat differences between sites of different contamination level, but it seems more probable that differences in habitat, diet or ecosystem structure are associated with changed antioxidant concentrations rather than the direct effects of radiolysis products.
References
- 1.Monaghan P., Metcalfe N. B., Torres R. 2009. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol. Lett. 12, 75–92 10.1111/j.1461-0248.2008.01258.x (doi:10.1111/j.1461-0248.2008.01258.x) [DOI] [PubMed] [Google Scholar]
- 2.Weiss J. F., Landauer M. R. 2003. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology 189, 1–20 10.1016/S0300-483X(03)00149-5 (doi:10.1016/S0300-483X(03)00149-5) [DOI] [PubMed] [Google Scholar]
- 3.Esnault M.-A., Legue F., Chenal C. 2010. Ionizing radiation: advances in plant response. Environ. Exp. Bot. 68, 231–237 10.1016/j.envexpbot.2010.01.007 (doi:10.1016/j.envexpbot.2010.01.007) [DOI] [Google Scholar]
- 4.Møller A. P., Surai P., Mousseau T. A. 2005. Antioxidants, radiation and mutation as revealed by sperm abnormality in barn swallows from Chernobyl. Proc. R. Soc. B 272, 247–253 10.1098/rspb.2004.2914 (doi:10.1098/rspb.2004.2914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaka R., Vandecasteele C. M., Misset M. T. 2002. Effects of low chronic doses of ionizing radiation on antioxidant enzymes and G6PDH activities in Stipa capillata (Poaceae). J. Exp. Bot. 53, 1979–1987 10.1093/jxb/erf041 (doi:10.1093/jxb/erf041) [DOI] [PubMed] [Google Scholar]
- 6.Vandenhove H., Vanhoudt N., Cuypers A., van Hees M., Wannijn J., Horemans N. 2010. Life-cycle chronic gamma exposure of Arabidopsis thaliana induces growth effects but no discernable effects on oxidative stress pathways. Plant Physiol. Biochem. 48, 778–786 10.1016/j.plaphy.2010.06.006 (doi:10.1016/j.plaphy.2010.06.006) [DOI] [PubMed] [Google Scholar]
- 7.Bonisoli-Alquati A., Mousseau T. A., Møller A. P., Caprioli M., Saino N. 2010. Increased oxidative stress in barn swallows from the Chernobyl region. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 155, 205–210 10.1016/j.cbpa.2009.10.041 (doi:10.1016/j.cbpa.2009.10.041) [DOI] [PubMed] [Google Scholar]
- 8.Bonisoli-Alquati A., Møller A. P., Rudolfsen G., Saino N., Caprioli M., Ostermiller S., Mousseau T. A. 2011. The effects of radiation on sperm swimming behavior depend on plasma oxidative status in the barn swallow (Hirundo rustica). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 159, 105–112 10.1016/j.cbpa.2011.01.018 (doi:10.1016/j.cbpa.2011.01.018) [DOI] [PubMed] [Google Scholar]
- 9.Choppin G. R., Liljenzin J.-O., Rydberg J. 1995. Radiochemistry and nuclear chemistry. Oxford, UK: Butterworth-Heinemann [Google Scholar]
- 10.Hancock J. T., Desikan R., Neill S. J., Cross A. R. 2004. New equations for redox and nano-signal transduction. J. Theor. Biol. 226, 65–68 10.1016/j.jtbi.2003.08.003 (doi:10.1016/j.jtbi.2003.08.003) [DOI] [PubMed] [Google Scholar]
- 11.Schafer F. Q., Buettner G. R. 2001. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 30, 1191–1212 10.1016/S0891-5849(01)00480-4 (doi:10.1016/S0891-5849(01)00480-4) [DOI] [PubMed] [Google Scholar]
- 12.Meyer A. J., May M. J., Fricker M. 2001. Quantitative in vivo measurement of glutathione in Arabidopsis cells. Plant J. 27, 67–78 10.1046/j.1365-313x.2001.01071.x (doi:10.1046/j.1365-313x.2001.01071.x) [DOI] [PubMed] [Google Scholar]
- 13.Smith C. V., Jones D. P., Guenthner T. M., Lash L. H., Lauterburg B. H. 1996. Compartmentation of glutathione: implications for the study of toxicity and disease. Toxicol. Appl. Pharmacol. 140, 1–12 10.1006/taap.1996.0191 (doi:10.1006/taap.1996.0191) [DOI] [PubMed] [Google Scholar]
- 14.Hosoda M., Tokonami S., Sorimachi A., Monzen S., Osanai M., Yamada M., Kasiwakura I., Akiba S. 2011. The time variation of dose rate artificially increased by the Fukushima nuclear crisis. Sci. Rep. 1, 87. 10.1038/srep00087 (doi:10.1038/srep00087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malmezat T., Breuille D., Capitan P., Mirand P. P., Obled C. 2000. Glutathione turnover is increased during the acute phase of sepsis in rats. J. Nutr. 130, 1239–1246 [DOI] [PubMed] [Google Scholar]
- 16.Chesser R. K., et al. 2000. Concentrations and dose rate estimates of 134137Cesium and 90Strontium in small mammals at Chernobyl, Ukraine. Environ. Toxicol. Chem. 19, 305–312 [Google Scholar]
- 17.Geras'kin S. A., Fesenko S. V., Alexakhin R. M. 2008. Effects of non-human species irradiation after the Chernobyl NPP accident. Environ. Int. 34, 880–897 10.1016/j.envint.2007.12.012 (doi:10.1016/j.envint.2007.12.012) [DOI] [PubMed] [Google Scholar]
- 18.Wickliffe J. K., Bickham A. M., Rodgers B. E., Chesser R. K., Phillips C. J., Gaschak S. P., Chizhevsky I., Boker R. J. 2003. Exposure to chronic, low-dose rate γ-radiation at Chernobyl does not induce point mutations in Big Blue mice. Environ. Mol. Mutagen. 42, 11–18 10.1002/em.10170 (doi:10.1002/em.10170) [DOI] [PubMed] [Google Scholar]