Abstract
Familiarity plays an important role in the evolution of sociality and cooperation. Familiar individuals may gain a reputation for participating in, or defecting from, cooperative tasks. Previous research suggests that long-term familiarity with territorial neighbours benefits breeders. We tested the hypothesis that great tits (Parus major) are more likely to join in neighbours' nest defence if those neighbours are familiar from the previous year. We show that neighbours that shared a territory boundary the previous year are more likely to join their neighbours' nest defence than neighbours that did not share a boundary before. Closer neighbours did not differ from distant neighbours in their latency to join. For familiar neighbours that joined, there was no difference in call rate in relation to whether one or both members of the focal pair were familiar. First-time breeders (by definition unfamiliar) did not join each other's nest defence. This is the first evidence of a relationship between familiarity and joining in nest defence. Such direct benefits of familiarity may have important implications in the evolution of sociality.
Keywords: cooperation, nest defence, Parus major, familiarity, mobbing
1. Introduction
The social environment is important not only to group-living animals, but also to territorial species that are generally considered to have limited social interactions. Stable relationships with territorial neighbours are expected to lead to reduced aggression [1], and repeated interactions with the same individuals may aid the evolution of cooperation [2].
For many bird species, nest predation has a major impact on nesting success and hence, fitness [3,4]. Nest defence in passerine birds usually takes the form of predator mobbing. Mobbing involves an attack on a predator by prey individuals in order to drive it away. It is widespread among vertebrates, including fish, birds and mammals [5–7]. The more individuals participate in a mob, the higher the chance of deterring the predator [8,9]. Joint predator mobbing in territorial neighbours has been documented in passerine birds [10] including great tits [11].
Stable territorial relationships benefit breeders through reduced aggression between neighbours (known as the ‘Dear Enemy Phenomenon’ [12]). They may also benefit from undirected, mutually beneficial actions, such as alarm calls in response to predators [13]. There is some debate as to whether joint mobbing is driven by reciprocity [10,14], or by-product mutualism (sensu [15]). If it is driven by reciprocity, stable, long-term neighbours may benefit from reputations built up over repeated interactions. Great tits (Parus major) join group mobs in winter [16] and mob at nests of other pairs during breeding [11]. Recent findings suggest that long-term familiarity improves reproductive success in the great tit [17]. In this experiment, we tested the hypothesis that long-term familiarity between territorial neighbours is positively related to joining behaviour in predator mobbing. We then ask within the individuals that did join, how does distance affect latency to join and how does the degree of familiarity affect intensity of mobbing behaviour (call rate)?
2. Material and methods
(a). Field protocol
The experiment took place in Wytham Woods, Oxfordshire between 12 and 27 May 2011. A population of great tits breeding in nest-boxes was monitored during the breeding season as in Perrins [18]. Breeder identities were determined when the young were 8–12-days old (hatching = day 1) from a previously fitted metal British Trust for Ornithology ring or electronic passive integrated transponder tag. Age was determined from plumage [19] as first year or older than 1 year. Trials were performed blind with respect to neighbour familiarity status to avoid researcher bias. This was achieved by assessing pairs of nests for familiarity of the breeders that were older than 1 year after the trials took place. Pairs of neighbouring nests consisted of three groups:
— Familiar. Adults were older than 1 year and at least one member from each nest had been neighbours in the previous year.
— Unfamiliar. Adults were older than 1 year, none of which had been neighbours in the previous year.
— First year. Adults were 1 year old and therefore were unfamiliar under our definition.
Trials were performed when nestlings were 17 days old (mean:16.5 days; s.e.: 0.245), or as close to 17 days as possible, if nest asynchrony posed a risk that one nest would fledge before the trial. First, the experimenter (A.M.G.-Z.) verified that nestlings were alive and had not fledged. The adults were then marked temporarily with non-toxic, acrylic paint in order to identify which nest-box they came from (adapted from Krams et al. [10]). The paint was applied to a piece of adhesive insulation foam placed inside the nest-box entrance, so that the birds would mark themselves when entering and leaving the nest-box. The birds exhibited little neophobia towards the marking foam, and feeding continued without interruption. Once marking on all birds was confirmed by observation, the trials took place. In the trials, the experimenter stood directly beneath the box, which hung from the tree 2–3 m above the ground. On approach, the experimenter noisily moved dead leaves on the ground with her feet, then scraped the bark of the tree with a wooden pole and, finally, scraped the pole against the woodcrete nest-box, a sound that often elicits alarm calls from parents during routine nest monitoring (A. M. Grabowska-Zhang, personal observation). The sequence of movements was designed to imitate the sounds of approach, interest in the nest and an attempt to enter the nest. Previous studies have shown a strong correlation between avian responses to humans and model predators [20]. Also, parent birds that respond strongly to humans suffer lower nest predation [21,22] and male great tits in our study population have a similar response to human observer together with chick distress call as they do to a model predator [23]. Great tits often mob humans when they ring chicks (A. M. Grabowska-Zhang, personal observation) or monitor the nest. The trial started when at least one of the parents at the focal nest started mobbing (this occurred in every trial), and lasted for 5 min. Mobbing in great tits involves emitting repeated alarm calls, pivoting on the perch, frequent hops between perches while approaching the predator and, sometimes, exaggerated flights. Mobbing by neighbours was recorded when the bird was heard (and subsequently seen) or seen and could be identified as belonging to the marked nest-box during observation. Latency to join and count of neighbour calls in the second minute after joining was recorded. The trial was repeated at the neighbouring nest after a period of about 1 h (mean: 63.8 min; s.e.: 2.3 min).
After the trials, distances between boxes were calculated from nest-box GPS coordinates using MapInfo v. 8.5 software. Breeding territories were estimated using Dirichlet tessellation, which has been shown to approximate measures obtained with mapped territories [24]. Territories of experimental birds were matched against their territories in 2010, to verify between-year familiarity through being neighbours.
(b). Statistical methods
We used R (v. 2.10.1) [25] for statistical analyses. We tested for differences between groups using parametric and non-parametric tests (detailed in §3). We modelled the latency to join using a Cox proportional hazards (PH) model. To avoid pseudoreplication, one observation from each nest pair was randomly removed, and the model was applied to the reduced dataset. We repeated the randomization and the model 1000 times to obtain mean parameter estimates and their confidence intervals. We tested for differences in call rate of neighbours that joined depending on the degree of familiarity. Responding birds were compared in two groups depending on whether they were familiar with one or both individuals in the focal nest. Unfamiliar birds that joined were excluded from the analysis, as that occurred only twice in the dataset.
3. Results
(a)The. occurrence of joining
For pairs of nests where each contained at least one familiar individual, in 12 out of 16 trials (seven out of eight nest pairs), at least one neighbour joined the mob. Individuals from the unfamiliar group joined the mob in just two out of 16 trials (one out of eight nest pairs). No neighbours joined the mob in first-years' nests (figure 1). Fisher's exact probability test [26] yielded p < 0.001. No unmarked great tits were observed to mob during the trials (see the electronic supplementary material). Mean distances between pairs of nests did not differ between groups (ANOVA: F2, 21 = 1.04, p = 0.372).
Figure 1.
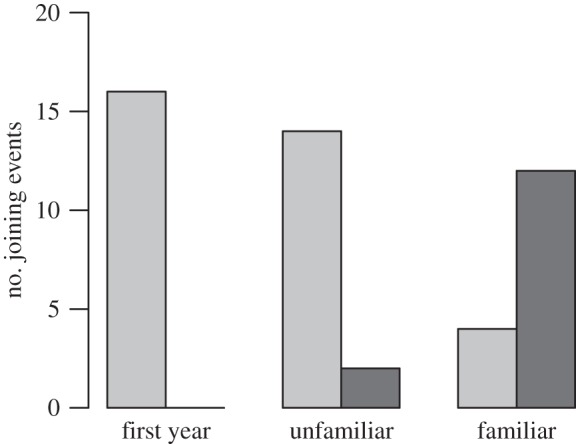
Outcomes of the trials. ‘First year’ refers to nests (eight pairs) where all adults were 1 year old breeders. ‘Unfamiliar’ nests (eight pairs) contained adults older than 1 year that had not been neighbours the year before. ‘Familiar’ nests (eight pairs) had adults older than 1 year and at least one member from each nest had been neighbours the year before. Light grey bars, did not join; dark grey bars, joined.
(b). Latency to join
We asked whether, among birds that joined (n = 14), distance affects time to joining. The Cox PH model yielded no evidence that neighbours from more distant nests joined nest defence later than closer neighbours (table 1).
Table 1.
Average parameter estimates and their CI from 1000 iterations of Cox PH model for the effects of distance on the latency to join.
| coefficient | parameter estimate | lower CI | upper CI |
|---|---|---|---|
| distance | −0.0139 | −0.0552 | 0.0906 |
(c). Neighbour call rate
Neighbour alarm call rate was compared between birds familiar with one or both individuals from the focal nest. There was no difference in calls per minute between the two groups (Mann–Whitney test: U = 4, n1 = 8, n2 = 4, p = 0.52).
4. Discussion
We demonstrate a significant influence of prior familiarity on joining behaviour during mobbing. Birds from familiar nests were more likely to join than neighbours from unfamiliar nests. Within the familiar nest pairs that joined, call rate did not differ between individuals that joined one or two familiar individuals. Hence, we have no evidence that the intensity of mobbing behaviour is affected by the degree of familiarity, although a small sample size limits the power of this analysis. Overall, these results indicate clear differences in behaviour towards familiar individuals. Joining in nest defence may be one of the mechanisms underlying the higher reproductive success of great tits that have familiar neighbours [17].
Familiar neighbours may have had more interactions with each other over time, and therefore more opportunity to build up a good reputation, which theoretically [2] aids the evolution of cooperation. While we cannot state why first-year birds never joined mobbing, we show that mobs of first-time breeders do not elicit a joining response. Recent work suggests that social network structure affects the likelihood of reciprocity being stable in a population [27]. First-time breeders spent the winter dispersing from their natal area, unlike the more sedentary older birds, and as newcomers to the settlement area, their relationships with neighbours may be less stable. Further work could investigate the social interactions of adults during winter flocking, to see whether associations are more likely to give rise to joining behaviour.
Overall, distant and close joining neighbours did not differ in latency to join. This suggests that variation in neighbour distance does not affect the neighbour's travel time towards the mob or delay between arrival near the neighbours' nest and joining the mob. This effect suggests by-product mutualism may not be operating here. Mutualism stems from an ultimately selfish behaviour; birds join their neighbours because their own nest is close enough to be at risk. Under this scenario, distant neighbours would invest less in joining than close neighbours. It is possible that the variation in distance in our sample was not sufficient to detect a distance effect, as pairs of nests were on average 70.1 m apart (s.e.: 4.7 m), perhaps too close for distance to affect the result.
We found that familiar neighbours were more likely to join in mobbing an intruder on an adjacent territory, and conclude that familiarity is important in social interactions. While determining the mechanism responsible for joining was not the main aim of our study, we cannot exclude the possibility that an interplay between selfish mutualistic responses and reciprocity-based reactions is involved in the system. Future studies may be able to tease apart the underlying processes.
Acknowledgements
This research was funded by NERC through a PhD studentship to A.M.G.-Z., and by ERC grant ADG 250164 to B.C.S. We thank Andy Gosler and John Quinn for advice on experimental design, and Indrikis Krams and two anonymous referees for comments.
References
- 1.Temeles E. J. 1994. The role of neighbors in territorial systems: when are they dear enemies. Anim. Behav. 47, 339–350 10.1006/anbe.1994.1047 (doi:10.1006/anbe.1994.1047) [DOI] [Google Scholar]
- 2.Trivers R. L. 1971. The evolution of reciprocal altruism. Q. Rev. Biol. 46, 35–57 10.1086/406755 (doi:10.1086/406755) [DOI] [Google Scholar]
- 3.Ricklefs R. E. 1969. An analysis of nesting mortality in birds. Smithson. Contrib. Zool. 9, 1–48 10.5479/si.00810282.9 (doi:10.5479/si.00810282.9) [DOI] [Google Scholar]
- 4.McCleery R. H., Perrins C. M. 1991. Effects of predation on the numbers of great tits Parus major. In Bird population studies: relevance to conservation and Management (eds Perrins C. M., Lebreton J.-D., Hirons G. M.), pp. 129–147 Oxford, UK: Oxford University Press [Google Scholar]
- 5.Graw B., Manser M. B. 2007. The function of mobbing in cooperative meerkats. Anim. Behav. 74, 507–517 10.1016/j.anbehav.2006.11.021 (doi:10.1016/j.anbehav.2006.11.021) [DOI] [Google Scholar]
- 6.Curio E. 1978. Adaptive significance of avian mobbing. I. Teleonomic hypotheses and predictions. J. Comp. Ethol. 48, 175–183 10.1111/j.1439-0310.1978.tb00254.x (doi:10.1111/j.1439-0310.1978.tb00254.x) [DOI] [Google Scholar]
- 7.Pitcher T. J., Green D. A., Magurran A. E. 1986. Dicing with death: predator inspection behavior in minnow shoals. J. Fish. Biol. 28, 439–448 10.1111/j.1095-8649.1986.tb05181.x (doi:10.1111/j.1095-8649.1986.tb05181.x) [DOI] [Google Scholar]
- 8.Flasskamp A. 1994. The adaptive significance of avian mobbing. V. An experimental test of the move on hypothesis. Ethology 96, 322–333 10.1111/j.1439-0310.1994.tb01020.x (doi:10.1111/j.1439-0310.1994.tb01020.x) [DOI] [Google Scholar]
- 9.Pettifor R. A. 1990. The effects of avian mobbing on a potential predator, the European kestrel, Falco tinnunculus. Anim. Behav. 39, 821–827 10.1016/s0003-3472(05)80945-5 (doi:10.1016/s0003-3472(05)80945-5) [DOI] [Google Scholar]
- 10.Krams I., Krama T., Igaune K., Mand R. 2008. Experimental evidence of reciprocal altruism in the pied flycatcher. Behav. Ecol. Sociobiol. 62, 599–605 10.1007/s00265-007-0484-1 (doi:10.1007/s00265-007-0484-1) [DOI] [Google Scholar]
- 11.Zimmermann U., Curio E. 1988. Two conflicting needs affecting predator mobbing by great tits, Parus major. Anim. Behav. 36, 926–932 10.1016/s0003-3472(88)80175-1 (doi:10.1016/s0003-3472(88)80175-1) [DOI] [Google Scholar]
- 12.Fisher J. B. 1954. Evolution and bird sociality. In Evolution as a process (eds Huxley J., Hardy A. C., Ford E. B.), pp. 71–83 London, UK: Allen & Unwin [Google Scholar]
- 13.Clutton-Brock T. 2009. Cooperation between non-kin in animal societies. Nature 462, 51–57 10.1038/nature08366 (doi:10.1038/nature08366) [DOI] [PubMed] [Google Scholar]
- 14.Wheatcroft D. J., Krams I. 2009. Avian mobbing: byproduct mutualism not reciprocal altruism response. Trends Ecol. Evol. 24, 5–6 10.1016/j.tree.2008.09.002 (doi:10.1016/j.tree.2008.09.002) [DOI] [PubMed] [Google Scholar]
- 15.Russell A. F., Wright J. 2009. Avian mobbing: byproduct mutualism not reciprocal altruism. Trends Ecol. Evol. 24, 3–5 10.1016/j.tree.2008.09.003 (doi:10.1016/j.tree.2008.09.003) [DOI] [PubMed] [Google Scholar]
- 16.Hinde R. A. 1952. The behaviour of the great tit (Parus major) and some other related species. Behaviour 2, 1–201 [Google Scholar]
- 17.Grabowska-Zhang A. M., Wilkin T. A., Sheldon B. C. 2012. Effects of neighbor familiarity on reproductive success in the great tit (Parus major). Behav. Ecol. 23, 322–333 10.1093/beheco/arr189 (doi:10.1093/beheco/arr189) [DOI] [Google Scholar]
- 18.Perrins C. M. 1965. Population fluctuations and clutch-size in the great tit, Parus major L. J. Anim. Ecol. 34, 601–647 10.2307/2453 (doi:10.2307/2453) [DOI] [Google Scholar]
- 19.Svensson L. 1992. Identification guide to European passerines. Stockholm, Sweden: Bonniers [Google Scholar]
- 20.Eckert C. G., Weatherhead P. J. 1987. Male characteristics, parental quality and the study of mate choice in the red-winged blackbird (Agelaius phoeniceus). Behav. Ecol. Sociobiol. 20, 35–42 10.1007/bf00292164 (doi:10.1007/bf00292164) [DOI] [Google Scholar]
- 21.Andersson M., Wiklund C. G., Rundgren H. 1980. Parental defense of offspring: a model and an example. Anim. Behav. 28, 536–542 10.1016/s0003-3472(80)80062-5 (doi:10.1016/s0003-3472(80)80062-5) [DOI] [Google Scholar]
- 22.Greig-Smith P. W. 1980. Parental investment in nest defense by stonechats (Saxicola-torquata). Anim. Behav. 28, 604–619 10.1016/s0003-3472(80)80069-8 (doi:10.1016/s0003-3472(80)80069-8) [DOI] [Google Scholar]
- 23.Radford A. N., Blakey J. K. 2000. Intensity of nest defence is related to offspring sex ratio in the great tit Parus major. Proc. R. Soc. Lond. B 267, 535–538 10.1098/rspb.2000.1033 (doi:10.1098/rspb.2000.1033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkin T. A., Garant D., Gosler A. G., Sheldon B. C. 2006. Density effects on life-history traits in a wild population of the great tit Parus major: analyses of long-term data with GIS techniques. J. Anim. Ecol. 75, 604–615 10.1111/j.1365-2656.2006.01078.x (doi:10.1111/j.1365-2656.2006.01078.x) [DOI] [PubMed] [Google Scholar]
- 25.R Development Core Team 2005. R: a language and environment for statistical computing (2.10.1). Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 26.Freeman G. H., Halton J. H. 1951. Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika 38, 141–149 10.2307/2332323 (doi:10.2307/2332323) [DOI] [PubMed] [Google Scholar]
- 27.van Doorn G. S., Taborsky M. 2011. The evolution of generalized reciprocity on social interaction networks. Evolution 66, 651–664 10.1111/j.1558-5646.2011.01479.x (doi:10.1111/j.1558-5646.2011.01479.x) [DOI] [PubMed] [Google Scholar]


