Abstract
Given the physiological limits to egg size, large-bodied non-avian dinosaurs experienced some of the most extreme shifts in size during postnatal ontogeny found in terrestrial vertebrate systems. In contrast, mammals—the other dominant vertebrate group since the Mesozoic—have less complex ontogenies. Here, we develop a model that quantifies the impact of size-specific interspecies competition on abundances of differently sized dinosaurs and mammals, taking into account the extended niche breadth realized during ontogeny among large oviparous species. Our model predicts low diversity at intermediate size classes (between approx. 1 and 1000 kg), consistent with observed diversity distributions of dinosaurs, and of Mesozoic land vertebrates in general. It also provides a mechanism—based on an understanding of different ecological and evolutionary constraints across vertebrate groups—that explains how mammals and birds, but not dinosaurs, were able to persist beyond the Cretaceous–Tertiary (K–T) boundary, and how post-K–T mammals were able to diversify into larger size categories.
Keywords: allometry, body mass, Mesozoic vertebrates, size-specific competition
1. Introduction
Dinosaurs and mammals have successively dominated terrestrial life for more than 200 Myr. Yet, they differ in the most fundamental biological trait—reproduction, with dinosaurs being oviparous, and mammals viviparous. A peculiar constraint on oviparous taxa is that offspring (total clutch sizes) are very small relative to adults (compared with similar-sized viviparous taxa) [1,2]. This occurs because of upper limits to eggshell thickness (the shell must be sufficiently thin to allow gaseous exchange) [3,4]. Not surprisingly, scaling exponents from adult–neonate mass allometries are lower among extant herpetofauna and birds (approx. 0.4–0.7) than mammals (approx. 0.8–1) (reviewed in [5]). Among extinct dinosaurs, the adult-to-neonate mass ratio estimated for a approximately 4 tonne titanosaur was 2500 : 1, over two orders of magnitude greater than that of the Asian elephant, Elephas maximus [1].
Thus, dinosaurs have more complex ontogenetic life histories than similar-sized mammals, implying more extensive ecological niche shifts through their development [6]. Previously, it was hypothesized that the ability of dinosaurs to disperse into a wider variety of niches, coupled with higher reproductive rates [7], meant their populations were more resilient to environmental perturbations, which played a large part in their dominance of terrestrial life for ca 180 Myr [2].
However, wider intraspecific niche breadths imply more interspecific niche overlaps, hence greater potential for competition [6]. This competition should be especially pronounced in assemblages comprising very large taxa, whose offspring are considerably smaller than the adults. Here, we develop a simple, deterministic model to explore the influence of size-specific competition on populations of differently sized dinosaurs and mammals, and its implications for body size distributions of the dominant terrestrial vertebrate groups of the Mesozoic and Cenozoic.
2. Material and methods
Our simulated dinosaur assemblage comprises species from 27 size categories (populations), from log2M = −9 to 17 (approx. 2 g to 131 tonnes). Our mammal assemblage comprises 24 categories (up to log2M = 14, approx. 16 tonnes). These ranges represent the smallest and largest estimated body masses of extinct dinosaurs and mammals, respectively [8,9]. Each population was structured according to size classes of log2M increments from neonate to adult, where Mneonate was estimated from Madult using allometric equations (scaling exponents are 0.6 for dinosaurs, and 0.9 for mammals; see above). Taking into account inter- and intraspecific allometric effects on size-specific mortality, reproductive output [5] and abundance [10] (see electronic supplementary material, part A), our model estimates changes in population abundances owing to competition-induced mortalities among similarly sized individuals. Competition is strictly interspecific, in that abundances of each mass class are reduced by the frequency occurrence of that class among other populations in the assemblage, weighted by the Lotka–Volterra competition coefficient α. Values for α are non-empirical, simply reflecting the number of individuals of a mass class assumed to die owing to competition from one other individual of that class. We defined unique α values for interactions among dinosaurs (αDD) or mammals (αMM), and among each other (αDM and αMD). Finally, we explore implications of a mass extinction event, such as that occurred at the Cretaceous–Tertiary (K–T) boundary, which primarily affected large-bodied land animals [11,12]. To mimic the K–T, we set initial conditions to exclude all individuals larger than an arbitrary mass threshold of 25 kg.
3. Results
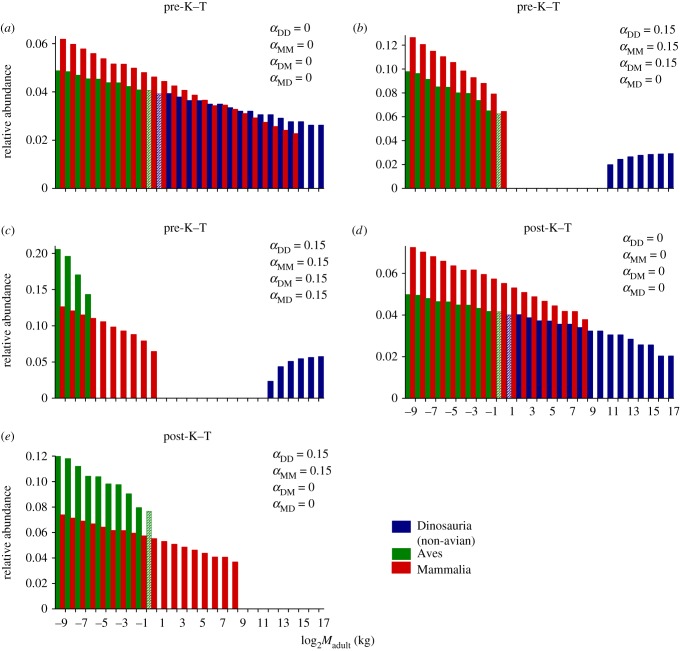
The ecological relevance of relatively small offspring in dinosaurs is most pronounced among larger mass classes. Around the mass range where increases in dinosaur Madult no longer result in major increases in Mneonate, simulated populations include substantially more ontogenetic niche steps, and interspecific size (niche) overlaps, compared with similarly sized mammals (see electronic supplementary material, figure S2). The impact on model outcomes is clear: while species abundances decrease steadily with increases in Madult (figure 1a), size-specific competition (positive αDD) reduces population abundances of intermediate-sized (approx. 1–1000 kg) dinosaur species towards extinction (figure 1b). Niche overlap frequencies are reduced among the largest taxa, because some individuals escape the competition trap, and so their populations persist even when faced with competition. Mammals, with fewer interspecific niche overlaps, do not suffer population extinctions in any mass range (for αMM = αDD). Large mammal species are, however, excluded by competition from dinosaur individuals of similar masses (positive αDM; figure 1b). Interestingly, when competition from mammals on dinosaurs is included (positive αMD), populations of small-bodied dinosaur species also experience the greatest losses (figure 1c). This probably occurs because at small Madult, even subtle differences in ontogenetic complexity between viviparous and oviparous groups equate to slightly higher competition pressure on dinosaurs.
Figure 1.
Modelled relative population abundances of dinosaur and mammal species distributed along a mass gradient: (a) without competition, abundance scales negatively with Madult; (b) size-specific competition among dinosaurs (αDD) causes extinctions in the intermediate mass range, but not among mammals (αMM)—mammals are excluded from larger mass categories by competition from dinosaurs (αDM); and (c) competition from mammals on dinosaurs (αMD) contributes to extinctions of small-bodied dinosaurs. Post-K–T scenarios, i.e. initially excluding all individuals above an extinction mass threshold of 25 kg: (d) with no competition dinosaur populations have higher recovery rates than mammals, but (e) when competition is operating, only mammals and small dinosaur populations re-establish. Different colours for dinosaurs and birds are presented only for visual effect (dashed bars represent the mass range where Mesozoic non-avian dinosaurs and birds overlap in figure 2).
Under a scenario without competition, our simulation mimicking K–T extinctions predicts better recovery rates for large dinosaurs than mammals (figure 1d), because the small Mneonate of the former ensures that even the largest species comprised mass classes beneath the extinction mass threshold. This result is consistent with the idea that large-bodied dinosaurs were more resilient to environmental perturbation than similar-sized mammals, because their small offspring facilitated higher reproductive rates [2]. However, with size-specific competition among surviving individuals, dinosaur populations fail to re-establish at larger size classes (figure 1e). Under this scenario, mammal populations are able to re-establish over a range of mass categories, and even diversify into larger mass categories (assuming zero competition from the ‘now extinct’ dinosaurs, i.e. αDM = 0).
4. Discussion
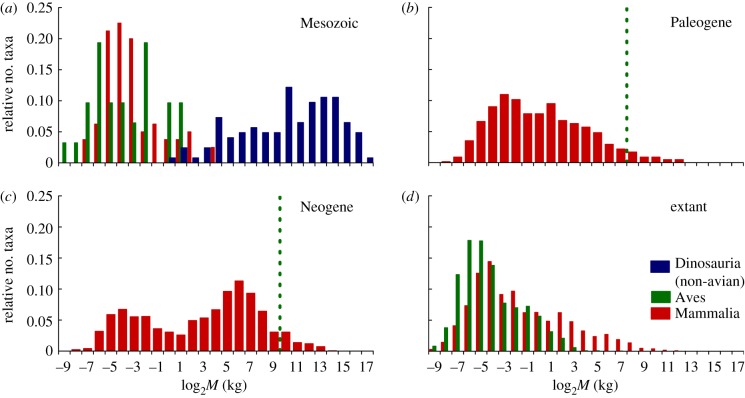
These results, in particular that abundances of intermediate-sized (approx. 1–1000 kg) dinosaurs decrease at a faster rate than similarly sized mammals, are robust to changes in a variety of life-history parameters, including differences in survivorship and reproductive output (see electronic supplementary material, part B). Thus, we expect that, within terrestrial assemblages featuring large-bodied, oviparous animals, size-specific competition among species resulted in dominance of the largest taxa, because they include life stages that escape such competition traps. Small taxa (less than 1 kg) may be successful because their shorter life stages entail fewer niche overlaps among species, and because small animals can more easily escape competition by exploiting a wider diversity of niches [13]. These findings are broadly consistent with fossil evidence for body mass–species richness (M–S) distributions in the Mesozoic, which are characterized by disproportionately high diversity among larger mass classes (principally non-avian dinosaurs), and a paucity of intermediate-sized species (figure 2a) [8,14]. Small size classes were occupied mainly by mammals and birds, which reached a maximum mass of approximately 20–30 kg despite relatively high taxonomic diversity [15,16]. Among non-avian dinosaurs, dominance of large taxa is especially evident among sauropods, a group in which gigantism is an evolutionary hallmark, with species smaller than 4–5 tonnes being almost entirely restricted to island dwarf forms [8]. The gap in the intermediate range may be due to sampling or taphonomic bias, e.g. underrepresentation of small ornithischians and theropods (but see Carrano [17]), or was filled by another vertebrate group. However, to account for the observed gap, up to 98 per cent of ‘missing’ taxa would have to be in the less than 1000 kg mass range (table 1), supporting that the observed gap is a realistic representation of Mesozoic assemblages.
Figure 2.
Body mass–species richness distributions of (a) non-avian dinosaurs, birds and mammals of the Mesozoic (b,c) mammals of the Cenozoic; and (d) extant mammals and birds. (b,c) Dashed green lines indicate the upper mass class occupied by dinosaurs (birds) since the K–T. Species richness of each mass category is presented relative to that of the whole group (for data compilation, see electronic supplementary material, part C).
Table 1.
Simulated assemblage composition needed to shift the Mesozoic terrestrial vertebrate M–S pattern from bimodal (figure 2a) to a more typical right-skewed distribution (details given in electronic supplementary material, part C).
| mass range of taxa (kg) | observed |
additional taxa needed for right-skew distribution |
||
|---|---|---|---|---|
| number of taxa | assemblage (%) | number of taxa | assemblage (%) | |
| <1 | 93 | 39.7 | 106 | 45.3 |
| 1–1000 | 80 | 34.2 | 124 | 53.0 |
| combined <1000 | 173 | 73.9 | 230 | 98.3 |
| >1000 | 61 | 26.1 | 4 | 1.7 |
Size-specific competition with dinosaurs also probably excluded Mesozoic mammals from larger mass classes. Our results mimic post-K–T diversification of mammals into larger mass classes in the Paleogene through the Cenozoic (figure 2b,c) [18]—mammals only contracted to present-day distributions later with Quaternary climate change and spread of humans (figure 2d). Cenozoic vertebrate distributions were never as skewed towards larger taxa. A narrow size gap (approx. 0.5–32 kg) for the Neogene, similar to the anomalous approximately 0.25–4 kg gap hypothesized for extant mammals [19], emerges only owing to an abundance of large Carnivora in our datasets.
Whether extreme size shifts—a consequence of disproportionately small eggs/neonates [1,3,8], as well as limited parental care and absence of suckling—in dinosaurs translated into ecological niche shifts during ontogeny is uncertain, but this phenomenon is common today [6] among, inter alia, herpetiles, birds and mammals, including the largest herbivore, the African elephant, Loxodonta africana [20]. The resultant size-specific competition could easily have precluded non-avian dinosaurs from recovery following catastrophic K–T events, because if extinctions mainly affected animals above a mass threshold [11,12], then they simply did not have sufficient diversity below this threshold to refill their niches. Small dinosaurs were also limited by competition pressure, including from small mammals (as it is, many adopted a fundamentally different, airborne, niche). Even though larger dinosaurs re-established at various stages of the Cenozoic in the form of terror birds and certain ratites [21] (figure 2b,c), they never again reached masses needed to escape size-specific competition. Because mammals were never limited across the critical mass range, population recovery after the K–T crisis [18] facilitated contemporary dominance of viviparity over oviparity among terrestrial vertebrates.
Acknowledgements
This is contribution no. 123 the DFG Research Unit 533 The Biology of Sauropod Dinosaurs. We thank Martin Sander, Matt Sponheimer, Jacqueline Codron and two anonymous reviewers for valuable comments.
References
- 1.Sander P. M., Peitz C. 2008. Upper Cretaceous titanosaur nesting sites and their implications for sauropod dinosaur reproductive biology. Palaeontogr. Abt. A Palaeozool.-Stratigr. 284, 69–107 [Google Scholar]
- 2.Janis C. M., Carrano M. 1992. Scaling of reproductive turnover in archosaurs and mammals: why are large terrestrial mammals so rare? Ann. Zool. Fenn. 28, 201–216 [Google Scholar]
- 3.Seymour R. S. 1979. Dinosaur eggs: gas conductance through the shell, water loss during incubation and clutch size. Paleobiology 5, 1–11 [Google Scholar]
- 4.Ar A., Rahn H., Paganelli C. V. 1979. The avian egg: mass and strength. Condor 81, 331–337 10.2307/1366955 (doi:10.2307/1366955) [DOI] [Google Scholar]
- 5.Hendriks A. J., Mulder C. 2008. Scaling of offspring number and mass to plant and animal size: model and meta-analysis. Oecologia 155, 705–716 10.1007/s00442-007-0952-3 (doi:10.1007/s00442-007-0952-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werner E. E., Gilliam J. F. 1984. The ontogenetic niche and species interactions in size-structured populations. Annu. Rev. Ecol. Syst. 15, 393–425 10.1146/annurev.es.15.110184.002141 (doi:10.1146/annurev.es.15.110184.002141) [DOI] [Google Scholar]
- 7.Werner J., Griebeler E. M. 2011. Reproductive biology and its impact on body size: comparative analysis of mammalian, avian and dinosaurian reproduction. PLoS ONE 6, e28442. 10.1371/journal.pone.0028442 (doi:10.1371/journal.pone.0028442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sander P. M., et al. 2011. Biology of the sauropod dinosaurs: the evolution of gigantism. Biol. Rev. 86, 117–155 10.1111/j.1469-185X.2010.00137.x (doi:10.1111/j.1469-185X.2010.00137.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sander P. M., Clauss M. 2008. Sauropod gigantism. Science 322, 200–201 10.1126/science.1160904 (doi:10.1126/science.1160904) [DOI] [PubMed] [Google Scholar]
- 10.Damuth J. 1981. Population density and body size in mammals. Nature 290, 699–700 10.1038/290699a0 (doi:10.1038/290699a0) [DOI] [Google Scholar]
- 11.Robertson D. S., McKenna M. C., Toon O. B., Hope S., Lillegraven J. A. 2004. Survival in the first hours of the Cenozoic. Geol. Soc. Am. Bull. 116, 760–768 10.1130/B25402.1 (doi:10.1130/B25402.1) [DOI] [Google Scholar]
- 12.Archibald J. D. 1996. Dinosaur extinction and the end of an era. New York, NY: Columbia University Press [Google Scholar]
- 13.Hutchinson G. E., MacArthur R. H. 1959. A theoretical ecological model of size distributions among species of animals. Am. Nat. 93, 117–125 10.1086/282063 (doi:10.1086/282063) [DOI] [Google Scholar]
- 14.Peczkis J. 1995. Implications of body-mass estimates for dinosaurs. J. Vert. Paleontol. 14, 520–533 10.1080/02724634.1995.10011575 (doi:10.1080/02724634.1995.10011575) [DOI] [Google Scholar]
- 15.Luo Z.-X., Yuan C.-X., Meng Q.-J., Ji Q. 2011. A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature 476, 442–445 10.1038/nature10291 (doi:10.1038/nature10291) [DOI] [PubMed] [Google Scholar]
- 16.Hone D. W. E., Dyke G. J., Haden M., Benton M. J. 2008. Body size evolution in Mesozoic birds. J. Evol. Biol. 21, 618–624 10.1111/j.1420-9101.2007.01483.x (doi:10.1111/j.1420-9101.2007.01483.x) [DOI] [PubMed] [Google Scholar]
- 17.Carrano M. T. 2006. Body-size evolution in the Dinosauria. In Amniote paleobiology: perspectives on the evolution of mammals, birds, and reptiles (eds Carrano M. T., Blob R. W., Gaudin T., Wible J. R.), pp. 225–268 Chicago, IL: University of Chicago Press [Google Scholar]
- 18.Smith F. A., et al. 2010. The evolution of maximum body size of terrestrial mammals. Science 330, 1216–1219 10.1126/science.1194830 (doi:10.1126/science.1194830) [DOI] [PubMed] [Google Scholar]
- 19.Kelt D. A., Meyer M. D. 2009. Body size frequency distributions in African mammals are bimodal at all spatial scales. Glob. Ecol. Biogeogr. 18, 19–29 10.1111/j.1466-8238.2008.00422.x (doi:10.1111/j.1466-8238.2008.00422.x) [DOI] [Google Scholar]
- 20.Woolley L.-A., Page B., Slotow R. 2011. Foraging strategy within African elephant family units: why body size matters? Biotropica 43, 489–495 10.1111/j.1744-7429.2010.00733.x (doi:10.1111/j.1744-7429.2010.00733.x) [DOI] [Google Scholar]
- 21.Feduccia A. 2003. ‘Big bang’ for tertiary birds? Trends Ecol. Evol. 18, 172–176 [Google Scholar]