Abstract
Plant defences against herbivores include direct defences such as secondary metabolites or physical structures (e.g. trichomes) as well as indirect defences mediated via mutualistic interactions with other organisms including ants. Production of both direct defences and rewards for mutualistic ants may be costly for a plant, and it has been suggested that trade-offs may exist between direct and ant-mediated defences. We have conducted a meta-analysis of 25 studies testing the above hypothesis and found a significant negative correlation between plant allocation to direct and ant-mediated defences. The strength of correlation was similar for across- and within-species comparisons, and for chemical and physical direct defences. However, trade-offs with direct defences were significant only in plants which offered to ants more costly rewards such as food bodies and/or domatia, but not in plants which attracted ants with relatively cheap extrafloral nectaries. Our results therefore support the hypothesis that plant investment in ant-mediated defences may reduce the requirement for direct chemical and physical defences, but only in plants which offer more costly rewards to their bodyguards.
Keywords: direct defences, herbivores, indirect defences, meta-analysis, mutualistic ants, trade-offs
1. Introduction
Many plants have evolved mutualistic interactions with ants that provide effective defence against herbivores in exchange for food (extrafloral nectaries (EFNs), food bodies) and/or refuge and nesting space (domatia) [1]. Production of these rewards can be costly to the plants [2], and Janzen [3] was the first to propose that plants which evolved symbiosis with ants might reduce their allocation to direct defences such as toxic chemicals because maintenance of both ant-mediated and direct defences is redundant and costly. In addition, some chemical defences may have negative effects on ants as well [4,5]. Although numerous studies have tested for potential trade-offs among ant-mediated and direct plant defences, their results have been mixed. Some studies reported reduced chemical defences in myrmecophytic plants [4,5], whereas no trade-offs between ant-mediated and direct defence were found by others [6,7].
Although most studies examined trade-offs among chemical and ant-mediated defences, Heil et al. [8] suggested that mechanical defences may show stronger trade-offs because they are often more costly than chemical defences and hence may be subject to strong counterselection if they become redundant. Moreover, Rudgers et al. [7] suggested that trade-offs with direct defences may be more probable when indirect defences are obligate rather than facultative. Obligate myrmecophytes usually offer multiple rewards to ants such as food bodies and domatia whereas many other plants attract ants using EFNs alone. Hence, the strength of trade-offs among direct and ant-mediated defences may vary depending on the type of rewards offered by plants to their bodyguards [9].
Here, we report the first meta-analysis testing the evidence of trade-offs among direct and ant-mediated plant defences. Specifically, we compare the strength of trade-offs at within- versus among-species level, between mechanical versus chemical direct defences and between plants offering different types of reward to ants.
2. Material and methods
We have conducted a literature search using the ISI Web of Science electronic database and the combinations of keywords ‘ant*’, ‘plant*’, ‘defen?e*’, ‘trade-off*’, ‘indirect’ and ‘direct’. We also searched for articles citing early key papers on the topic [10]. To be included in the analysis, the retrieved articles had to (i) examine relationship between at least one direct defence trait and at least one ant-mediated defence trait either within a single plant species or across several plant species and (ii) report Pearson's correlation coefficient between the two defence traits or other data such as the mean, measure of variance and test statistics that could be converted to the correlation coefficient. Our final database consisted of 25 studies published in 1973–2011 which reported 43 associations among direct and ant-mediated defence traits in 14 different plant genera (see electronic supplementary material, appendices S1 and S2).
Measures of mechanical defences included trichome density, epicuticular waxes, leaf toughness and thorn length. Measures of chemical defences included concentrations of plant secondary metabolites (either individual compounds or groups of compounds) or growth inhibition of insect herbivores reared on an artificial diet with added plant extracts. Indirect ant-mediated defences were measured either as presence and/or abundance of ants or of plant structures that attract ants (e.g. domatia, food bodies and EFNs). We distinguished between studies conducted within and across plant species. Within-species studies included ant exclusion experiments or studies comparing populations of plants differing in defensive traits. Across-species studies either compared direct defences in myrmecophytic and non-myrmecophytic species or examined continuous variation in direct and indirect defence traits across species; these studies were always conducted within the same plant genus, thus minimizing phylogenetic dependencies.
The meta-analysis was carried out using MetaWin v. 2.0 statistical software [11]. We used the z-transformed Pearson's product-moment correlation coefficient Zr as a common measure of association between direct and indirect defensive traits. Some studies reported Pearson's correlations directly, others reported F, t or χ2 statistics which were converted into r using the statistical calculator in MetaWin [11]. For comparisons of direct defences in two plants groups (e.g. in ant exclusion studies or comparisons of direct defences in non-myrmecophytic and myrmecophytic species), we first calculated standardized mean difference and then converted it into r [12].
Confidence intervals (95% CI) around mean effect sizes were constructed by bootstrapping method with 4999 iterations. Relationships between measures of direct and indirect defences were considered significant when the 95% CI of the z-transformed correlation coefficient did not overlap with the zero. Homogeneity analysis was used to test whether variation in effect sizes could be explained by the sampling error alone (total heterogeneity, Qt) and for testing the significance of the moderators (between-group heterogeneity, Qb). We used a mixed effects meta-analysis model for moderator analysis, which assumes random variation in effect among studies within a group, and fixed variation among groups [13]. As moderators, we tested level of study (within-species versus across-species comparisons), type of direct defences (chemical or mechanical) and type of reward (EFNs, domatia and/or food bodies) offered by the plants to ants. Robustness of results to publication bias was tested by calculating Rosenthal's fail-safe number (nfs) which indicates how many studies with non-significant results need to be added to the analysis to make the observed effect size non-significant [11]. Usually results are considered robust to publication bias when nfs > 5n + 10, where n is the number of observations included in the meta-analysis.
3. Results
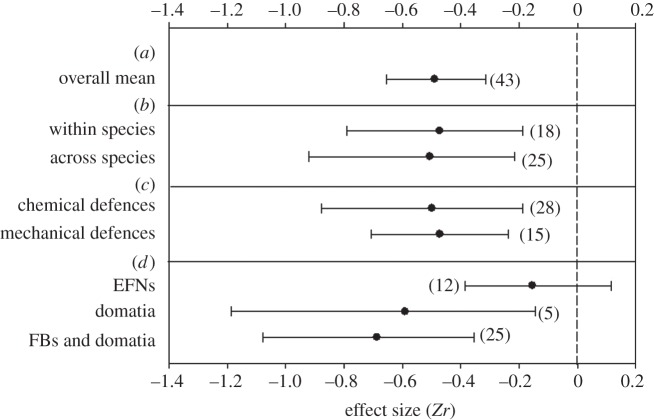
Overall z-transformed correlation between ant-mediated and direct defences was significantly negative (figure 1a). Rosenthal's fail-safe number was 3082, which is considerably higher than 5n + 10 (225) and suggests that the results of meta-analysis are robust against potential publication bias. Homogeneity analysis showed significant variation in effect size among studies that could not be explained by the sampling error alone (Qt = 341.87, d.f. = 42, p < 0.0001). The strength of correlation between ant-mediated and direct defences was not dependent on whether the comparison was conducted across or within plant species (figure 1b; Qb = 0.033, d.f. = 1, p = 0.856) or the type of direct defences (figure 1c; Qb = 0.019, d.f. = 1, p = 0.889). However, the type of reward offered by plants to ants had a significant effect on magnitude of the effect size (Qb = 6.736, d.f. = 2, p = 0.035). Significant correlations between direct and ant-mediated defences were observed only in plants which rewarded ants with food bodies and/or domatia, but not in plants which attracted ants with EFNs alone (figure 1d).
Figure 1.
Overall mean (a) and effect sizes (means ± 95% CIs) of relationship between measures of direct and ant-mediated defences in plants grouped by (b) level of study, (c) type of direct defences, and (d) type of rewards offered to ants (FBs, food bodies, EFNs, extrafloral nectaries). Effects are significantly different from 0 when 95% CI does not contain zero. Numbers in parentheses refer to the number of correlations for the specific groups.
4. Discussion
Our meta-analysis provides support for Janzen's hypothesis [3] that plants which have evolved symbiosis with ants relax their direct defences against herbivores. We showed that the strength of negative correlations with ant-mediated defences was similar for chemical and mechanical defences, contrary to the prediction by Heil et al. [8] that trade-offs are stronger between indirect and mechanical defences. These results are in agreement with the previous meta-analysis by Koricheva [12] who found no difference in fitness costs of chemical and mechanical defences. However, Koricheva et al. [14] showed little evidence of trade-offs among different types of direct plant defence, whereas the present study showed significant trade-offs among direct and indirect defences. Different types of direct defence may not be redundant because they are effective against different types of herbivore (e.g. specialists versus generalists, mammals versus insects). In contrast, ant-mediated defences are effective against most types of herbivore and have been shown to provide more effective protection against herbivory than direct defence [15]. Therefore, while it might be evolutionarily advantageous to maintain several types of direct defence, possession of both strong direct defences and ant-mediated defences may be redundant and costly in terms of resources and potentially harmful to the bodyguards themselves.
Significant negative correlations between direct and ant-mediated defences were found at both within- and across-species levels. At the within-species level, these correlations suggest the existence of ecological trade-offs and indicate that investment in both direct and ant-mediated defences is costly and individual plants tend to invest either in direct defences or in ant-mediated defences, but not both. In the across-species comparison, negative correlations between direct and ant-mediated defences are likely to represent evolutionary trade-offs and differences between plant species which have facultative versus obligate mutualistic interactions with ants. Facultative myrmecophiles normally invest fewer resources in ant attraction than obligate myrmecophytes [1,16]. As a result, facultative associations with ants do not provide the same level of protection from herbivores as obligate mutualism [17], thus requiring the maintenance of a full complement of direct defences. Higher investments of obligate myrmecophytes in rewards for ants result in more efficient ant-mediated defence, and this make direct defences evolutionary redundant, resulting in a negative across-species correlation between investment in ant defences and direct defences.
Interestingly, trade-offs among direct and ant-mediated defences were significant only for plants which offered domatia and/or food bodies as rewards to ants. Costs of production of food bodies are relatively high [2], and all plants which produced food bodies also had domatia (figure 1d), which might have further increased costs of rewards. In contrast, no negative correlations between direct and indirect defences were found in plants which attracted ants with EFNs alone. Costs of EFN production appear to be low [18,19], which makes it possible for EFN-bearing plants to maintain direct defences. Moreover, maintenance of direct defences in EFN-bearing plants is important because such plants tend to have facultative mutualistic interactions with ants [16] resulting in a less effective indirect defence.
In conclusion, our meta-analysis has resolved the controversy over the sources of variation in the magnitude of the reported trade-offs among direct and ant-mediated defences and showed that mutualistic interactions between plants and ants reduce the requirement for direct chemical and physical defences. However, trade-offs occurred only in plants providing more costly rewards to ants (hence, ‘you get what you pay for’). Ant-mediated defences represent just one type of indirect defence in plants and it remains to be shown whether significant trade-offs exist among direct defences and other types of indirect defence, such as production of volatile organic compounds to attract carnivores.
Acknowledgements
We are grateful to Paulo S. Oliveira and two anonymous referees for helpful comments on the manuscript.
References
- 1.Rico-Gray V., Oliveira P. S. 2007. The ecology and evolution of ant–plant interactions. Chicago, IL: The University of Chicago Press [Google Scholar]
- 2.Heil M., Fiala B., Linsenmair K. E., Zotz G., Menke P., Maschwitz U. 1997. Food body production in Macaranga triloba (Euphorbiaceae): a plant investment in anti-herbivore defence via mutualistic ant partners. J. Ecol. 85, 847–861 10.2307/2960606 (doi:10.2307/2960606) [DOI] [Google Scholar]
- 3.Janzen D. H. 1966. Coevolution of mutualism between ants and acacias in Central America. Evolution 20, 249–275 10.2307/2406628 (doi:10.2307/2406628) [DOI] [PubMed] [Google Scholar]
- 4.Heil M., Fiala B., Linsenmair K. E., Boller T. 1999. Reduced chitinase activities in ant plants of the genus Macaranga. Naturwissenschaften 86, 146–149 10.1007/s001140050589 (doi:10.1007/s001140050589) [DOI] [Google Scholar]
- 5.Dyer L. A., Dodson C. D., Beihoffer J., Letourneau D. K. 2001. Trade-offs in antiherbivore defenses in Piper cenocladum: ant mutualists versus plant secondary metabolites. J. Chem. Ecol. 27, 581–592 10.1023/A:1010345123670 (doi:10.1023/A:1010345123670) [DOI] [PubMed] [Google Scholar]
- 6.Letourneau D. K., Barbosa P. 1999. Ants, stem borers, and pubescence in Endospermum in Papua New Guinea. Biotropica 31, 295–302 10.1111/j.1744-7429.1999.tb00141.x (doi:10.1111/j.1744-7429.1999.tb00141.x) [DOI] [Google Scholar]
- 7.Rudgers J. A., Strauss S. Y., Wendel J. F. 2004. Trade-offs among anti-herbivore resistance traits: insights from Gossypieae (Malvaceae). Am. J. Bot. 91, 871–880 10.3732/ajb.91.6.871 (doi:10.3732/ajb.91.6.871) [DOI] [PubMed] [Google Scholar]
- 8.Heil M., Delsinne T., Hilpert A., Schürkens S., Andary C., Linsenmair K. E., Sousa S., McKey D. 2002. Reduced chemical defence in ant-plants? A critical re-evaluation of a widely accepted hypothesis. Oikos 99, 457–468 10.1034/j.1600-0706.2002.11954.x (doi:10.1034/j.1600-0706.2002.11954.x) [DOI] [Google Scholar]
- 9.Piovia-Scott J. 2011. Plant phenotype influences the effect of ant mutualists on a polymorphic mangrove. J. Ecol. 99, 327–334 10.1111/j.1365-2745.2010.01728.x (doi:10.1111/j.1365-2745.2010.01728.x) [DOI] [Google Scholar]
- 10.Rehr S. S., Feeny P. P., Janzen D. H. 1973. Chemical defence in Central American non-ant-acacias. J. Anim. Ecol. 42, 405–416 10.2307/3294 (doi:10.2307/3294) [DOI] [Google Scholar]
- 11.Rosenberg M. S., Adams D. C., Gurevitch J. 2000. MetaWin: statistical software for meta-analysis, v. 2.0. Sunderland, MA: Sinauer [Google Scholar]
- 12.Koricheva J. 2002. Meta-analysis of sources of variation in fitness costs of plant antiherbivore defenses. Ecology 83, 176–190 10.2307/2680130 (doi:10.2307/2680130) [DOI] [Google Scholar]
- 13.Gurevitch J., Hedges L. V. 2001. Meta-analysis: combining the results of independent experiments. In Design and analysis of ecological experiments (eds Scheiner S. M., Gurevitch J.), pp. 347–369 New York, NY: Oxford University Press [Google Scholar]
- 14.Koricheva J., Nykänen H., Gianoli E. 2004. Meta-analysis of trade-offs among plant antiherbivore defenses: are plants jacks-of-all-trades, masters of all. Am. Nat. 163, E64–E75 10.1086/382601 (doi:10.1086/382601) [DOI] [PubMed] [Google Scholar]
- 15.Massad T. J., Fincher R. T., Smilanich A. M., Dyer L. 2011. A quantitative evaluation of major plant defense hypotheses, nature versus nurture, and chemistry versus ants. Arthropod Plant Interact. 5, 125–139 10.1007/s11829-011-9121-z (doi:10.1007/s11829-011-9121-z) [DOI] [Google Scholar]
- 16.Schemske D. W. 1983. Limits to specialization and coevolution in plant–animal mutualisms. In Coevolution (ed. Nitecki M. H.), pp. 67–109 Chicago, IL: University of Chicago Press [Google Scholar]
- 17.Rosumek F. B., Silveira F. A. O., de S. Neves F., de U. Barbosa N. P., Diniz L., Oki Y., Pezzini F., Fernandes G. W., Cornelissen T. 2009. Ants on plants: a meta-analysis of the role of ants as biotic defenses. Oecologia 160, 537–549 10.1007/s00442-009-1309-x (doi:10.1007/s00442-009-1309-x) [DOI] [PubMed] [Google Scholar]
- 18.O'Dowd D. J. 1979. Foliar nectar production and ant activity on a neotropical tree, Ochroma pyramidale. Oecologia 43, 233–248 10.1007/BF00344773 (doi:10.1007/BF00344773) [DOI] [PubMed] [Google Scholar]
- 19.Katayama N., Suzuki N. 2011. Anti-herbivory defense of two Vicia species with and without extrafloral nectaries. Plant Ecol. 212, 743–752 10.1007/s11258-010-9862-2 (doi:10.1007/s11258-010-9862-2) [DOI] [Google Scholar]