Abstract
Adverse drug reactions are the fifth most frequent cause of deaths in developed countries, effectively a global epidemic. However, progress in ameliorating the problem has been slow. Pharmacovigilance currently operates without clear objectives in relation to individual decisions, with no protocol (although risk management plans are a great step forward), with obscure materials and methods used for making decisions, with very limited reasoning and discussion, and little or no follow up and audit of the results. Problems include under-reporting, poor quality reports, underuse of the latest communications technology and suboptimal individual feedback to reporters. Assessment of causality is poor, impeding decision-making. After signal detection, more active measures to assess the risk to public health are needed. Other essential factors include precision about the ways in which data are prepared and transformed into databases, the recognition of secondary effects, which may be more obvious than the primary effect, but not so easy to link causally, and cognisance of all kinds of interactions. Areas that should be developed include pharmacoepidemiology, knowledge finding (through data mining), and communication and systems technology. The general way forward seems clear: a rigorous way of documenting all the steps, from getting reports of harms into regulatory databases to assessing their effects on public health, is essential and should be publicly reviewed for weaknesses. In turn, matters would be much improved by input on benefit/harm perceptions from patient groups, influencing decisions about what should be the true targets for regulatory and pharmacovigilance activities, avoiding second guessing by regulators.
Introduction
Modern pharmacovigilance was heralded in the 1960s by the thalidomide disaster, a devastating and obvious fetal abnormality produced by use of the drug in pregnancy. It is now clear that adverse drug reactions are effectively a global epidemic. In spite of the apparent rarity of serious reactions to individual drugs, overall they are the fifth most frequent cause of deaths in the USA [1]. The frequency is similar in the UK, with large economic effects on health care [2]. Similar figures have been obtained in several other countries. In spite of this knowledge, progress in ameliorating the problem has been very slow. What are the reasons for this?
Current problems
After the pioneering work of David Finney [3] and others, much pharmacovigilance has concentrated on individual case harm reports (ICHRs, commonly known as individual case safety reports, ICSRs), which are provided, mostly voluntarily, by health-care practitioners around the world to national regulatory authorities or via pharmaceutical companies, who are obliged to report (and paradoxically may be more likely to report) events that are not necessarily causally related to their drug. In spite of a collection of currently in excess of 6.5 million ICHRs in the WHO Global Database (‘Vigibase’) in the Uppsala Monitoring Centre, Sweden, reporting rates are generally acknowledged to be no more than 5–10% overall, although they may be as high as 70% for some serious adverse events. Furthermore, reporting methods have not developed greatly from the original very limited paper-based formulations, which acquired little information, and the same fields are still used for storing the information in databases. The ICH E2B initiative [4] saw more fields added, with the opportunity for free-text descriptions, but analysis of the data depends on the reporting terms selected on data input. Routine signal searches are generally initiated using those transformed terms. A revised version of E2B, E2B(R3), was released for consultation in September 2011.
This creation of an active networking between health-care professionals and regulators, in order to safeguard patients, was revolutionary in the late 1960s, but lack of development using the latest communications technology has not been optimized for reporting by physicians and others, and has also left the capture of qualitative information and the concerns of patients woefully lacking. This includes a lack of dialogue and useful individual feedback to reporters. It is not surprising that reporting rates remain low.
Following the detection of a signal that something could be wrong with a drug, more active measures to assess that risk further are necessary for evaluation of its effect on public health, in other words whether the problem is going to affect many people. Because of the rarity of serious adverse events, observational studies are normally used, although some suggest that large prospective studies are necessary, even if they are expensive. In either case, the reasons for decisions to take further action are not usually made public. Few have addressed this matter, but some have made firm proposals for decision making [5]–[10]. At the heart of the matter is how one determines a primary probability (notional) of a causal link between a drug and an adverse event, the seriousness of the perceived public health risk and the availability of resources to investigate it. The judgement to take things further is heavily weighted by the frequent comment that ICHRs are ‘only anecdotal’ and ‘poor quality evidence’.
This is a dangerous catch 22. ICHRs are the chief means by which new hypotheses about rare events are brought to our attention, so we need to have a clear, open procedure to determine what signals should be further investigated and when. The causality guidelines offered by Bradford Hill in 1965 [11], [12] and the EIDOS and DoTS proposals [7], [9] form a strong basis for these considerations and should be transparently used and evaluated for their merits. Moreover, causality logic should be a basic subject in all medical schools, since it is at the root of successful diagnosis. In addition to this logic, I believe that three other factors are essential in pharmacovigilance and in medicine in general:
to be precise about the ways in which data are prepared and transformed into databases;
to be able to recognize secondary effects, which may be more obvious than the primary effect, but not so easy to link causally;
to be cognizant of interactions, not only between drugs but between drugs and diseases and even environmental factors. Non-drug interactions may be written off as confounders when they are actually co-precipitating factors, such as those that are important determinants of medication errors.
Pharmacovigilance currently forms a sort of sociological experiment that operates, as far as health-care professionals and the public are concerned, without clear objectives in relation to individual decisions, with no protocol (although risk management plans are a great step forward), with obscure materials and methods used for making decisions, with very limited reasoning and discussion, and little or no follow-up and audit of the results.
The general way forward seems clear: a rigorous way of documenting all the steps, from getting ICHRs into regulatory databases to assessing the effects on public health, is essential and should be publicly reviewed for weaknesses. In turn, matters would be much improved if patient groups were to have an input, by giving their benefit/harm perceptions, and therefore influencing decisions about what should be the true targets for regulatory and pharmacovigilance activities, so avoiding second guessing by regulators.
Developing some specific areas of pharmacovigilance
There have been many advances in tools that have been proposed to aid the overall tasks of pharmacovigilance, and here I shall critically review some of them.
Pharmacoepidemiology
Advances in pharmacoepidemiology have been dramatic over the last two decades, particularly in observational epidemiology and meta-analysis. Together these two areas have partially addressed a major challenge for epidemiologists: many adverse reactions are too rare to be seen as statistically significant until considerable exposure has taken place, and such exposure is uncontrolled in day-to-day clinical practice. On the other hand, a major advantage of observational epidemiology is that it does tackle the use of drugs in unselected populations. However, a considerable danger arises in assuming that even a large study can determine lack of causality in a few patients. Importantly, what it can do is to assess the relative probabilities below which any risk merges into the background, and occasionally a study may provide useful information about low risk groups with shared characteristics. It is these low-risk groups that should be studied further, for instance by genotyping and examining other aspects, such as medication errors. Such studies should also form part of our investigation of similar patients collected via ICHRs.
To find sufficient patient exposure, longitudinal health-care data are used. While overall data quality testing is done on databases, for each investigation different quality issues are relevant, and in each specific area of quality problems may arise. Transparency in how quality is examined should be an important part of observational studies, as should the potential for biases.
Since epidemiology is considered elsewhere in this issue of the Journal, I shall comment on only one further matter. Obsession with one kind of tool (for example, observational vs. prospective studies) should be firmly resisted. It is the right tool for the job that is important and different methods should not be considered as competitive. Reasons for differences in findings need to be considered carefully. Two recent studies of the hypothesis that bisphosphonates may be associated with oesophageal cancer gave different results using almost the same data sets and very similar methods [13], [14]. This difference may have been due to different exposure times [15].
Knowledge finding (data mining)
Data mining is an important tool in determining how patients who have an adverse event while taking a particular drug differ from background (often expressed as observed : expected ratios and called disproportionality when the ratio is statistically significant). Almost all work on this has been done on data collections of ICHRs, and there is good evidence that mining is an important aid to sifting large numbers of reports. In spite of exhortations to the contrary, disproportional findings are considered by some to be signals before clinical review. It seems wrong to do this in heterogeneous collections of ICHRs without looking for other criteria that support causality. There are several requirements for assessing the usefulness of data mining in ICHR datasets:
the characteristics of the dataset mined and the effects of changing numerators and denominators over time;
the importance of multiple testing in producing false positive results;
the optimum thresholds to ensure acceptable positive and negative predictive values;
how these should be judged;
other requirements, such as stratification and pattern recognition.
The Uppsala Monitoring Centre has also for several years used data mining for adverse events (but not designated adverse events) reported in longitudinal health-care records. Data quality, terminology, missing data, the relatively small numbers of exposed patients and loss to follow-up in some databases are some of the challenges, together with the problem of using multiple tests. However, thresholds are not a problem here, since the patients can be compared with their own unexposed time (self-controls) as well as with all the other patients in the database. This is close to a combination of a signalling tool and an analytical observational study in one. The new question here is: What strength is added by having both statistically significant self-controls and a comparison with other enrolled patients? Another question is whether, if data mining in health-care data sets becomes routine, care will need to be taken in using confirmatory studies in the same data set. I do not think that simply dividing the data set provides an answer, since this will lose very necessary statistical power.
Assessment of effectiveness and harms
This is currently done in a Delphic fashion, based on consensus opinion of experts examining data, although what data we do not know. However, value judgements of this sort are not easily validated or reproducible, nor can they easily be used to compare drugs, a critical piece of information for health-care providers and patients. Moreover, it often seems that considerable weight is given to a current signal of an adverse event rather than the total profile of the drug. Needless to say, evidence of efficacy from clinical trials is used as a surrogate for effectiveness in regular clinical practice and benefit and harm as experienced by patients is not assessable, since there is little information, except in a few databases like DiPEx [16]. If we had the data, how would we assess them?
The Uppsala Monitoring Centre is working on a semi-quantitative method that may be able to achieve this, but much needs to be done in this key area. A critical role for clinical pharmacologists is always to be comprehensive in their consideration of drugs. Only they have the right skills to piece together all our knowledge about a drug and to set it in a clinical context.
Communication and systems technology
‘Intellectual masturbation’ has been defined as ‘Spending time and brainpower on something that you are powerless to change’[17]. Given that a huge amount of effort and knowledge goes into finding out about how drugs work for good and ill, the very limited results on improving the individual patient's benefit to harm balance seem to suggest self-gratification rather than a productive exercise. There can be only one answer to this paradox (that what we know is not what the patient should know), and that is a concentrated effort to improve our communications and educational methods, using and developing all the media and technological supports that are available to us (Figure 1). This also implies more widespread use of social media.
Figure 1.
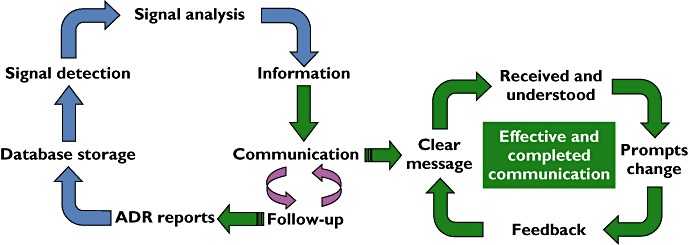
Pharmacovigilance activities. Blue arrows – what we do now; green arrows – what we should be doing
Serious research into and audit of communications science may be much more fruitful than providing more data that are irrelevant, in that they are not used to best effect, or at all.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 2.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, Farrar K, Park BK, Breckenridge AM. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15–9. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finney DJ. The design and logic of a monitor of drug use. J Chronic Dis. 1965;18:77–98. doi: 10.1016/0021-9681(65)90054-8. [DOI] [PubMed] [Google Scholar]
- 4.International Conference on Harmonization. Clinical Safety Data Management: Data Elements for Transmission of Individual Case Safety Reports (ICH E2B(R2)) Geneva: International Conferences on Harmonization, 5 February 2001. Available at http://www.ich.org/products/guidelines/efficacy/efficacy-single/article/maintenance-of-the-clinical-safety-data-management-including-data-elements-for-transmission-ofbrin.html (last accessed 3 January 2012)
- 5.Edwards IR, Lindquist M, Wiholm BE, Napke E. Quality criteria for early signals of possible adverse drug reactions. Lancet. 1990;336:156–8. doi: 10.1016/0140-6736(90)91669-2. [DOI] [PubMed] [Google Scholar]
- 6.Waller PC, Evans SJ. A model for the future conduct of pharmacovigilance. Pharmacoepidemiol Drug Saf. 2003;12:17–29. doi: 10.1002/pds.773. [DOI] [PubMed] [Google Scholar]
- 7.Aronson JK, Ferner RE. Joining the DoTS: new approach to classifying adverse drug reactions. BMJ. 2003;327:1222–5. doi: 10.1136/bmj.327.7425.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aronson JK, Price D, Ferner RE. A strategy for regulatory action when new adverse effects of a licensed product emerge. Drug Saf. 2009;32:91–8. doi: 10.2165/00002018-200932020-00002. [DOI] [PubMed] [Google Scholar]
- 9.Ferner RE, Aronson JK. EIDOS: a mechanistic classification of adverse drug effects. Drug Saf. 2010;33:15–23. doi: 10.2165/11318910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Tang A. Evaluating the evidence base in pharmacovigilance decision making. PhD Thesis, University of Portsmouth. Available at http://eprints.port.ac.uk/2191/1/Amy_Tang-_Electronic_PhD_thesis_-_November2010.pdf (last accessed 3 January 2012)
- 11.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howick J, Glasziou P, Aronson JK. The evolution of evidence hierarchies: what can Bradford Hill's ‘guidelines for causation’ contribute? J R Soc Med. 2009;102:186–94. doi: 10.1258/jrsm.2009.090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green J, Czanner G, Reeves G, Watson J, Wise L, Beral V. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: nested case-control study. BMJ. 2010;341:c4444. doi: 10.1136/bmj.c4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardwell CR, Abnet CC, Cantwell MM, Murray LJ. Exposure to oral bisphosphonates and risk of esophageal cancer. JAMA. 2010;304:657–63. doi: 10.1001/jama.2010.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wysowski DK. Oral bisphosphonates and oesophageal cancer. BMJ. 2010;341:c4506. doi: 10.1136/bmj.c4506. [DOI] [PubMed] [Google Scholar]
- 16.Health talk on-line. Available at http://www.healthtalkonline.org/ (last accessed 3 January 2012)
- 17. http://JargonDatabase.com Intellectual masturbation. Available at http://www.jargondatabase.com/Category/Business/Management-Jargon/Intellectual-Masturbation (last accessed 3 January 2012)


