Abstract
Apicomplexan parasites, including Plasmodium falciparum and Toxoplasma gondii (the causative agents of malaria and toxoplasmosis, respectively), are responsible for considerable morbidity and mortality worldwide. These pathogenic protozoa replicate within an intracellular vacuole inside of infected host cells, from which they must escape to initiate a new lytic cycle. By integrating cell biological, pharmacological, and genetic approaches, we provide evidence that both Plasmodium and Toxoplasma hijack host cell calpain proteases to facilitate parasite egress. Immunodepletion or inhibition of calpain-1 in hypotonically lysed and resealed erythrocytes prevented the escape of P. falciparum parasites, which was restored by adding purified calpain-1. Similarly, efficient egress of T. gondii from mammalian fibroblasts was blocked by either small interfering RNA–mediated suppression or genetic deletion of calpain activity and could be restored by genetic complementation.
Apicomplexan parasites are obligate intra-cellular pathogens that exhibit complex life cycles involving distinct sexual and asexual stages of growth. The asexual phase is made up of a lytic cycle in which parasites establish an intracellular niche within the host: Plasmodium species infect erythrocytes, whereas Toxoplasma gondii infects nucleated animal cells. The process of schizogony in Plasmodium (endodyogeny in Toxoplasma) involves replication within a specialized “parasitophorous vacuole” to yield multiple daughter parasites (1, 2). The resulting merozoites (tachzyoites in Toxoplasma) must escape from this vacuole and the host cell to invade uninfected cells and continue the infection. Egress from the infected cell is a rapid event, requiring only seconds at the end of the ~36- to 48-hour intracellular life cycle (3, 4). Both calcium (5, 6) and proteases (4, 7–10) have been implicated in escape from the parasitophorous vacuole and/or host cell membranes.
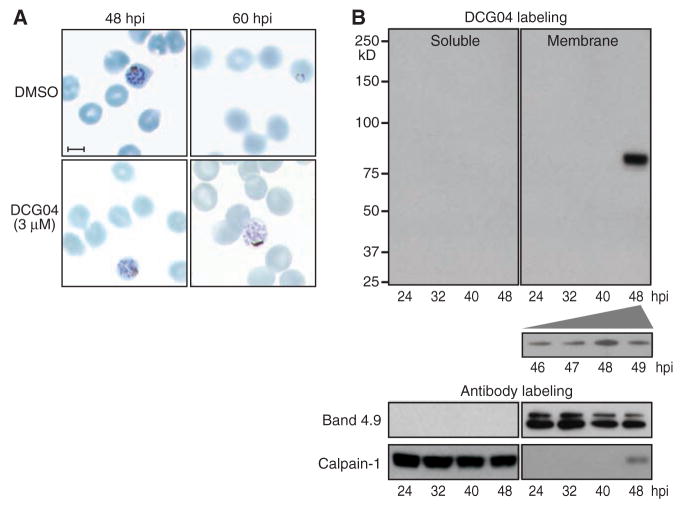
In studies on P. falciparum, DCG04 (a biotinylated derivative of the nonspecific papain family protease inhibitor E64) labels multiple proteases in parasite lysates, including falcipain-1 (11). Although falcipain-1 is most abundant in extracellular merozoites, treatment of living P. falciparum cultures with DCG04 revealed a specific block in schizont-stage parasites (Fig. 1A). DCG04-treated merozoites developed normally (12) but became trapped within intact red blood cell (RBC) membranes. Cysteine proteases may play a role in host cell rupture (3, 4), suggesting the feasibility of exploiting an activity-based probe such as DCG04 for affinity purification of the relevant enzyme(s).
Fig. 1.
Host calpain-1 is associated with P. falciparum egress. (A) Human RBCs were infected with 3D7 strain parasites, synchronized in sorbitol, treated at 42 hours postinfection (hpi) with either dimethyl sulfoxide (control) or 3 μM DCG04, and fixed and stained 6 to 18 hours later. Normal schizonts were evident in both samples at 48 hours, but whereas controls emerged to establish new ring stage infections by 60 hours, parasites treated with DCG04 appeared unable to egress, remaining arrested as schizonts. Scale bar, 5 μm. (B) Synchronized infected cultures were treated with 3 μM DCG04 for 2 hours beginning at various times throughout the intraerythrocytic life cycle, followed by incubation with 0.02% saponin to permeabilize the RBC and parasitophorous vacuole membrane (but not parasites). Parasites were removed by centrifugation, and the remaining material fractionated to yield a host + parasitophorous vacuole membrane pellet and a soluble fraction. Blotting identified proteins biotinylated by DCG04 (labeling any active cysteine protease), as well as the RBC membrane marker band 4.9, and calpain-1 (a cytoplasmic RBC protein when inactive). The single prominent ~80-kD band observed in membranes isolated from RBCs harboring schizont-stage parasites was identified as host cell calpain-1 (table S1).
To focus on those proteases most likely to be involved in parasite egress, infected cultures were labeled with DCG04, treated with saponin to permeabilize the RBC and parasitophorous vacuole membranes, centrifuged briefly to remove parasite cells (which are not permeabilized by saponin), and pelleted to produce soluble and membrane-associated fractions (13). The ability of saponin to lyse the RBC and parasitophorous vacuole membranes selectively, while sparing the parasite plasma membrane, was confirmed by assaying leakage of a cytoplasmic green fluorescent protein (GFP) marker. Immunoblotting with antibodies to GFP, the parasite plasma membrane marker MSP1, the digestive vacuole marker plasmepsin-II, and the erythrocyte membrane marker stomatin showed efficient separation of erythrocyte components from parasite material (fig. S1).
Biotinylated DCG04 detected a single reactive peak of ~80 kD specific to membranes isolated from erythrocytes infected with schizont-stage parasites and maximal at ~48 hours postinfection (Fig. 1B). Mass spectrometry of DCG04-labeled material purified from a streptavidin affinity column unequivocally identified human calpain-1, based on 27 peptides (42% coverage) (table S1). Failure to label other cysteine proteases such as SERAs, falcipains, or other digestive vacuole enzymes (despite their abundance in infected cultures) confirms the removal of parasites and that digestive vacuole contents were not released during egress. DCG04 also failed to label the parasite’s endogenous calpain, an essential cytoplasmic protease required for early cell cycle progression, but not for egress (14).
Of the two common calcium-regulated cysteine proteases in human cells, calpains 1 and 2, only the former is found in erythrocytes, where it is the most abundant cysteine protease (15) (erythrocytes lack the hydrolytic enzymes associated with lysosomes). Calpains are normally inactive cytosolic enzymes, but upon binding calcium they become proteolytically active and associate with membranes (16, 17). Probing DCG04-labeled subcellular fractions of P. falciparum–infected erythrocytes with anti–calpain-1 antibody revealed abundant calpain-1 in the erythrocyte cytoplasm throughout the parasite growth cycle (Fig. 1B), but membrane-associated enzyme was found only during late schizogony. The observed pattern of membrane association coincides precisely with calpain activation as detected by the activity-based probe DCG04 (Fig. 1B).
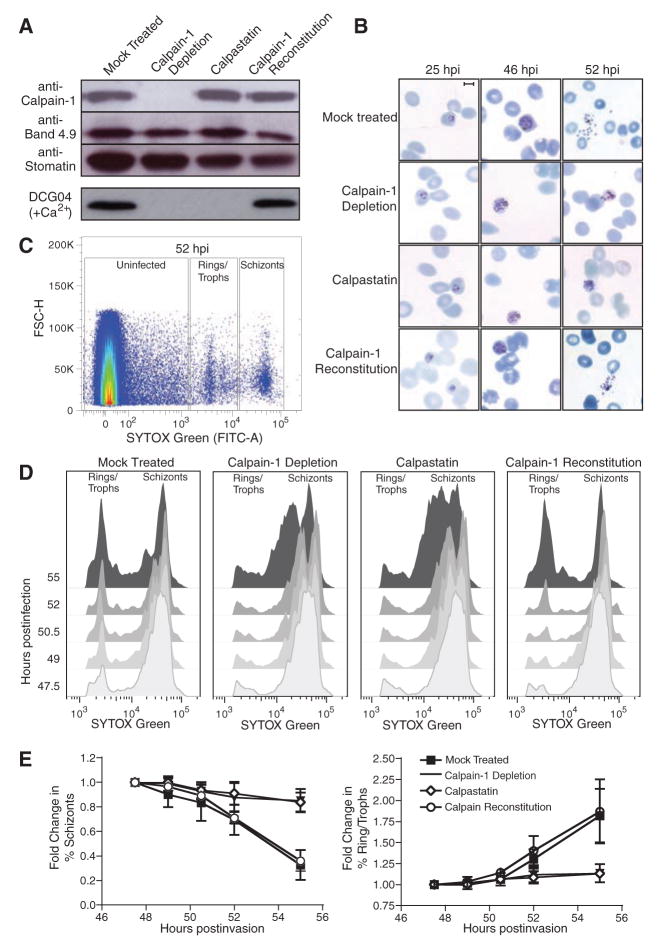
As malaria parasites can replicate in hypotonically lysed and resealed erythrocytes (18), we employed a biochemical/cell biological approach to assess the role of calpains in P. falciparum egress. Lysed RBCs were incubated with anti–calpain-1 antibody conjugated to protein G Sepharose beads, allowing depletion of all detectable calpain-1 before resealing (Fig. 2A). Calpain-1 was also reconstituted into immunodepleted RBCs by resealing in the presence of purified, inactive calpain-1 (without calcium). Control samples were lysed and treated with unconjugated Sepharose (mock) or beads conjugated to anti-flotillin, and a mock-depleted sample was resealed in the presence of the 14-kD, non-cell permeable domain I of the highly specific calpain inhibitor calpastatin (19–21). DCG04 labeling of calcium-treated resealed erythrocytes confirmed that calpain-1 activity was removed by immunodepletion, that calpastatin loading blocked calpain-1 activity, and that reconstitution with calpain-1 restored activity to approximately wild-type (WT) levels (Fig. 2A).
Fig. 2.
Host calpain-1 is required for P. falciparum egress. Uninfected RBCs were hypotonically lysed, immunodepleted with monoclonal anti–calpain-1 conjugated to Sepharose (or mock-treated with Sepharose beads only), and resealed under hypotonic conditions in the presence of 1 μM calpastatin, purified calpain-1, or buffer alone. (A) Immunoblots showed specific removal of calpain-1 in immunodepleted RBCs and reconstitution with purified enzyme; equal loading was confirmed with the use of anti-band 4.9 and anti-stomatin. Labeling of resealed RBCs with 5 μM DCG04 in the presence of calcium demonstrated that calpain activity was eliminated by either immunodepletion or calpastatin loading; activity was restored by reconstitution with exogenous calpain-1. (B) Purified schizont-stage parasites were mixed with resealed RBCs and incubated for various times before fixation. Giemsa-stained smears showed incomplete egress from calpain-depleted or calpastatin-loaded cells, but proper egress from RBCs reconstituted with purified calpain-1. Scale bar = 5 μm. (C) During late schizogony, infected resealed RBCs were harvested at 90-min intervals, fixed, and stained for flow cytometry based on forward scatter (FSC-H) and parasite DNA content (SYTOX-Green). Boxes indicate gates defining uninfected cells, rings/trophozoites, and schizont-stage parasites (sample plot; see fig. S2B). (D) Flow cytometric data was gated to exclude uninfected erythrocytes and converted to a two-dimensional plot highlighting the progress of calpain-reconstituted cultures from schizonts to ring-stage infection as efficiently as mock treated controls, in contrast to the arrest of calpain-depleted and calpastatin-treated cells in schizogony, indicating an egress defect. (E) Quantitation of flow cytometric data (percentage of rings/trophozoites or schizonts relative to time t = 0, ± SE, from four independent experiments).
Incubation of lysed and resealed erythrocytes with highly synchronized P. falciparum schizonts produced ~80% of the parasitemia levels observed during parallel infection of control erythrocytes (fig. S2A) (18). In calpain-1–depleted or calpastatin-loaded cells, however, development of intracellular parasites was arrested during late schizogony, and parasites failed to egress (Fig. 2B). Very few ring stage parasites were observed in calpain-1–depleted or calpastatin-loaded cells, even as late as 75 hours postinvasion. Flow cytometry of DNA content in infected RBCs was used as a quantitative measure of the intraerythrocytic P. falciparum life cycle (22), permitting the discrimination of schizonts from ring/trophozoite stages and uninfected RBCs (Fig. 2C and fig. S2B). Both resealed (mock treated) erythrocytes and erythrocytes immunodepleted by an antibody unrelated to calpain (anti-flotillin) (fig. S3) displayed normal kinetics of parasite growth and egress, progressing from ring stages (1 N) to multinucleated schizonts (~16 N), followed by egress and reinvasion to produce rings in fresh erythrocytes ~50 hours postinfection (Fig. 2, D and E). Calpastatin-loaded and calpain-1 immunodepleted erythrocytes progressed to schizonts with normal kinetics but arrested in late schizogony, producing few new ring-stage infections (the broad “schizont” peak observed at late time points probably represents dying parasites). Erythrocyte ghosts reconstituted with purified calpain-1 after immunodepletion restored the transition from schizonts to rings.
Thus, calpain-1 activity is required for efficient egress of P. falciparum parasites from infected human erythrocytes in vitro, but it is difficult to assess the importance of calpain-mediated parasite egress in vivo because RBCs are not readily amenable to genetic manipulation. Calpain-1 deficiency has not been described in humans, and P. falciparum will not infect rodent erythrocytes. P. yoelii will infect calpain-1 knockout (KO) mice (23), but these parasites preferentially invade reticulocytes (24), which express both calpain-1 and calpain-2 (25) (National Center for Biotechnology Information GEO profile GDS2655), in contrast to human erythrocytes, which express calpain-1 only (15). Genetic deletion of either calpain-2 (26) or the CAPNS1 regulatory subunit required for activity of both calpains (27) produces embryonic lethality in mice.
To further explore the importance of calpains during parasite infection, we turned to the apicomplexan parasite T. gondii, which is related to Plasmodium but able to invade, establish its parasitophorous vacuole, and replicate in virtually any nucleated cell (28). WT fibroblasts are typically lysed by T. gondii parasites ~48- to 60-hours postinfection, releasing free tachyzoites into the medium, whereupon they invade new host cells. This process is readily visualized in living cultures using transgenic parasites expressing a fluorescent protein reporter (29). Although CAPNS1 KO mice die as embryos (27), KO cell lines and small interfering RNA (siRNA) approaches provide alternative experimental routes to assess the importance of calpain for T. gondii egress.
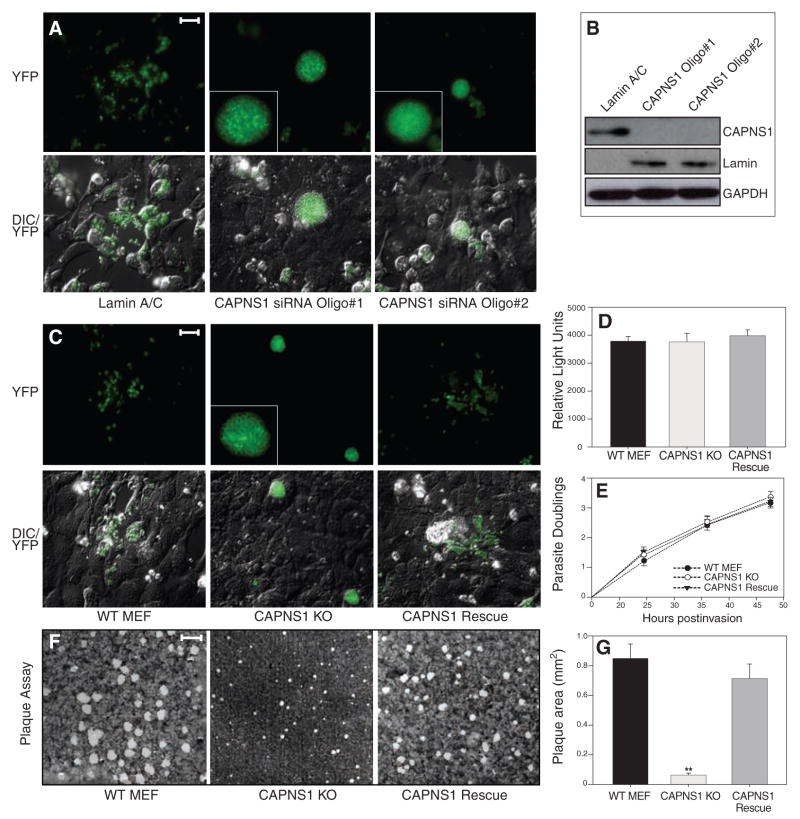
Most mammalian cells express both calpain-1 and 2, but targeting the regulatory subunit CAPNS1 permits the elimination of both. Two oligonucleotides were employed (independently) for siRNA studies in U2OS human host cells, both of which produced >20-fold reduction in CAPNS1 transcript levels and even greater protein depletion (Fig. 3B). Many extracellular T. gondii tachyzoites could be seen after infection of control cultures, but very few were observed after transfection with either of the CAPNS1 siRNAs (Fig. 3A). Rather, these cultures were characterized by host cells packed full of fluorescent parasites unable to egress efficiently. These grossly swollen cells often detached from the monolayer and could be found floating in the culture medium giving the appearance of a hot air balloon convention.
Fig. 3.
Host calpain facilitates the egress of T. gondii. (A) U2OS cells were transfected with siRNAs targeting either the common calpain regulatory subunit CAPNS1 (two different siRNAs) or lamin A/C, infected 24 hours post-transfection with T. gondii parasites expressing yellow fluorescent protein (YFP), and examined by fluorescence microscopy 56 hours later. Parasites ruptured out of most infected cells in controls, but not the knockdown cultures, where infected cells were grossly swollen with intracellular parasites. Insets show enlarged images of parasite-containing vacuoles. Scale bar, 30 μm. (B) Suppression of CAPNS1 protein levels was assessed in uninfected cells 72 hours post transfection using antibodies to CAPNS1, lamin A/C, and glyceraldehyde phosphate dehydrogenase (GAPDH). (C) YFP parasites were used to follow replication and egress in CAPNS1 KO host cells. By 56 hours postinfection, parasites had ruptured out of most cells in the WT and CAPNS1 rescue cultures, whereas CAPNS1 KO cells were heavily infected but unlysed. Scale bar, 30 μm. (D) To follow infection, cells were inoculated with luciferase-expressing transgenic parasites, producing comparable numbers of intracellular tachyzoites 4 hours postinfection. (E) To assay intracellular replication, host cell monolayers were infected with parasites, and parasitophorous vacuoles were scored every 12 hours for the number of parasites per vacuole, demonstrating comparable growth rates throughout the intracellular growth cycle (represented as log2 parasite count). (F) Confluent fibroblast monolayers were infected with T. gondii tachyzoites, and plaques were stained with crystal violet at 7 days, demonstrating comparable rates of infection but very small plaques in the CAPNS1 KO cultures, as a consequence of the egress defect in this mutant host cell line. Scale bar, 2 mm. (G) Quantitation of plaque size revealed a substantial decrease in the CAPNS1 KOs versus WT and CAPNS1 rescued cells (average radius of 20 randomly selected plaques per sample ± SE). Asterisks indicate the statistical significance using a Student’s two-tailed t test comparing CAPNS1 KO to WT, which yielded a P value of 1.91 × 10−7.
Fibroblast cell lines derived from CAPNS1 KO mouse embryos lack both calpain-1 and -2 activity (30), and infection of these cells with T. gondii produced the same swollen cell phenotype observed in CAPNS1 knock-down experiments (Fig. 3C). Transgenic expression of CAPNS1 in the KO mutants restores calpain-1 and -2 activity (30) and also complemented the T. gondii egress defect. Parasite tachyzoites were readily able to invade (Fig. 3D) and replicate (Fig. 3E) in WT, CAPNS1 KOs, and CAPNS1-complemented fibroblasts, demonstrating that the impact of host cell calpains on T. gondii infection is specific to egress. Plaque assays showed a ~13-fold reduction in plaque size for T. gondii in CAPNS1 mutants versus parental MEF cells or CAPNS1-complemented KOs (Fig. 3, F and G). In contrast to P. falciparum, which rarely emerge from calpain-depleted erythrocytes (Fig. 2), some T. gondii parasites did eventually manage to escape from calpain-deficient fibroblasts, yielding a small plaque phenotype.
In summary, in addition to the many roles that parasite-encoded cysteine proteases play in the biology of infection and pathogenesis (25), the apicomplexans Plasmodium falciparum and Toxoplasma gondii both exploit host cell calpains to facilitate escape from the intracellular parasitophorous vacuole and/or host plasma membrane. The precise mechanism of calpain-mediated parasite egress is unknown, but calpains play a role in remodeling of the cytoskeleton and plasma membrane during the migration of mammalian cells (31), and activated calpain-1 can degrade erythrocyte cytoskeletal proteins in vitro and during P. falciparum infection in vivo (fig. S4). The calcium responsible for calpain activation during parasite infection may be supplied through the action of a parasite-encoded perforin recently implicated in T. gondii egress (32). The parasitophorous vacuole was labeled by the calcium-specific dye Fluo-4-AM during late schizogony, and depletion of internal calcium with the membrane-permeant chelator EGTA-AM blocked parasite egress, whereas removal of calcium from the culture medium did not (fig. S5). We suggest a model in which a calcium signal triggered late during parasite infection activates host cell calpain, which relocalizes to the host plasma membrane, cleaving cytoskeletal proteins to facilitate parasite egress (fig. S6). Because parasites that fail to escape from their host cells are unable to proliferate, this suggests an intriguing strategy for anti-parasitic therapeutics.
Supplementary Material
Footnotes
www.sciencemag.org/cgi/content/full/1171085/DC1
Materials and Method
References
20 January 2009; accepted 10 March 2009
Published online 2 April 2009;
10.1126/science.1171085
Include this information when citing this paper.
References and Notes
- 1.Nishi M, Hu K, Murray JM, Roos DS. J Cell Sci. 2008;121:1559. doi: 10.1242/jcs.021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu K, et al. Mol Biol Cell. 2002;13:593. doi: 10.1091/mbc.01-06-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glushakova S, Yin D, Li T, Zimmerberg J. Curr Biol. 2005;15:1645. doi: 10.1016/j.cub.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 4.Salmon BL, Oksman A, Goldberg DE. Proc Natl Acad Sci USA. 2001;98:271. doi: 10.1073/pnas.011413198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black MW, Arrizabalaga G, Boothroyd JC. Mol Cell Biol. 2000;20:9399. doi: 10.1128/mcb.20.24.9399-9408.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagamune K, et al. Nature. 2008;451:207. doi: 10.1038/nature06478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadley T, Aikawa M, Miller LH. Exp Parasitol. 1983;55:306. doi: 10.1016/0014-4894(83)90027-9. [DOI] [PubMed] [Google Scholar]
- 8.Wickham ME, Culvenor JG, Cowman AF. J Biol Chem. 2003;278:37658. doi: 10.1074/jbc.M305252200. [DOI] [PubMed] [Google Scholar]
- 9.Arastu-Kapur S, et al. Nat Chem Biol. 2008;4:203. doi: 10.1038/nchembio.70. [DOI] [PubMed] [Google Scholar]
- 10.Yeoh S, et al. Cell. 2007;131:1072. doi: 10.1016/j.cell.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 11.Greenbaum DC, et al. Science. 2002;298:2002. doi: 10.1126/science.1077426. [DOI] [PubMed] [Google Scholar]
- 12.Glushakova S, Mazar J, Hohmann-Marriott MF, Hama E, Zimmerberg J. Cell Microbiol. 2009;11:95. doi: 10.1111/j.1462-5822.2008.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Materials and methods are available as supporting material on Science Online.
- 14.Russo I, Oksman A, Vaupel B, Goldberg DE. Proc Natl Acad Sci USA. 2009;106:1554. doi: 10.1073/pnas.0806926106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasini EM, et al. Blood. 2006;108:791. doi: 10.1182/blood-2005-11-007799. [DOI] [PubMed] [Google Scholar]
- 16.Croall DE, Ersfeld K. Genome Biol. 2007;8:218. doi: 10.1186/gb-2007-8-6-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goll DE, Thompson VF, Li H, Wei W, Cong J. Physiol Rev. 2003;83:731. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 18.Murphy SC, et al. PLoS Med. 2006;3:e528. doi: 10.1371/journal.pmed.0030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanna RA, Campbell RL, Davies PL. Nature. 2008;456:409. doi: 10.1038/nature07451. [DOI] [PubMed] [Google Scholar]
- 20.Moldoveanu T, Gehring K, Green DR. Nature. 2008;456:404. doi: 10.1038/nature07353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gil-Parrado S, et al. Biol Chem. 2003;384:395. doi: 10.1515/BC.2003.045. [DOI] [PubMed] [Google Scholar]
- 22.Bianco AE, Battye FL, Brown GV. Exp Parasitol. 1986;62:275. doi: 10.1016/0014-4894(86)90032-9. [DOI] [PubMed] [Google Scholar]
- 23.Hanspal M, Goel VK, Oh SS, Chishti AH. Mol Biochem Parasitol. 2002;122:227. doi: 10.1016/s0166-6851(02)00104-4. [DOI] [PubMed] [Google Scholar]
- 24.Weiss L, Johnson J, Weidanz W. Am J Trop Med Hyg. 1989;41:135. [PubMed] [Google Scholar]
- 25.Rosenthal PJ. Int J Parasitol. 2004;34:1489. doi: 10.1016/j.ijpara.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Dutt P, et al. BMC Dev Biol. 2006;6:3. doi: 10.1186/1471-213X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arthur JS, Elce JS, Hegadorn C, Williams K, Greer PA. Mol Cell Biol. 2000;20:4474. doi: 10.1128/mcb.20.12.4474-4481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roos DS, Donald RG, Morrissette NS, Moulton AL. Methods Cell Biol. 1994;45:27. doi: 10.1016/s0091-679x(08)61845-2. [DOI] [PubMed] [Google Scholar]
- 29.Joiner KA, Roos DS. J Cell Biol. 2002;157:557. doi: 10.1083/jcb.200112144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dourdin N, et al. J Biol Chem. 2001;276:48382. doi: 10.1074/jbc.M108893200. [DOI] [PubMed] [Google Scholar]
- 31.Huttenlocher A, et al. J Biol Chem. 1997;272:32719. doi: 10.1074/jbc.272.52.32719. [DOI] [PubMed] [Google Scholar]
- 32.Kafsack BFC, et al. Science. 2009;323:530. doi: 10.1126/science.1165740. published online 18 December 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.We thank R. W. Doms, M. Marti, and M. Klemba for critical discussions; the Penn Proteomics Core for mass spectrometry; and M. A. Lampson for help with imaging. P. falciparum expressing GFP were provided by O. S. Harb. P.H.D. and D.P.B. are funded by National Research Service Awards, and D.S.R. is an Ellison Medical Foundation Senior Scholar in Global Infectious Disease, supported by grants from NIH. D.C.G. was supported by the Ritter Foundation, the Penn Genome Frontiers Institute, and the Penn Institute for Translational Medicine and Therapeutics.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.