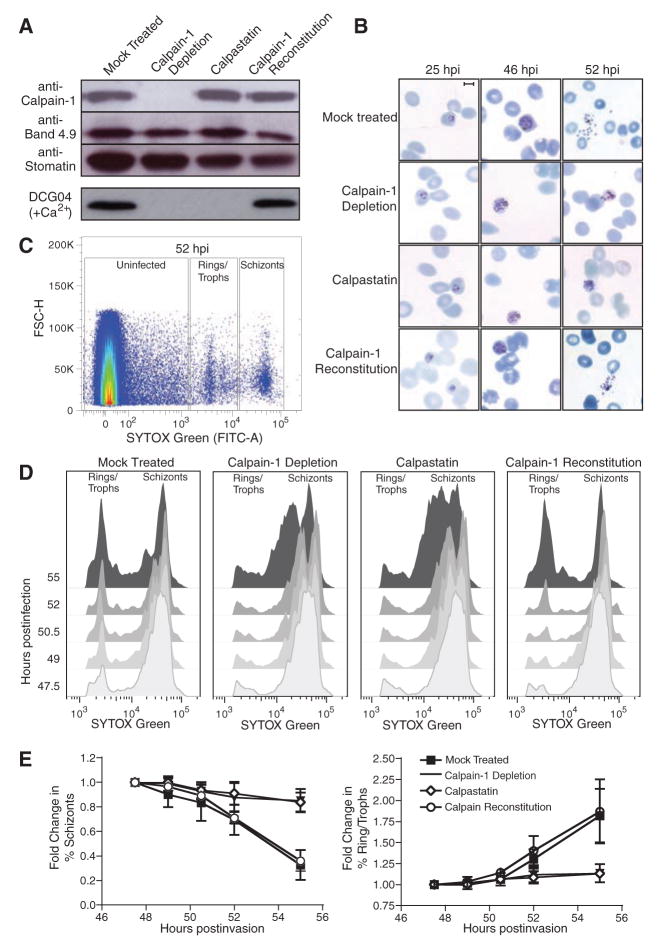
Fig. 2.
Host calpain-1 is required for P. falciparum egress. Uninfected RBCs were hypotonically lysed, immunodepleted with monoclonal anti–calpain-1 conjugated to Sepharose (or mock-treated with Sepharose beads only), and resealed under hypotonic conditions in the presence of 1 μM calpastatin, purified calpain-1, or buffer alone. (A) Immunoblots showed specific removal of calpain-1 in immunodepleted RBCs and reconstitution with purified enzyme; equal loading was confirmed with the use of anti-band 4.9 and anti-stomatin. Labeling of resealed RBCs with 5 μM DCG04 in the presence of calcium demonstrated that calpain activity was eliminated by either immunodepletion or calpastatin loading; activity was restored by reconstitution with exogenous calpain-1. (B) Purified schizont-stage parasites were mixed with resealed RBCs and incubated for various times before fixation. Giemsa-stained smears showed incomplete egress from calpain-depleted or calpastatin-loaded cells, but proper egress from RBCs reconstituted with purified calpain-1. Scale bar = 5 μm. (C) During late schizogony, infected resealed RBCs were harvested at 90-min intervals, fixed, and stained for flow cytometry based on forward scatter (FSC-H) and parasite DNA content (SYTOX-Green). Boxes indicate gates defining uninfected cells, rings/trophozoites, and schizont-stage parasites (sample plot; see fig. S2B). (D) Flow cytometric data was gated to exclude uninfected erythrocytes and converted to a two-dimensional plot highlighting the progress of calpain-reconstituted cultures from schizonts to ring-stage infection as efficiently as mock treated controls, in contrast to the arrest of calpain-depleted and calpastatin-treated cells in schizogony, indicating an egress defect. (E) Quantitation of flow cytometric data (percentage of rings/trophozoites or schizonts relative to time t = 0, ± SE, from four independent experiments).