Abstract
Purpose
To establish normative metabolite ratios throughout the newborn brain using 3D MR Spectroscopic Imaging.
Materials and Methods
MRI and MRSI have been valuable tools for assessing normal and abnormal neuronal maturation for newborns. In this study, we performed whole brain 3D MRSI in addition to comprehensive anatomic and other functional imaging methods to examine maturation. 55 newborn subjects (28.4 ± 2.6 weeks post-conceptional age at birth, 34.1 ± 3.1 weeks post-conception age at scan, 32 males and 23 females) had high quality MRSI studies (104 exams) and normal neurodevelopmental outcome (NMS=0, MDI>85) at age 12 months.
Results
The NAA to Cho ratio increased significantly with age for all regions. Lac to NAA ratio decreased significantly with age in the regions of thalamus, basal ganglia, cortical spinal tract, and parietal white matter, and showed a decreasing trend in the other regions.
Conclusion
Brain metabolites can be obtained through in vivo 3D MRSI and used to monitor newborn brain maturation.
Keywords: newborn, brain, spectroscopy, mrsi
Introduction
Magnetic resonance imaging (MRI) techniques have been shown to provide valuable anatomic information in the newborn brain, but the techniques for metabolic analyses of brains of prematurely born infants are much less developed. The identification and analysis of metabolic markers for assessment of normal brain development, as provided by MR Spectroscopic Imaging (MRSI), could be an important method to improve assessments of neonatal brain development and injury (1–7). Although single voxel proton spectroscopy can be performed in prematurely born neonates, the required size of the voxel would encompass mixed brain regions of varying maturity, therefore, yielding inaccurate measurements. 3D point-resolved spectroscopic imaging (PRESS-MRSI) can provide accurate measurements of metabolite levels in smaller voxels throughout the brain, but it is more complex than commercially available single voxel and 2D MRSI techniques and, therefore, the data acquired require a longer acquisition time and more extensive and time-consuming processing. This study utilized automated processing methods for 3D-PRESS MRI to rapidly analyze the spectral arrays acquired via cerebral MRSI of prematurely born neonates. The rapid post-processing methods allowed the analysis of all spectra in <30 minutes per patient and provided metabolite ratios for multiple small (1 cc) and specific anatomic ROIs, allowing metabolite levels to be more easily assessed on a routine basis. This study reports metabolite ratios, obtained by 3D PRESS-MRSI, from 14 regions of gray and white matter of a group of prematurely born neonates who had normal neuromotor outcomes at age 12 months. Through the presented optimized acquisition, processing and analysis methods, this study establishes a baseline of the normal metabolite ratios for prematurely born newborns, which are crucial in evaluating their normal brain development and effects upon this development of complications of premature birth and its therapies, together with other imaging methods.
Methods
Patient Selection
As part of an ongoing study of brain injury in prematurely born neonates, 217 patients were prospectively enrolled in this study. The studies were performed in an MR compatible incubator with a specialized neonatal head coil to provide a temperature-controlled, well-monitored, safe environment and to improve image quality (8). Motor outcome was assessed at 1 year of age using a neuromotor score (NMS) of 0–5 as previously defined (9); cognitive outcome was measured using the mental development index (MDI) of the Bayley Scales of Infant Development II. From this cohort, 55 newborn subjects (28.4 ± 2.6 weeks post-conceptional age at birth, 34.1 ± 3.1 weeks post-conception age at scan, 32 males and 23 females) had high quality MRSI studies (104 exams) and normal neurodevelopmental outcome (NMS=0, MDI>85) at age 12 months: these MRSI exams are the subject of this report. Other subjects were excluded due to NMS>0 at age of 12 months. The initial MR was performed as soon as the neonate was judged to be stable enough to be imaged safely. A second MR was performed prior to discharge in 49 patients; in 6 patients, a second MR could not be obtained due to logistical issues of patients back transporting to their own local hospitals. Sedation using pentobarbital was available for newborns who moved excessively during the scan and with full parental consent.
Our committee on human research approved the study protocol and informed consent was obtained from parents.
MRI Techniques
A series of standard MR scans were performed for clinical assessment of the neonatal patient on 1.5 T GE Signa Echospeed scanner (GE Healthcare, Waukesha, USA) using a custom built neonatal birdcage head coil that include 1) T1 weighted sagittal and axial spin-echo images with TR/TE of 500/11, 4mm thickness, 1 excitation, 192×256 encoding matrix; 2) T2 weighted axial dual echo, spin-echo with TR of 3sec, TE of 60 and 120ms, 192×256 encoding matrix, 4mm thickness. A spin-echo echo-planar diffusion sequence was also performed for tissue microstructure analyses, which is not a part of this current study. A multivoxel 3D MR spectroscopy scan was performed to obtain metabolite levels covering most of the brain using PRESS acquisition with band selective inversion with gradient dephasing (BASING) lactate editing method (10,11). The uniformity of the selected region was obtained by slightly overexciting the prescribed region and shaped with fixed very selective saturation (VSS) pulses (12) around the PRESS selection and graphically prescribed saturation bands on top of subcutaneous lipid regions around the scalp. The acquisition parameters are 144ms/1s (TE/TR), 1cc, 8×8×8 array, and an acquisition time of 17 minutes. The total examination time was approximately 1 hour. Sedation was given with parental consent if the subject is moving excessively and causing motion artifacts on the imaging series.
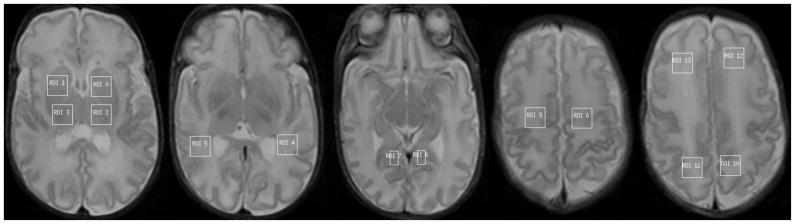
Regions of interests (ROI’s) were drawn bilaterally on T2 images for thalamus, basal ganglia, ventral (temporal) visual association tract, calcarine grey matter, corticospinal tract, parietal white matter, and frontal white matter (see Figure 1).
Figure 1.
ROI’s in basal ganglia (0,1), thalamus (2,3), visual association tract (4,5), calcarine grey matter (6,7), corticospinal tract (8,9), parietal white matter (10,11), and frontal white matter (12,13), drawn on the T2 images.
Data Analysis
Spectroscopy data were analyzed using ratios of peak heights using methods previously detailed by Nelson SJ (13). After a Gaussian spectral apodization of 2 Hz and automatic phasing using the residual water signal, the peak heights were obtained using an automated local maxima detection algorithm. Spectra with choline peak of SNR of 5 or less are excluded due to poor data quality.
Statistical analysis was performed using the statistical package SAS version 9.1 (SAS Institute, Cary, NC). A p-value of 5% or less is defined for statistical significance. The MRS parameters in regions of interests at different ages are the independent parameters. To account for the intra-subject correction due to multiple regions of interests as well as repeated measurements from the same infants, a random effects model is used. In this model, we included neonate’s identification numbers as the random effects. The fixed effects were the gestational age of MRI scans, gestational age of birth, regions of interests, sedation and interactions. The least squared means and standard deviations are used to describe the expected values as well as their variations. The estimated slope of gestational age of MRI scans represents the developmental trends of the corresponding parameters. For all the estimated slopes, a p-value was provided for the null hypothesis of no age related trends, which weren’t adjusted for possible multiple hypothesis tests.
Results
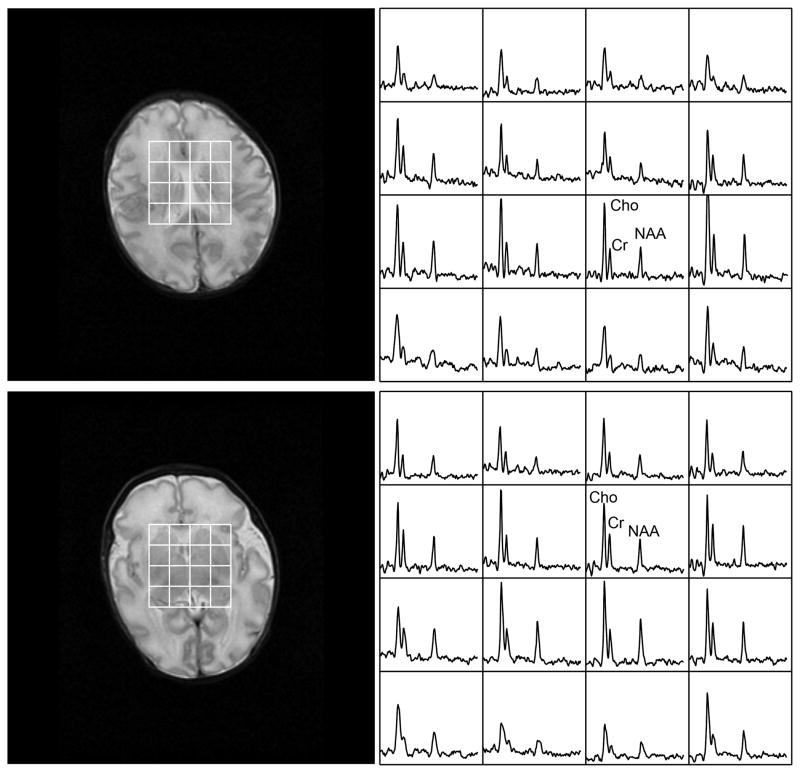
Most of the subjects had adequate imaging and spectroscopy exams without sedation, and 28 patients had to be sedated to reduce excessive motion artifacts. Of the 104 exams, selective voxels (<5%) were excluded due to lipid contamination resulting from the limited coverage of the 8×8×8 PRESS selection. A representative processed spectrum is shown in Figure 2.
Figure 2.
A selected processed spectral array with corresponding T2 image that is representative of data acquired from the patient population. The subject was born after 32 weeks of gestation and scanned at 34 weeks.
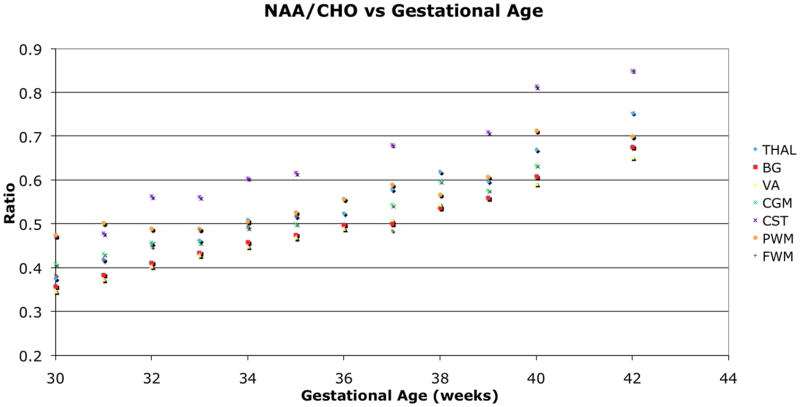
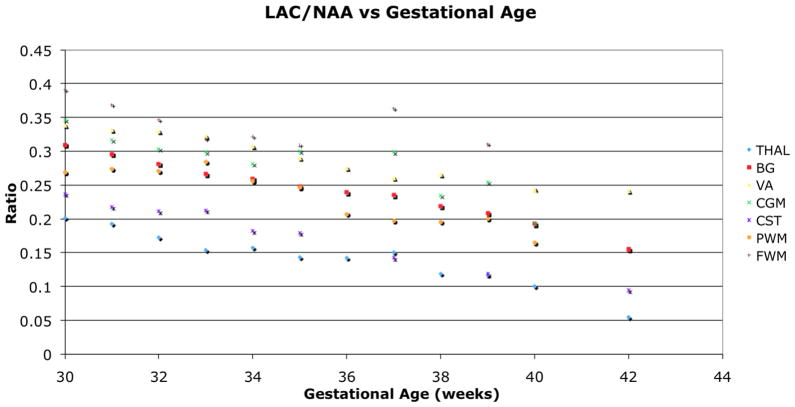
The left and right ROIs were found to be statistically similar and combined for the analysis. The NAA to Cho ratio increased significantly (p<0.001) with age for all regions (Figure 3). Lac to NAA ratio decreased with age in the regions of THAL, BG, CST, and PWM, and showed a decreasing trend in the other regions (Figure 4). Lac to Cho ratio only decreased significantly with age in the CST, and varied differently for other regions (Figure 5). The cortical spinal tracts had the highest NAA/Cho ratio and the temporal visual association tract had the lowest NAA/Cho. The summary of the respective changes is summarized in Table 1 and peak ratios at the various ages for different locations are presented in Table 2.
Figure 3.
NAA to Cho ratio change with age. All regions showed significant increase (p<0.001) with age as NAA increases and Cho decreases through brain maturation.
Figure 4.
Lac to NAA ratio decreased with age in the regions of THAL, BG, CST, and PWM, and showed a decreasing trend in the other regions.
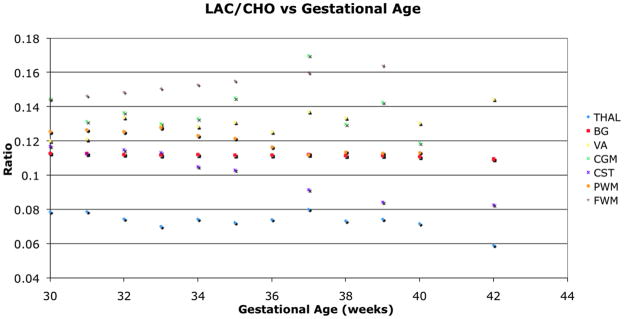
Figure 5.
Lac to Cho ratio only decreased significantly with age for CST, and varied differently for other regions.
Table 1.
Summary of metabolite ratio changes with respect to age in weeks.
| NAA/Cho | Lac/NAA | Lac/Cho | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Regions | Slope | StdErr | p value | Slope | StdErr | p value | Slope | StdErr | p value |
| THAL | 0.028 | 0.0020 | <0.0001 | −0.0087 | 0.0024 | <0.001 | −0.00024 | 0.0011 | 0.83 |
| BG | 0.023 | 0.0022 | <0.0001 | −0.011 | 0.0031 | <0.001 | −0.00016 | 0.0012 | 0.90 |
| VA | 0.024 | 0.0039 | <0.0001 | −0.011 | 0.0058 | 0.072 | 0.00093 | 0.0026 | 0.73 |
| CGM | 0.019 | 0.0035 | <0.0001 | −0.0083 | 0.0052 | 0.12 | 0.00083 | 0.0029 | 0.78 |
| CST | 0.029 | 0.0040 | <0.0001 | −0.013 | 0.0030 | <0.0001 | −0.0036 | 0.0018 | 0.049 |
| PWM | 0.017 | 0.0044 | <0.001 | −0.0099 | 0.0042 | 0.0042 | −0.0015 | 0.0020 | 0.45 |
| FWM | 0.018 | 0.0049 | 0.001 | −0.0076 | 0.0070 | 0.29 | 0.0022 | 0.0032 | 0.49 |
Table 2.
Metabolite ratio in various regions for early birth.
| 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THAL | NAA/Cho | 0.37±0.022 | 0.42±0.035 | 0.45±0.034 | 0.46±0.033 | 0.51±0.040 | 0.52±0.032 | 0.52±0.0067 | 0.58±0.035 | 0.62±0.061 | 0.60±0.015 | 0.67±0.010 |
| Lac/NAA | 0.20±0.015 | 0.19±0.025 | 0.17±0.018 | 0.15±0.015 | 0.16±0.026 | 0.14±0.017 | 0.14±0.029 | 0.15±0.016 | 0.12±0.023 | 0.12±0.011 | 0.10±0.0071 | |
| Lac/Cho | 0.079±0.0055 | 0.079±0.0077 | 0.074±0.0061 | 0.070±0.0063 | 0.074±0.0082 | 0.072±0.0071 | 0.074±0.012 | 0.08±0.0076 | 0.073±0.0088 | 0.074±0.0046 | 0.072±0.0024 | |
| BG | NAA/Cho | 0.36±0.012 | 0.38±0.023 | 0.41±0.022 | 0.43±0.016 | 0.46±0.024 | 0.48±0.017 | 0.50±0.027 | 0.50±0.016 | 0.54±0.035 | 0.56±0.0077 | 0.61±0.0036 |
| Lac/NAA | 0.31±0.0072 | 0.30±0.0076 | 0.28±0.0080 | 0.27±0.0068 | 0.26±0.0098 | 0.25±0.0077 | 0.24±0.018 | 0.23±0.0071 | 0.22±0.019 | 0.21±0.0052 | 0.19±0.0012 | |
| Lac/Cho | 0.11±0.0041 | 0.11±0.00043 | 0.11±0.00051 | 0.11±0.0004 | 0.11±0.00059 | 0.11±0.00055 | 0.11±0.0017 | 0.11±0.00044 | 0.11±0.0011 | 0.11±0.00038 | 0.11±0.00017 | |
| VA | NAA/Cho | 0.35±0.0060 | 0.37±0.0057 | 0.40±0.0060 | 0.43±0.0057 | 0.45±0.0078 | 0.47±0.0058 | 0.49±0.016 | 0.51±0.0061 | 0.54±0.015 | 0.59±0.0025 | |
| Lac/NAA | 0.34±0.0087 | 0.33±0.0082 | 0.33±0.0087 | 0.32±0.0082 | 0.31±0.011 | 0.29±0.0084 | 0.28±0.022 | 0.26±0.0091 | 0.27±0.022 | 0.24±0.00000 | ||
| Lac/Cho | 0.12±0.0083 | 0.12±0.0062 | 0.13±0.011 | 0.13±0.0056 | 0.13±0.011 | 0.13±0.0086 | 0.13±0.0023 | 0.14±0.0094 | 0.13±0.0020 | 0.13±0.0018 | ||
| CGM | NAA/Cho | 0.41±0.0066 | 0.43±0.044 | 0.46±0.020 | 0.46±0.030 | 0.49±0.023 | 0.50±0.037 | 0.58±0.014 | 0.60±0.0059 | 0.58±0.0059 | 0.63±0.047 | |
| Lac/NAA | 0.35±0.029 | 0.32±0.050 | 0.30±0.046 | 0.30±0.042 | 0.28±0.064 | 0.30±0.040 | 0.30±0.089 | 0.23±0.047 | 0.25±0.029 | 0.19±0.027 | ||
| Lac/Cho | 0.14±0.015 | 0.13±0.021 | 0.14±0.24 | 0.13±0.016 | 0.13±0.032 | 0.14±0.020 | 0.17±0.076 | 0.13±0.025 | 0.14±0.020 | 0.12±0.014 | ||
| CST | NAA/Cho | 0.47±0.030 | 0.48±0.039 | 0.56±0.033 | 0.56±0.048 | 0.60±0.039 | 0.62±0.027 | 0.68±0.031 | 0.71±0.026 | |||
| Lac/NAA | 0.24±0.015 | 0.22±0.015 | 0.21±0.016 | 0.21±0.017 | 0.18±0.014 | 0.18±0.022 | 0.14±0.010 | 0.12±0.018 | ||||
| Lac/Cho | 0.12±0.0039 | 0.11±0.0040 | 0.11±0.0043 | 0.11±0.0041 | 0.10±0.0049 | 0.10±0.0062 | 0.91±0.00091 | 0.08±0.0048 | ||||
| PWM | NAA/Cho | 0.47±0.040 | 0.50±0.055 | 0.49±0.060 | 0.49±0.050 | 0.50±0.036 | 0.53±0.045 | 0.56±0.0030 | 0.59±0.034 | 0.57±0.0023 | 0.61±0.049 | 0.71±0.017 |
| Lac/NAA | 0.27±0.025 | 0.27±0.021 | 0.27±0.014 | 0.28±0.022 | 0.26±0.019 | 0.25±0.030 | 0.21±0.034 | 0.20±0.012 | 0.20±0.030 | 0.20±0.025 | 0.16±0.0068 | |
| Lac/Cho | 0.13±0.0037 | 0.13±0.0030 | 0.13±0.0031 | 0.13±0.0026 | 0.12±0.0041 | 0.12±0.0041 | 0.12±0.0081 | 0.11±0.00073 | 0.11±0.0071 | 0.11±0.0026 | 0.11±0.00064 | |
| FWM | NAA/Cho | 0.38±0.025 | 0.42±0.013 | 0.45±0.035 | 0.49±0.022 | 0.49±0.039 | 0.52±0.024 | 0.48±0.00000 | 0.55±0.019 | |||
| Lac/NAA | 0.39±0.026 | 0.37±0.013 | 0.35±0.036 | 0.32±0.022 | 0.32±0.040 | 0.31±0.024 | 0.36±0.00021 | 0.31±0.020 | ||||
| Lac/Cho | 0.14±0.00012 | 0.15±0.00006 | 0.15±0.00017 | 0.15±0.00011 | 0.15±0.00019 | 0.15±0.00012 | 0.16±0.00000 | 0.16±0.00009 |
Note: Missing data points in selective regions are due to limited spatial coverage (CST, FWM) and poor data quality (VA, CGM) from poorly shimmed regions.
Sedation had no effect on the metabolite ratios and their trends over the age range for all regions.
Discussion
In this study, we utilized 3D MRSI to report metabolic ratios of premature newborns with normative outcome. MR spectroscopy has been shown to be a valuable tool for evaluating brain injury in neonates (1,4,6,7). However the complexity in performing, processing, and analyzing 3D MRSI have largely limited the acquisition to 2D and single voxel studies of the neonatal brain. Also, as noted by McNatt et al (14), normative 1H MRS data in newborns are not well established and institutions rely on their own experiences and results to determine the severity of the injury. Various groups use LCModel to quantify metabolites (14,15), which can be difficult for many institutions to perform routinely in the clinical setting due to computationally intensive post-processing requirements. In addition, absolute metabolite quantitation using modeling also assumes a clear definition of peaks where the beginning and the end of the peak locations are set. This is always a challenge to establish with in vivo spectra. A normal metabolite baseline using simple metabolite ratios would facilitate differentiating between normal and abnormal brains in premature neonates. Only newborns with normal neurological follow-up examination at 1 year of age were included in this study in order to establish a normal baseline, to which proton MRS of future premature newborns and newborns with birth complications can be compared. It is already known that NAA/Cho ratio increases as the brain matures, which has been previously reported at specific time points (1,16). This study provides evaluation of metabolite levels for the entire brain in prematurely born neonates with correlation to gestational ages from 27 weeks to full term. Although we recognize that a normal 12-month exam does not guarantee that the child will develop completely normally, it suggests that any subsequently developing abnormalities will be relatively mild (17). In this normative population, lactate level is barely visible, which confirms previous studies indicating injury with elevated levels of lactate (7).
NAA, a marker for neuronal activity, increases with brain maturity. Concurrently, Cho, a marker for membrane turnover and myelination, and LAC, a marker of anaerobic respiration, decrease with age as the rapid brain growth of the neonate slows in infancy; these findings have been reported previously using single voxel techniques (1,15,18). The study demonstrated that NAA/Cho significantly increased with age for all the regions of interest across the entire premature newborn brain. Among the regions, cortical spinal tract’s highest ratio inferred that it had the highest neuronal activity and possibly matured first. In comparison, the temporal visual association pathway showed the lowest ratio, as it is a relatively immature pathway at this age.
Also, the parietal white matter had higher NAA/Cho ratio than the frontal white matter, possibly demonstrating the sensory pathway is quicker to mature compared to the motor pathway (19). Lac/NAA ratio should decrease with age, as the rapid growth of the newborn brain starts to slow allowing adequate ATP production for growth and metabolism by aerobic respiration and starts rely on glucose for energy (20). Figure 4 demonstrates that the Lac/NAA ratio decreased over time in all regions, but this was not always statistically significant. The lack of significance is likely due to the limited patient population as individuals have different metabolic rates, which will contribute to the variability of the data. In addition, the provided gestational age is an approximate estimation of the actual age of the newborn; this can contribute to the variability as a few days of neonatal brain development may yield different results. It could also result from the fact that some regions, such as the frontal white matter and temporal visual association tracts, remain relatively immature at 42 weeks (19); longer follow-up may be necessary to demonstrate this phenomenon. We expect all regions to show significant decreasing Lac/NAA as we continue to build our newborn metabolite database with more premature newborns, particularly as a wider adjusted age range is included.
The variation in Lac/Cho ratio shown in Figure 5 is expected, as Lac and Cho both decrease with maturity, but the precise biochemical changes that underly these decreases are not known and are likely to vary somewhat from region to region. Individuals mature slightly differently, so the decrease in lactate and choline levels will vary, resulting in no correlation with gestational age. The included premature newborns in this study have normative neuromotor outcome are slightly older (approximately 34 weeks post-conception at scan with 28 weeks at birth), which may suggest that newborns with higher gestational age have a better chance of having a normal growth trajectory in comparison to younger premature infants. Further studies are ongoing to investigate the cohorts with poor outcome of newborns with abnormal neuromotor scores, indicative of abnormal growth.
As expected, sedation had no effect on the overall trend of metabolite levels in correlation with age. Unlike previously reported (21), the metabolite levels for patients with and without sedation did not differ. It is possible that with large amount of data, that variability is not significant.
This study demonstrated the feasibility of the 3D MRSI method to analyze the spatial and temporal variations of brain cellular metabolite levels in preterm infants. This study establishes a baseline for regional metabolite levels, which may be used to assess any association of brain injury with regional metabolite ratios at similar post-conceptual ages and with neurodevelopmental outcome. The current study is still limited by the incomplete coverage of the brain with 8×8×8 matrix at 1cc due to scan time (17 minutes) concerns as demonstrated by missing data points for CST and FWM. Even with this long duration, there were usable spectral data in all exams due the robustness of the technique. Spectroscopy has a small amount of motion correction built into the analysis due to the phase correction algorithm. With the development of newer phased array coil technology and associated parallel imaging (22) and fast acquisition techniques (23), it is now possible to obtain similar quality of 3D MRSI with full brain coverage at much shorter scan times of 10 minutes or less and, thus, to assess the value of this technique in clinical practice.
Acknowledgments
This work was supported by NIH grant 1R01NS046432, 1R01EB009756
References
- 1.Kimura H, Fujii Y, Itoh S, et al. Metabolic Alterations in the Neonate and Infant Brain during Development - Evaluation with Proton Mr Spectroscopy. Radiology. 1995;194(2):483–489. doi: 10.1148/radiology.194.2.7529934. [DOI] [PubMed] [Google Scholar]
- 2.Barkovich AJ, Miller SP, Bartha A, et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol. 2006;27(3):533–547. [PMC free article] [PubMed] [Google Scholar]
- 3.Fan G, Wu Z, Chen L, Guo Q, Ye B, Mao J. Hypoxia-ischemic encephalopathy in full-term neonate: correlation proton MR spectroscopy with MR imaging. Eur J Radiol. 2003;45(2):91–98. doi: 10.1016/s0720-048x(02)00021-9. [DOI] [PubMed] [Google Scholar]
- 4.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357(19):1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 5.Cecil KM, Jones BV. Magnetic resonance spectroscopy of the pediatric brain. Top Magn Reson Imaging. 2001;12(6):435–452. doi: 10.1097/00002142-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Barkovich AJ, Hajnal BL, Vigneron D, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol. 1998;19:143–150. [PMC free article] [PubMed] [Google Scholar]
- 7.Barkovich AJ, Baranski K, Vigneron D, et al. Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. AJNR Am J Neuroradiol. 1999;20(8):1399–1405. [PMC free article] [PubMed] [Google Scholar]
- 8.Dumoulin CL, Rohling KW, Piel JE. Magnetic resonance imaging compatible neonate incubator. Magnetic Resonance Engineering. 2002;15:117–128. [Google Scholar]
- 9.Hajnal BL, Sahebkar-Moghaddam F, Barnwell AJ, Barkovich AJ, Ferriero DM. Early prediction of neurologic outcome after perinatal depression. Pediatr Neurol. 1999;21(5):788–793. doi: 10.1016/s0887-8994(99)00101-0. [DOI] [PubMed] [Google Scholar]
- 10.Luyten PR, Marien AJ, den Hollander JA. Acquisition and quantitation in proton spectroscopy. NMR Biomed. 1991;4(2):64–69. doi: 10.1002/nbm.1940040206. [DOI] [PubMed] [Google Scholar]
- 11.Star-Lack J, Nelson SJ, Kurhanewicz J, Huang LR, Vigneron DB. Improved water and lipid suppression for 3D PRESS CSI using RF band selective inversion with gradient dephasing (BASING) Magn Reson Med. 1997;38(2):311–321. doi: 10.1002/mrm.1910380222. [DOI] [PubMed] [Google Scholar]
- 12.Tran TK, Vigneron DB, Sailasuta N, et al. Very selective suppression pulses for clinical MRSI studies of brain and prostate cancer. Magn Reson Med. 2000;43(1):23–33. doi: 10.1002/(sici)1522-2594(200001)43:1<23::aid-mrm4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Nelson SJ. Analysis of volume MRI and MR spectroscopic imaging data for the evaluation of patients with brain tumors. Magn Reson Med. 2001;46(2):228–239. doi: 10.1002/mrm.1183. [DOI] [PubMed] [Google Scholar]
- 14.McNatt SA, McComb JG, Nelson MD, Bluml S. Proton magnetic resonance spectroscopy of hydrocephalic infants. Pediatr Neurosurg. 2007;43(6):461–467. doi: 10.1159/000108788. [DOI] [PubMed] [Google Scholar]
- 15.Kreis R, Hofmann L, Kuhlmann B, Boesch C, Bossi E, Huppi PS. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 2002;48(6):949–958. doi: 10.1002/mrm.10304. [DOI] [PubMed] [Google Scholar]
- 16.Vigneron DB, Barkovich AJ, Noworolski SM, et al. Three-dimensional proton MR spectroscopic imaging of premature and term neonates. AJNR Am J Neuroradiol. 2001;22(7):1424–1433. [PMC free article] [PubMed] [Google Scholar]
- 17.Mercuri E, Barnett A, Rutherford M, et al. Neonatal cerebral infarction and neuromotor outcome at school age. Pediatrics. 2004;113(1 Pt 1):95–100. doi: 10.1542/peds.113.1.95. [DOI] [PubMed] [Google Scholar]
- 18.Bartha AI, Yap KRL, Miller SP, et al. The Normal Neonatal Brain: MR Imaging, Diffusion Tensor Imaging, and 3D MR Spectroscopy in Healthy Term Neonates. AJNR Am J Neuroradiol. 2007;28(6):1015–1021. doi: 10.3174/ajnr.A0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tau GZ, Peterson BS. Normal Development of Brain Circuits. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vannucci SJ, Hagberg H. Hypoxia-ischemia in the immature brain. J Exp Biol. 2004;207(Pt 18):3149–3154. doi: 10.1242/jeb.01064. [DOI] [PubMed] [Google Scholar]
- 21.Wang ZJ, Vigneron DB, Miller SP, et al. Brain metabolite levels assessed by lactate-edited MR spectroscopy in premature neonates with and without pentobarbital sedation. AJNR Am J Neuroradiol. 2008;29(4):798–801. doi: 10.3174/ajnr.A0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozturk E, Banerjee S, Majumdar S, Nelson SJ. Partially parallel MR spectroscopic imaging of gliomas at 3T. Conf Proc IEEE Eng Med Biol Soc. 2006;1:493–496. doi: 10.1109/IEMBS.2006.259793. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham CH, Vigneron DB, Chen AP, et al. Design of flyback echo-planar readout gradients for magnetic resonance spectroscopic imaging. Magn Reson Med. 2005;54(5):1286–1289. doi: 10.1002/mrm.20663. [DOI] [PubMed] [Google Scholar]