Summary
Enterotoxigenic Escherichia coli (ETEC) causes human morbidity and mortality in developing nations and is an emerging threat to food safety in developed nations. The ETEC heat-labile enterotoxin (LT) not only causes diarrheal disease by deregulating host adenylate cyclase, but also enhances ETEC adherence to intestinal epithelial cells. The mechanism governing this LT pro-adherence phenotype is unclear. Here we investigated intestinal epithelial cell signal transduction pathways activated by ETEC and quantified the relative importance of these host pathways to LT-induced ETEC adherence. We show that ETEC activates both NF-κB and mitogen-activated protein kinase (MAPK) signaling pathways through mechanisms that are primarily dependent upon LT. LT-induced NF-κB activation depends upon the cAMP-dependent activation of the Ras-like GTPase Rap1 but is independent of protein kinase A (PKA). By using inhibitors of these pathways, we demonstrate that inhibiting the p38 MAPK prevents LT from increasing ETEC adherence. By contrast, the LT pro-adherence phenotype appears unrelated to both LT-induced Rap1 activity and to subsequent NF-κB activation. We speculate that LT may alter host signal transduction to induce the presentation of ligands for ETEC adhesins in such a way that promotes ETEC adherence. Our findings provide insight into previously unexplored functions of LT and their relative importance to ETEC virulence.
Keywords: Enterotoxigenic Escherichia coli, heat-labile enterotoxin, MAPK, NF-κB, Rap1
Introduction
Enterotoxigenic Escherichia coli (ETEC) causes enterotoxin-induced diarrhea and is a source of human morbidity and mortality (Turner et al., 2006). ETEC causes both travelers’ diarrhea (Rowe et al., 1970) and infectious diarrhea in developing nations (Sears et al., 1996). ETEC is considered an emerging cause of food-borne disease in developed nations (Beatty et al., 2006) and also causes disease in pigs (Moxley et al., 1998), further negatively impacting the food supply.
ETEC virulence is associated with the production of colonization factors (adhesins) and with one or more enterotoxins, including heat-labile (LT) and heat-stable forms (Fleckenstein et al., 2010). The enzymatic activity of these enterotoxins causes diarrhea by inducing water and electrolyte loss from the intestines of infected patients (Sears et al., 1996). LT, like the closely-related cholera toxin (CT), consists of an enzymatically-active A subunit and five B subunits that mediate binding to host GM1 ganglioside receptors (Finkelstein et al., 1974). The A subunit possesses an ADP-ribosylation activity that modifies the Gsα protein, leading to deregulation of adenylate cyclase and uncontrolled production of 3′5′-cyclic adenosine monophosphate (cAMP). Increased cAMP concentrations activate protein kinase A (PKA)-dependent pathways, resulting in activation of the cystic fibrosis transmembrane conductance regulator (CFTR), and ultimately causing chloride and water secretion (Sears et al., 1996).
In addition to its important role in causing secretory diarrhea, LT also provides a competitive advantage to ETEC adherence to cultured intestinal epithelial cells (Johnson et al., 2009), as well as to colonization of the murine (Allen et al., 2006) and porcine (Berberov et al., 2004) gut. We previously implicated PKA-dependent host pathways as important to LT-mediated enhancement of ETEC adherence (Johnson et al., 2009). However, the mechanism underlying this LT pro-adherence phenotype is unclear. PKA has many downstream targets, including NF-κB and mitogen-activated protein kinases (MAPKs). CT alters the activity of pathways regulated by various MAPKs including p38, JNK, and ERK (Chakraborty et al., 2008). ETEC alters the interleukin-8 response, an NF-κB-regulated pro-inflammatory cytokine in intestinal epithelial cells (Huang et al., 2004).
Here we determined the extent to which ETEC infection of intestinal adenocarcinoma HCT-8 cells activates NF-κB and MAPK pathways. We also quantified the dependence upon LT expression of the ability of ETEC to modulate host signal transduction pathways. We show that ETEC activates both NF-κB and MAPK signaling pathways through mechanisms primarily dependent upon LT. LT-induced NF-κB activation depends upon the cAMP-dependent activation of the Ras-like GTPase Rap1 but is independent of PKA. By using inhibitors of these pathways, we demonstrate that inhibiting the p38 MAPK prevents LT from increasing ETEC adherence. By contrast, the LT pro-adherence phenotype appears unrelated to LT-induced Rap1 activity and subsequent NF-κB activation.
Results
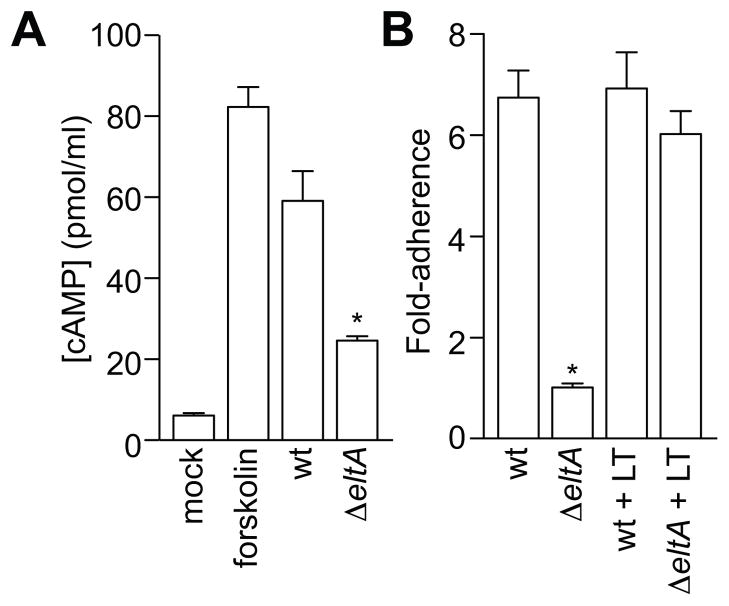
LT enhances ETEC H10407 adherence to HCT-8 cells
We showed previously that ETEC strains possessing LT adhere more avidly to cultured intestinal epithelial cells and induce the production of higher levels of cAMP, as compared with ETEC strains lacking LT (Johnson et al., 2009). To begin to determine the mechanism governing LT-induced adherence, we further developed assays using both the prototypical human isolate ETEC H10407 (Evans et al., 1975) and HCT-8 cells, a cell line derived from a human ileocecal colorectal adenocarcinoma (Tompkins et al., 1974) frequently used to study both ETEC and Vibrio cholerae.
We first determined whether ETEC was able to increase cAMP production in HCT-8 cells. To do this, we measured cAMP concentrations in cell lysates after infecting cells with either wild type (wt) or LT-deficient ΔeltA ETEC H10407. As expected, forskolin, a potent activator of adenylate cyclase, increased cAMP production approximately 8-fold (Fig. 1A). Similarly, infecting HCT-8 cells with wt ETEC significantly increased cAMP production (~6-fold) from HCT-8 cells. By contrast, infecting cells with ΔeltA ETEC increased cAMP production only ~2-fold.
Figure 1. LT enhances ETEC H10407 adherence to HCT-8 cells.
A. Quantification of cAMP (pmol/ml) obtained from HCT-8 cell lysates treated with forskolin or infected with ETEC H10407 strains possessing (wild-type; wt) or lacking LT (ΔeltA). Data are presented as mean ± SEM, n = 3, with asterisks (*) used to indicate a statistically significant difference from wt ETEC (p < 0.05, Student’s t test). B. Quantification of ETEC adherence (relative to the adherence of ΔeltA) to HCT-8 cells after a 1 h infection in the absence or presence of 100 ng/ml LT. Data are presented as mean ± SEM, n = 4, with asterisks (*) used to indicate a statistically significant difference from wt ETEC (p < 0.05, Student’s t test).
We also performed bacterial adherence assays to verify that LT expression enhances ETEC adherence to HCT-8 cells. Relative to the adherence of ΔeltA ETEC (set to 1.0), wt ETEC adherence was increased 6.7 ± 0.5-fold (Fig. 1B). This difference in adherence between wt and ΔeltA ETEC was independent of the multiplicity of infection (MOI) used in infection experiments (not shown). By exposing HCT-8 cells to purified LT holotoxin, we augmented ΔeltA adherence to levels not significantly different (6.0 ± 0.5-fold) from wt ETEC. By contrast, adding purified LT to wt ETEC induced no further increase in adherence. Thus, LT enhances both cAMP production and ETEC adherence to HCT-8 cells.
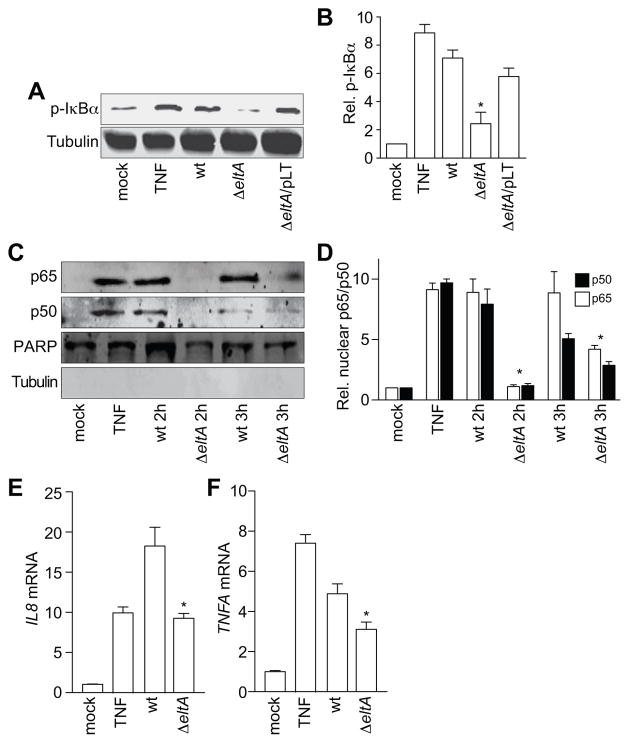
ETEC induces LT-dependent NF-κB activation
The NF-κB pathway plays a central role in activating pro-inflammatory host responses to pathogens. To determine whether ETEC activates NF-κB signaling in HCT-8 cells, we performed infection experiments and then quantified the extent of phosphorylation of the NF-κB inhibitory subunit IκBα. We used TNF-α (10 ng/ml, 30′) as a positive control to induce NF-κB activation. Wild-type, but not ΔeltA ETEC, induced a significant increase in IκBα phosphorylation after a 2 h post-infection (Fig. 2A–B). We obtained similar data at 3 h post-infection (not shown). Infecting HCT-8 cells with ΔeltA complemented with an LT-expression plasmid (ΔeltA/pLT), restored IκBα phosphorylation to a level similar to that observed with wild-type ETEC, suggesting that maximal NF-κB activation during ETEC infection depends upon LT (Fig. 2A–B).
Figure 2. ETEC induces LT-dependent NF-κB activation.
A. Immunoblotting of phosphorylated IκBα after treating HCT-8 cells with TNF-α (20 ng/ml, 30′) or infecting them with either wt, ΔeltA, or ΔeltA/pLT ETEC for 2 h. B. Quantification of relative phosphorylated IκBα levels shown in panel A. Data are presented as mean ± SEM, n = 5, with asterisks (*) used to indicate a statistically significant difference from wt ETEC (p < 0.05, Student’s t test). C. Immunoblotting of nuclear-localized NF-κB p65 and p50 subunits after treating HCT-8 cells with TNF-α (20 ng/ml, 30′) or infecting them with either wt or ΔeltA ETEC for 2 or 3 h. PARP and tubulin immunoblotting were used to normalize nuclear protein concentrations and to demonstrate the absence of contaminating cytoplasmic proteins in the nuclear fractions, respectively. D. Quantification of nuclear p65 and p50 data shown in panel C. Data are presented as mean ± SEM, n = 3, with asterisks (*) used to indicate a statistically significant difference from wt ETEC (p < 0.05, Student’s t test). E–F. Quantification of the relative abundance of IL8 (E) and TNFA (F) transcripts after treating HCT-8 cells with TNF-α (20 ng/ml, 30′) or infecting them with ETEC (2 h). Data are presented as mean ± SEM, n = 3, with asterisks (*) used to indicate a statistically significant difference from wt ETEC (p < 0.05, Student’s t test).
We next determined whether LT-induced IκBα phosphorylation was sufficient to induce the nuclear translocation of the NF-κB p50/p65 subunits during ETEC infection. To do this, we infected HCT-8 cells with ETEC and, after 2 or 3 h, fractionated the cells to obtain nuclear extracts. We quantified poly(ADP-ribose) polymerase (PARP) to normalize the concentrations of nuclear protein fractions. The lack of tubulin in the nuclear fraction demonstrated the absence of significant cross-contamination from cytoplasmic proteins (Fig. 2C). Wild-type, but not ΔeltA ETEC, significantly promoted the nuclear translocation of the NF-κB p50/p65 subunits at 2 h post-infection (Fig. 2D). We observed that after a 3 h infection, ΔeltA ETEC also induced a modest amount of p50/p65 nuclear translocation.
NF-κB regulates the transcription of genes encoding pro-inflammatory cytokines, including IL-8 and TNF-α. To demonstrate the functional significance of LT-dependent NF-κB activation, we quantified IL8 and TNFA transcript abundance. These transcripts were both significantly enriched in HCT-8 cells infected with wt ETEC, by comparison with cells infected with ΔeltA ETEC (Fig 2E–F).
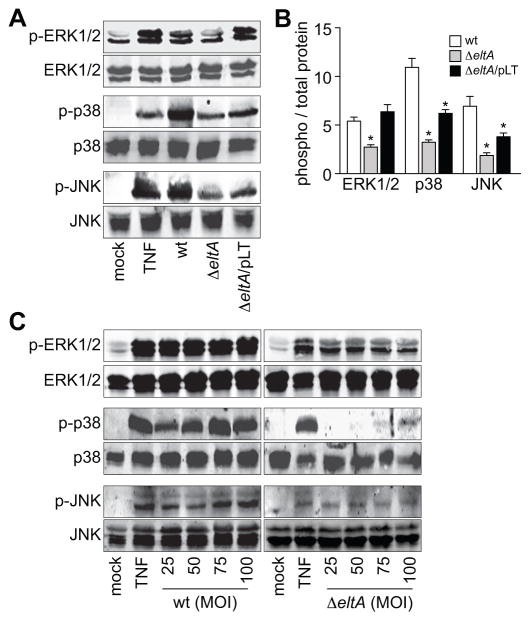
ETEC induces LT-dependent MAPK activation
In addition to NF-κB, mitogen-activated protein kinases (MAPKs; e.g. p38 MAPK, ERK1/2, and JNK) also regulate host responses to bacterial infection. To determine whether ETEC activates p38 MAPK, ERK1/2, and/or JNK, we performed infection experiments. We examined MAPK activation by quantifying the activation-associated phosphorylation of p38 MAPK, ERK1/2, and JNK using phospho-specific antibodies. As expected, treating cells with TNF-α induced the phosphorylation of p38, ERK1/2, and JNK. These MAPKs were also strongly activated by wt ETEC infection (Fig. 3A–B). By contrast to wt ETEC, infecting cells with ΔeltA ETEC induced only slight increases in ERK1/2, p38, and JNK phosphorlyation (Fig. 3A–B). Complementing ΔeltA with pLT fully restored ERK1/2 activation to levels similar to that observed after wt ETEC infection, and partially restored both p38 and JNK activation. These data suggest that MAPK activation during ETEC infection is dependent primarily upon LT. Differences between the wt and the complementation strains may be attributable to the levels of LT produced and/or secreted by wt vs. ΔeltA/pLT ETEC. We performed additional infection experiments by titrating the MOI (from 25 to 100) of both wt and ΔeltA ETEC. At all MOIs tested, the magnitude of MAPK activation was significantly higher in cells infected with wt as compared with cells infected with ΔeltA ETEC (Fig. 3C).
Figure 3. ETEC induces LT-dependent MAPK activation.
A. Immunoblotting of total and phosphorylated ERK1/2, p38, and JNK after treating HCT-8 cells with TNF-α (20 ng/ml, 30′) or infecting them with either wt, ΔeltA, or ΔeltA/pLT ETEC for 2 h. B. Quantification of relative phosphoprotein abundance from data shown in panel A. Data are presented as mean ± SEM, n = 3–4, with asterisks (*) used to indicate a statistically significant difference from wt ETEC (p < 0.05, Student’s t test). C. Immunoblotting of total and phosphorylated ERK1/2, p38, and JNK after treating HCT-8 cells with TNF-α (20 ng/ml, 30′) or infecting them with variable amounts of either wt or ΔeltA ETEC (MOIs ranging from 25–100).
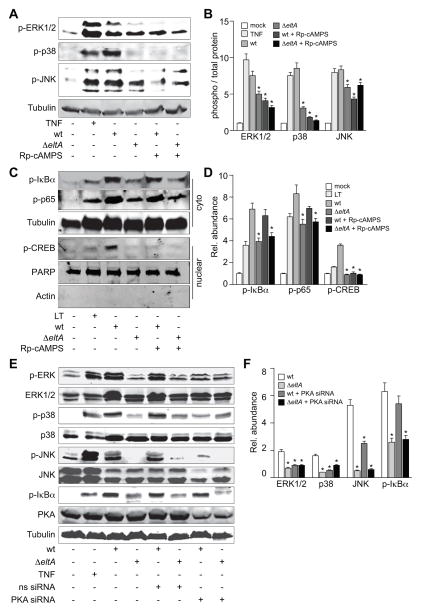
ETEC activation of MAPKs, but not NF-κB, is PKA-dependent
Because wt, but not ΔeltA ETEC strongly activated NF-κB and the p38/ERK/JNK MAPKs, we next determined the extent to which these phenotypes are dependent upon the activity of host protein kinase A (PKA). To do this, we used Rp-cAMPS, a specific inhibitor of PKA, to block the cAMP-mediated PKA activation induced by LT during ETEC infection. Pre-treating HCT-8 cells with Rp-cAMPS decreased the MAPK phosphorylation normally induced by wt ETEC (Fig. 4A–B), indicating that ETEC activation of MAPKs is PKA-dependent.
Figure 4. ETEC activation of MAPKs, but not NF-κB, is PKA-dependent.
A. Immunoblotting of phosphorylated ERK1/2, p38, and JNK after treating HCT-8 cells with TNF-α (20 ng/ml, 30′) or infecting them with either wt or ΔeltA ETEC for 2 h. Where indicated, HCT-8 cells were first treated with Rp-cAMPS (200 μM, 1 h). B. Quantification of relative phosphoprotein abundance from data shown in panel A. Data are presented as mean ± SEM, n = 3–5, with asterisks (*) used to indicate a statistically significant difference from wt ETEC (p < 0.05, Student’s t test). C. Immunoblotting of phosphorylated IκBα, phosphorylated p65, and phosphorylated nuclear CREB after treating HCT-8 cells with LT (1 μg/ml, 30′) or infecting them with either wt or ΔeltA ETEC for 2 h. Where indicated, HCT-8 cells were first treated with Rp-cAMPS (200 μM, 1 h). D. Quantification of data shown in panel C. Data are presented as mean ± SEM, n =3, with asterisks (*) used to indicate a statistically significant difference from wt ETEC (p < 0.05, Student’s t test). E. Immunoblotting of phosphorylated p38, ERK1/2, JNK, IκBα, and nuclear NF-κB p65 after treating HCT-8 cells with TNF-α (20 ng/ml, 30′) or infecting them with wt or ΔeltA ETEC for 2 h. Where indicated, HCT-8 cells were first transfected (48 h prior to infection) with an siRNA duplex targeting PKA. Phosphorylated protein abundance was normalized to total protein abundance and/or to tubulin. F. Quantification of data shown in panel E. Data are presented as mean ± SEM, n = 3, with asterisks (*) used to indicate a statistically significant difference from wt ETEC (p < 0.05, Student’s t test).
PKA mediates the phosphorylation of the cAMP response element binding (CREB) protein on Ser133 (Lalli et al., 1994). We used this phosphorylation event, which takes place in the nucleus, as an additional marker of PKA activation. To determine whether wt ETEC infection activated PKA and induced CREB S133 phosphorylation, we performed infection experiments in the presence or absence of Rp-cAMPS. Wild-type, but not ΔeltA ETEC induced CREB S133 phosphorylation (Fig. 4C–D). This phosphorylation was blocked by first treating HCT-8 cells with Rp-cAMPS, suggesting that the LT-induced activation of MAPKs during ETEC infection depends upon PKA activation.
We also determined if PKA activation was required to induce the NF-κB pathway in HCT-8 cells infected with ETEC. By contrast to MAPK activation, treating HCT-8 cells with Rp-cAMPS did not block the phosphorylation of either IκBα or p65 induced by wt ETEC, suggesting that LT-induced NF-κB activation is independent of PKA activity (Fig. 4C–D).
To substantiate further the role of PKA in LT-induced MAPK activation during ETEC infection, we also used siRNA to knockdown PKA abundance in HCT-8. Knocking down PKA levels by ~50 %, reduced the magnitude of MAPK activation during ETEC infection, but had no significant impact upon NF-κB activation (Fig. 4E–F).
ETEC activation of NF-κB depends upon Rap1 activation
PKA-independent pathways also respond to changes in [cAMP]. While we observed LT-induced NF-κB activation to be independent of PKA, we considered whether NF-κB activation might still be responsive to elevated [cAMP] in infected HCT-8 cells. To test this idea, we pre-treated HCT-8 cells with 2′, 5′-dideoxyadenosine (DDA), a specific inhibitor of adenylate cyclase, and then infected these cells with wt ETEC or ΔeltA ETEC. Pre-treating cells with DDA significantly reduced the IκBα phosphorylation normally induced by wt ETEC (Fig. 5A–B). By contrast, DDA did not alter TNF-α-induced IκBα phosphorlyation. These data suggest that elevated [cAMP] induced by LT is sufficient to activate NF-κB during ETEC infection, possibly through a PKA-independent mechanism.
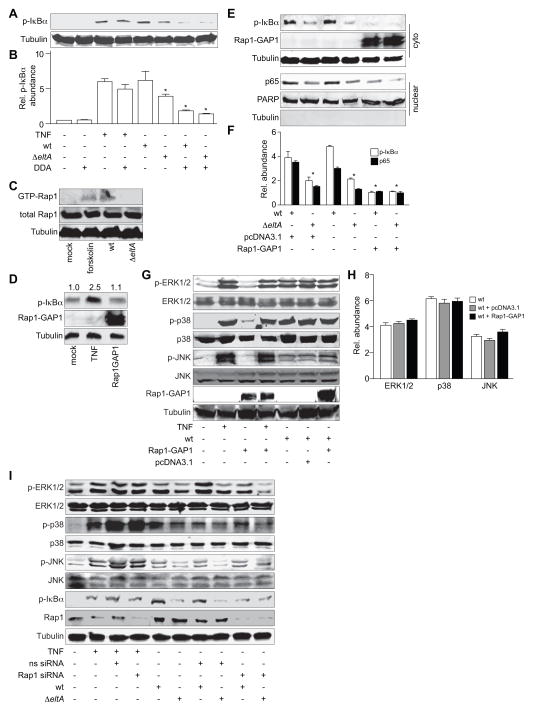
Figure 5. ETEC activation of NF-κB depends upon Rap1 activation.
A. Immunoblotting of phosphorylated IκBα after treating HCT-8 cells with TNF-α (20 ng/ml, 30′) or infecting them with either wt or ΔeltA ETEC for 2 h. Where indicated, HCT-8 cells were first treated with DDA (200 μM, 1 h). B. Quantification of data shown in panel A. Data are presented as mean ± SEM, n = 3, with asterisks (*) used to indicate a statistically significant difference from wt ETEC (p < 0.05, Student’s t test). C. Immunoblotting of GTP-bound (activated) and total Rap1 after treating HCT-8 cells with forskolin (10 μM, 1 h) or infecting them with either wt or ΔeltA ETEC for 2 h. D. Immunoblotting of phosphorylated IκBα after transfecting cells with a Rap1-GAP1 expression plasmid. The numbers above the immunoblots indicate normalized phosphorylated IκBα abundance. E. Immunoblotting of phosphorylated IκBα, nuclear p65, and Rap1-GAP1 after infecting HCT-8 cells with either wt or ΔeltA ETEC for 2 h, in the presence or absence of Rap1-GAP1 or empty vector. F. Quantification of IκBα phosphorylation and nuclear p65 levels shown in panel E. Data are presented as mean ± SEM, n = 3–4, with asterisks (*) used to indicate a statistically significant difference from wt ETEC (p < 0.05, Student’s t test). G. Immunoblotting of total and phosphorylated ERK1/2, p38, and JNK after infecting HCT-8 cells with wt ETEC for 2 h, in the presence or absence of Rap1-GAP1 or empty vector. Phosphorylated protein abundance was normalized to total protein abundance. H. Quantification of data shown in panel G. I. Immunoblotting of total and phosphorylated ERK1/2, p38, and JNK, phosphorylated IκBα, Rap1, and tubulin after treating HCT-8 cells with TNF-α (20 ng/ml, 30′) or infecting them with either wt or ΔeltA for 2 h. Where indicated, HCT-8 cells were first transfected (48 h prior to infection) with an siRNA duplex targeting Rap1 or a non-specific (ns) siRNA duplex.
In addition to PKA–dependent pathways, LT also has the potential to activate PKA–independent pathways such as those involving Epac (exchange protein directly activated by cAMP) proteins. Epac proteins are guanine exchange factors for specific Ras GTPases (Borland et al., 2009). cAMP binding to Epac1 induces Epac1 translocation to the plasma membrane where it binds to the Ras-like GTPase Rap1 (de Rooij et al., 1998, Ponsioen et al., 2009). Once activated, Rap1 regulates processes involved in regulating cellular adhesion (Caron, 2003), microtubule length and cortical actin, and cell barrier functions (Sehrawat et al., 2008).
Because elevated cAMP concentrations can activate Rap1 independently of PKA, we sought to determine if infecting HCT-8 cells with ETEC could activate Rap1 and if so, whether activating Rap1 would result in NF-κB activation. As expected, treating cells with forskolin activated Rap1. Wild-type, but not ΔeltA ETEC, also induced significant Rap1 activation (Fig. 5C).
Rap1-GAP1 is a GTPase-activating protein that inhibits Rap1 activation. We next transfected cells with a Rap1-GAP1 expression plasmid to block ETEC-induced Rap1 activation and determine the downstream impact on NF-κB activation. We first verified that expressing Rap1-GAP1 had no direct impact on the basal IκBα phosphorylation state in HCT-8 cells (Fig. 5D). Blocking Rap1 activation with Rap1-GAP1 significantly decreased both the IκBα phosphorylation and the subsequent nuclear translocation of the NF-κB p65 subunit normally induced by wt ETEC (Fig. 5E–F), suggesting that the Rap1 activation mediated by LT-induced increases in host [cAMP] causes NF-κB activation in infected intestinal epithelial cells.
We further determined whether blocking Rap1 activation by expressing Rap1-GAP1 would inhibit the MAPK pathway activation normally induced by LT during ETEC infection. By contrast to the impact of Rap1-GAP1 on NF-κB activation (Fig. 5E–F), over-expressing Rap1-GAP1 had no impact on p38, ERK1/2, or JNK activation (Fig. 5G–H), suggesting that LT-induced NF-κB, but not MAPK pathways are dependent upon Rap1 activation.
To corroborate our Rap1-GAP1 overexpression data, we also performed experiments in which we used siRNA to knockdown Rap1 abundance in HCT-8 cells. Depleting Rap1 abundance by ~70 % reduced the extent of IκBα phosphorylation induced by wt ETEC infection (Fig. 5I). By contrast, Rap1-knockdown had no significant impact upon MAPK activation (Fig. 5I), consistent with data obtained using Rap1-GAP1 overexpression, and thus reinforcing the notion that LT-induced NF-κB, but not MAPK pathways are dependent upon Rap1 activation.
LT-induced MAPK activation promotes ETEC adherence
LT promotes ETEC adherence to intestinal epithelial cells by increasing host [cAMP] and subsequently activating PKA (Johnson et al., 2009). Rp-cAMPS, a specific inhibitor of PKA activation (Rothermel et al., 1983), blocked the ability of LT to increase bacterial adherence to mammalian cells (Fig. 6A). Because our data indicated that ETEC activates the NF-κB and MAPK pathways, as well as Rap1, we next determined which of these signaling pathways contribute to the LT pro-adherence phenotype.
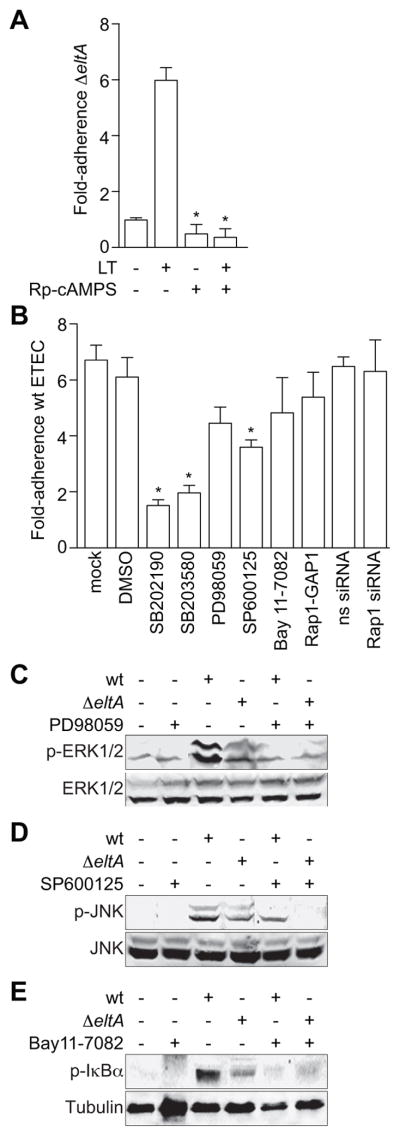
Figure 6. LT-induced MAPK activation promotes ETEC adherence.
A. Quantification of ΔeltA ETEC adherence to HCT-8 cells in the presence or absence of 100 ng/ml LT and/or200 μM Rp-cAMPS. Data are normalized to the magnitude of adherence of ΔeltA ETEC to untreated HCT-8 cells and represent the fold-change in adherence. Data are presented as mean ± SEM, n = 4, with asterisks (*) used to indicate a statistically significant difference from LT-treated cells (p < 0.05, Student’s t test). B. Quantification of wt ETEC adherence to HCT-8 cells after pre-treating cells (1 h) with 20 μM SB202190, 20 μM SB203580, 50 μM PD98059, 40 μM SP600125, 20 μM Bay 11-7082, or 48 h after transfecting Rap1-GAP1 plasmid or a Rap1 or a non-specific (ns) siRNA duplex. Data are normalized to the magnitude of adherence of wt ETEC to untreated HCT-8 cells and represent the fold-change in adherence. Data are presented as mean ± SEM, n = 3–8, with asterisks (*) used to indicate a statistically significant difference from wt ETEC (p < 0.05, Student’s t test). C–E. Immunoblotting of total and phosphorylated ERK1/2, JNK, or IκBα and tubulin after infecting cells with wt or ΔeltA ETEC for 2 h. Where indicated, HCT-8 cells were first treated with 50 μM PD98059 (panel C), 40 μM SP600125 (panel D), or 20 μM Bay11-7082 (panel E) for 1 h.
We pre-treated HCT-8 cells with specific inhibitors of NF-κB or MAPK pathways and then infected these cells with wt ETEC. Pre-treating cells with SB202190 and SB203580, two inhibitors of the p38 MAPK, significantly reduced wt ETEC adherence (78 and 74 % reduction, respectively; Fig. 6B). Inhibiting either ERK1/2 (with PD98059) or JNK (with SP60012) also had a measurable, but less significant impact upon ETEC adherence (35 and 60 % reduction, respectively). Thus, the ability of LT to promote adherence depends upon activating specific PKA/MAPK signaling pathways. We confirmed that pre-treating cells with these inhibitors appropriately blocked the activation of pathways normally induced by ETEC infection (Fig. 6C–E). Further, these inhibitors did not alter bacterial growth rates (data not shown), indicating that the reduced ETEC adherence was not related to impaired bacterial survival.
By contrast to the MAPK inhibitors, inhibiting NF-κB activation with Bay11-7082 did not significantly reduce wt ETEC adherence. We showed that LT-induced Rap1 activity mediates NF-κB activation in ETEC-infected intestinal epithelial cells (Fig. 5E–F). Neither over-expressing Rap1-GAP1 to block Rap1 activation, nor depleting Rap1 by using siRNA, had significant impact on ETEC adherence (Fig. 6B). Thus, ETEC activation of Rap1 and NF-κB appears unrelated to the LT pro-adherence phenotype.
Discussion
LT has been extensively characterized in terms of its structure, enzymology, and its role in causing secretory diarrhea (Fleckenstein et al., 2010). Less well characterized is the selective advantage that expressing LT confers to ETEC. Recent in vitro (Johnson et al., 2009) and in vivo (Allen et al., 2006, Berberov et al., 2004) studies suggest that LT promotes colonization of the small intestine by increasing the adherence of the pathogen to mammalian cells. Here we investigated host signal transduction pathways modulated by LT and their role in LT-induced ETEC adherence (Fig. 7).
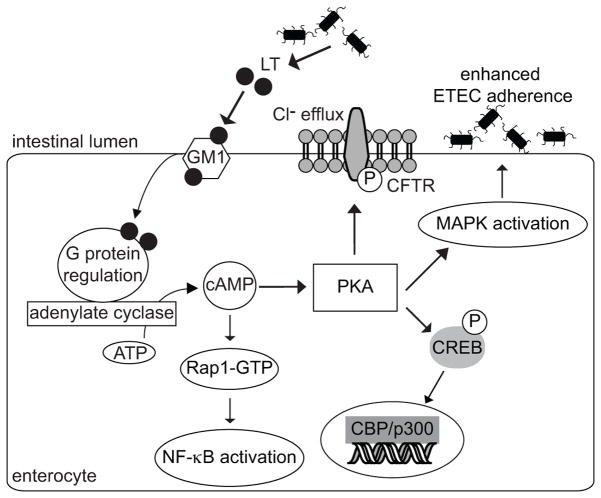
Figure 7. Intestinal epithelial cell signaling pathways altered by LT.
Elevated [cAMP] induced by LT deregulation of adenylate cyclase leads to PKA activation. Activated PKA results in CREB phosphorylation and the activation of the ERK1/2, p38, and JNK MAPKs. LT also activates NF-κB via a cAMP-dependent, but PKA-independent mechanism mediated by Rap1. Activating the p38 MAPK promotes ETEC adherence to intestinal epithelial cells through an unknown mechanism.
We found that ETEC activates the NF-κB pathway in an LT-dependent manner. NF-κB activation is a critical host response to infection (Li et al., 2002). Cells infected with wt ETEC had markedly higher levels of IL-8 and TNF-α expression, relative to both uninfected cells and to cells infected with ΔeltA ETEC, suggesting that ETEC activates innate immune responses through LT-dependent pathways.
We determined that NF-κB activation was dependent upon elevated [cAMP] induced by LT, but was independent of PKA activation. Rap1 is a member of the Ras family of small GTPases that can be activated by increased [cAMP] (de Rooij et al., 1998). Further studies showed that wt ETEC infection of intestinal epithelial cells activated Rap1. Inhibiting Rap1 with Rap1-GAP1 was sufficient to block ETEC-induced NF-κB activation, but not MAPK activation, suggesting that LT-induced NF-κB activation is instead regulated via Rap1.
It is well established that Rap1 is involved in multiple cellular functions, including ERK1/2 activation, cellular proliferation, integrin-mediated cell adhesion, and malignancy (Vossler et al., 1997). However, the role of Rap1 in regulating NF-κB has received less attention. Lipopolysaccharide (LPS) also induces Epac-mediated Rap1 activation, and this activation regulates NF-κB signaling in murine macrophages (Moon et al., 2007), although the mechanism is unknown. Epac-mediated Rap1 activation also positively regulates PI3K/Akt signaling (Jin et al., 2006, Misra et al., 2008), which can activate the NF-κB pathway directly (Madrid et al., 2001, Lin et al., 2011). Other reports indicate that Rap1 can activate the small GTPase Rac1 (Arthur et al., 2004), which can subsequently stimulate NF-κB (Sanlioglu et al., 2001, Matos et al., 2006). It will be interesting to determine in future studies what is the mechanism by which LT-induced Rap1 activation results in increased NF-κB activity.
We also demonstrated that infecting HCT-8 cells with ETEC activates the p38, ERK1/2, and JNK MAPKs. This phenotype was dependent upon LT-induced PKA activation. Vibrio cholerae infection of intestinal epithelial cells also activates MAPK pathways in a CT- and PKA-dependent manner (Bandyopadhaya et al., 2009). Because infecting cells with ΔeltA ETEC also induced a modest amount of MAPK and NF-κB activation, we speculate that other bacterial components, including LPS and flagellin (Guha et al., 2001), also contribute to this phenotype. However, by titrating the MOI of both wt and ΔeltA ETEC, we determined that the magnitude of MAPK activation was always significantly higher in cells infected with wt vs. cells infected with ΔeltA ETEC.
Because LT-induced activation of NF-κB and MAPKs proceeds through distinct mechanisms, we propose that each of host pathways may play a distinct role in ETEC-host interactions. Other recent studies have identified important differences in NF-κB and MAPK activation mediated by soluble LT vs. LT delivered via outer membrane vesicles (Chutkan et al., 2011). We further determined which of these host pathways were involved in LT-induced ETEC adherence.
Blocking the NF-κB and the Rap1 pathways with inhibitors or with siRNA did not impact ETEC adherence, suggesting that Rap1-mediated NF-κB activation does not significantly contribute to ETEC adherence.
By contrast, pre-treating cells with the p38 MAPK inhibitors SB202190 and SB203580 significantly inhibited ETEC adherence, thus implicating this host protein as a key contributor to ETEC-host cells interactions. Understanding the signal transduction events leading to the LT-induced pro-adherence phenotype via p38 MAPK activation awaits further experimentation. In the long-term, these, and related studies, may open new avenues for preventive therapies. Indeed, a fluorenone-based compound that inhibits LT-induced cAMP production has recently been shown to decrease ETEC adherence and colonization (Moen et al., 2010).
Experimental procedures
Bacterial strains
We used ETEC H10407 [serotype 078:H11; LT+ ST+ CFA/I+; (Evans et al., 1975)] and an H10407 mutant lacking LT expression [ΔeltA; (Allen et al., 2006)].
Chemicals and antibodies
We used p-IκBα, p38, p-p38, ERK1/2, p-ERK1/2, p-JNK, and p-CREB antibodies, PD98059, SB203580, as well as PKA siRNA from Cell Signaling. We used JNK and PARP antibodies from BD Biosciences. We used actin, tubulin, Rap1, p50 and p-p65 antibodies, as well as a Rap1 and a ‘scrambled’, non-specific siRNA duplex from Santa Cruz. We used a p65 antibody from Biolegend. We used forskolin, Rp-cAMPS, 2′5′-dideoxyadenosine (DDA), SB202190, SP600125, and Bay 11-7082 from Sigma. We used LT from List Biological Laboratories.
Cell culture and transfections
We maintained HCT-8 cells at 37 °C in 5 % CO2 in RPMI-1640 media supplemented with 10 % fetal bovine serum (FBS). For transfections, we seeded HCT-8 cells in 6-well plates, grew the cells to ~60 % confluence, and transfected them using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
Bacterial infections
We grew ETEC strains overnight without shaking at 37 °C in LB broth and then diluted the cultures 1:50 into serum- and antibiotic-free RPMI-1640 media for 3 h additional growth without shaking. We replaced cell culture medium with serum- and antibiotic-free RPMI-1640 medium prior to infections. We infected HCT-8 cells with ETEC at a multiplicity of infection of ~50 for 2 h, unless indicated otherwise, after which we washed the cells with cold PBS and lysed them for subsequent analyses.
Quantification of cAMP production
We infected HCT-8 cells with ETEC for 2 h. We used forskolin (20 μM) as a positive control to induce cAMP overexpression. We quantified cAMP concentrations by using a cAMP complete enzyme immunoassay kit according to the manufacturer’s instructions (Assay Designs).
Cell fractionation
We lysed HCT-8 cell pellets in ice-cold lysis buffer A (10.0 mM HEPES, 1.5 mM MgCl2, 10.0 mM KCl, 0.5 mM DTT, 0.05 % NP-40) supplemented with protease and phosphatase inhibitor cocktails (Thermo Scientific). After centrifugation, we collected the supernatant as the cytosolic fraction. We washed the nuclear pellet with ice-cold buffer A and resuspended the pellet in Buffer B (5.0 mM HEPES, 1.5 mM MgCl2, 0.2 mm EDTA, 0.5 mM DTT, 26 % glycerol, 300 mM NaCl). We passed these samples through 25 gauge needles to shear genomic DNA and then incubated the samples on ice for 40′ with intermittent vortexing, after which we collected the supernatant containing nuclear proteins by centrifugation. We quantified protein concentrations by using a BCA protein assay kit (Pierce).
Immunoblotting
We separated equal amounts of protein obtained from cytoplasmic or nuclear fractions by SDS-PAGE, transferred the protein to nitrocellulose membranes, and blocked the membranes in Odyssey blocking buffer (Li-Cor) for 1 h. We applied primary antibodies overnight at 4 °C and applied secondary antibodies (Li-Cor) for 1 h at room temperature before developing the blots using an Odyssey infrared imaging system (Li-Cor). We digitally quantified the resultant fluorescent signals and normalized the data to poly(ADP-ribose) polymerase (PARP) and actin or tubulin abundance to normalize protein loading among nuclear and cytoplasmic fractions, respectively.
Rap1 activation assay
We examined the Rap1 activation state by using a pull-down assay to capture GTP-bound Rap1 (de Rooij et al., 1998). We induced GST-RalGDS-RBD expression in E. coli BL21(DE3) with 1 mM isopropyl-β-D-thiogalactoside (IPTG) for 3 h at 37 °C and purified the over-expressed protein using a glutathione-sepharose column. We lysed HCT-8 cells in 50mM Tris-HCl (pH 7.4), 0.5 M NaCl, 1 % NP-40, 2.5 mM MgCl2, 10% glycerol and applied ~1.0 mg of the cell lysate to glutathione agarose-coupled GST-RalGDS-RBD beads for 2 h at 4 °C. We washed the beads 3 times with cold lysis buffer, and then eluted Rap1-GTP in SDS-PAGE loading buffer.
Real-Time Quantitative RT-PCR
We processed ETEC-infected HCT-8 cells with a SYBR Green Cells-to-CT kit, according to the manufacturer’s instructions (Ambion). We performed real-time PCR assays in a Power SYBR Green PCR Master Mix (Applied Biosystems) with the following primers: GAPDH (5′-AC2AG2TG2TCTC2TCTGACT2C & 5′-GTG2TCGT2GAG3CA2TG), IL-8 (5′-CTG2C2GTG2CTCTCT2G & 5′-C2T2G2CA4CTGCAC2T2), TNF-α (5′-TGCTC2TCAC3ACAC2AT & 5′-G2AG2T2GAC2T2G2TCTG2TA), with detection in a Fast 7500 system (Applied Biosystems). We calculated relative transcription levels using the ΔΔCt method by normalizing IL-8 and TNF-α data to GAPDH expression levels.
Bacterial adherence assays
We performed bacterial adherence assays as described previously (Johnson et al., 2009). Where indicated, we treated HCT-8 cells for 1 h with 50 μM ERK1/2 inhibitor PD98059, 20 μM p38 inhibitors SB202190 and SB203580, 40 μM JNK inhibitor SP600125, or 20 μM NF-κB inhibitor Bay 11-7082, prior to adding ETEC. We confirmed the efficacy of these inhibitors by monitoring the phosphorylation state of ERK1/2, JNK, and IκBα.
Acknowledgments
We thank J. Bos (University Medical Centre Utrecht) for Rap1 expression plasmids and reagents. We thank P. Stork (Oregon Health & Science University) for the Rap1-GAP1 expression plasmid. Supported by a subaward of grant P20 RR016443 from the NCRR of the U.S. National Institutes of Health.
Abbreviations
- cAMP
3′5′-cyclic adenosine monophosphate
- CFTR
cystic fibrosis transmembrane conductance regulator
- CT
cholera toxin
- DDA
2′5′-dideoxyadenosine
- ETEC
enterotoxigenic Escherichia coli
- IPTG
isopropyl-β-D-thiogalactoside
- LT
heat-labile enterotoxin
- MAPK
mitogen-activated protein kinase
- MOI
multiplicity of infection
- PARP
poly(ADP-ribose) polymerase
- PKA
protein kinase A
- ST
heat-stable toxin
References
- Allen KP, Randolph MM, Fleckenstein JM. Importance of heat-labile enterotoxin in colonization of the adult mouse small intestine by human enterotoxigenic Escherichia coli strains. Infect Immun. 2006;74:869–875. doi: 10.1128/IAI.74.2.869-875.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur WT, Quilliam LA, Cooper JA. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J Cell Biol. 2004;167:111–122. doi: 10.1083/jcb.200404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhaya A, Das D, Chaudhuri K. Involvement of intracellular signaling cascades in inflammatory responses in human intestinal epithelial cells following Vibrio cholerae infection. Mol Immunol. 2009;46:1129–1139. doi: 10.1016/j.molimm.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Beatty ME, Adcock PM, Smith SW, Quinlan K, Kamimoto LA, Rowe SY, et al. Epidemic diarrhea due to enterotoxigenic Escherichia coli. Clin Infect Dis. 2006;42:329–334. doi: 10.1086/499246. [DOI] [PubMed] [Google Scholar]
- Berberov E, Zhou Y, Francis D, Scott M, Kachman S, Moxley R. Relative importance of heat-labile enterotoxin in the causation of severe diarrheal disease in the gnotobiotic piglet model by a strain of enterotoxigenic Escherichia coli that produces multiple enterotoxins. Infect Immun. 2004;72:3914–3924. doi: 10.1128/IAI.72.7.3914-3924.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland G, Smith BO, Yarwood SJ. EPAC proteins transduce diverse cellular actions of cAMP. Br J Pharmacol. 2009;158:70–86. doi: 10.1111/j.1476-5381.2008.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron E. Cellular functions of the Rap1 GTP-binding protein: a pattern emerges. J Cell Sci. 2003;116:435–440. doi: 10.1242/jcs.00238. [DOI] [PubMed] [Google Scholar]
- Chakraborty K, Ghosh S, Koley H, Mukhopadhyay AK, Ramamurthy T, Saha DR, et al. Bacterial exotoxins downregulate cathelicidin (hCAP-18/LL-37) and human beta-defensin 1 (HBD-1) expression in the intestinal epithelial cells. Cell Microbiol. 2008;10:2520–2537. doi: 10.1111/j.1462-5822.2008.01227.x. [DOI] [PubMed] [Google Scholar]
- Chutkan H, Kuehn MJ. Context-dependent activation kinetics elicited by soluble versus outer membrane vesicle-associated heat-labile enterotoxin. Infect Immun. 2011;79:3760–3769. doi: 10.1128/IAI.05336-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Evans DG, Silver RP, Evans DJ, Jr, Chase DG, Gorbach SL. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975;12:656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RA, Boesman M, Neoh SH, LaRue MK, Delaney R. Dissociation and recombination of the subunits of the cholera enterotoxin (choleragen) J Immunol. 1974;113:145–150. [PubMed] [Google Scholar]
- Fleckenstein JM, Hardwidge PR, Munson GP, Rasko DA, Sommerfelt H, Steinsland H. Molecular mechanisms of enterotoxigenic Escherichia coli infection. Microbes Infect. 2010;12:89–98. doi: 10.1016/j.micinf.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Huang DB, DuPont HL, Jiang ZD, Carlin L, Okhuysen PC. Interleukin-8 response in an intestinal HCT-8 cell line infected with enteroaggregative and enterotoxigenic Escherichia coli. Clin Diagn Lab Immunol. 2004;11:548–551. doi: 10.1128/CDLI.11.3.548-551.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin A, Kurosu T, Tsuji K, Mizuchi D, Arai A, Fujita H, et al. BCR/ABL and IL-3 activate Rap1 to stimulate the B-Raf/MEK/Erk and Akt signaling pathways and to regulate proliferation, apoptosis, and adhesion. Oncogene. 2006;25:4332–4340. doi: 10.1038/sj.onc.1209459. [DOI] [PubMed] [Google Scholar]
- Johnson A, Kaushik R, Francis D, Fleckenstein J, Hardwidge P. Heat-labile enterotoxin promotes Escherichia coli adherence to intestinal epithelial cells. J Bacteriol. 2009;191:178–186. doi: 10.1128/JB.00822-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli E, Sassone-Corsi P. Signal transduction and gene regulation: the nuclear response to cAMP. J Biol Chem. 1994;269:17359–17362. [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Lin CH, Cheng HW, Ma HP, Wu CH, Hong CY, Chen BC. Thrombin induces NF-{kappa}B activation and IL-8/CXCL8 expression in lung epithelial cells by a Rac1-dependent PI3K/Akt pathway. J Biol Chem. 2011 doi: 10.1074/jbc.M110.112433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001;276:18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- Matos P, Jordan P. Rac1, but not Rac1B, stimulates RelB-mediated gene transcription in colorectal cancer cells. J Biol Chem. 2006;281:13724–13732. doi: 10.1074/jbc.M513243200. [DOI] [PubMed] [Google Scholar]
- Misra UK, Kaczowka S, Pizzo SV. The cAMP-activated GTP exchange factor, Epac1 upregulates plasma membrane and nuclear Akt kinase activities in 8-CPT-2-O-Me-cAMP-stimulated macrophages: Gene silencing of the cAMP-activated GTP exchange Epac1 prevents 8-CPT-2-O-Me-cAMP activation of Akt activity in macrophages. Cell Signal. 2008;20:1459–1470. doi: 10.1016/j.cellsig.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen ST, Blumentritt CA, Slater TM, Patel SD, Tutt CB, Estrella-Jimenez ME, et al. Testing the efficacy and toxicity of adenylyl cyclase inhibitors against enteric pathogens using in vitro and in vivo models of infection. Infect Immun. 2010;78:1740–1749. doi: 10.1128/IAI.01114-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon EY, Pyo S. Lipopolysaccharide stimulates Epac1-mediated Rap1/NF-kappaB pathway in Raw 264.7 murine macrophages. Immunol Lett. 2007;110:121–125. doi: 10.1016/j.imlet.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Moxley RA, Berberov EM, Francis DH, Xing J, Moayeri M, Welch RA, et al. Pathogenicity of an enterotoxigenic Escherichia coli hemolysin (hlyA) mutant in gnotobiotic piglets. Infect Immun. 1998;66:5031–5035. doi: 10.1128/iai.66.10.5031-5035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsioen B, Gloerich M, Ritsma L, Rehmann H, Bos JL, Jalink K. Direct spatial control of Epac1 by cyclic AMP. Mol Cell Biol. 2009;29:2521–2531. doi: 10.1128/MCB.01630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel JD, Stec WJ, Baraniak J, Jastorff B, Botelho LH. Inhibition of glycogenolysis in isolated rat hepatocytes by the Rp diastereomer of adenosine cyclic 3′,5′-phosphorothioate. J Biol Chem. 1983;258:12125–12128. [PubMed] [Google Scholar]
- Rowe B, Taylor J, Bettelheim KA. An investigation of traveller’s diarrhoea. Lancet. 1970;1:1–5. doi: 10.1016/s0140-6736(70)90520-9. [DOI] [PubMed] [Google Scholar]
- Sanlioglu S, Williams CM, Samavati L, Butler NS, Wang G, McCray PB, Jr, et al. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-alpha secretion through IKK regulation of NF-kappa B. J Biol Chem. 2001;276:30188–30198. doi: 10.1074/jbc.M102061200. [DOI] [PubMed] [Google Scholar]
- Sears CL, Kaper JB. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol Rev. 1996;60:167–215. doi: 10.1128/mr.60.1.167-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehrawat S, Cullere X, Patel S, Italiano J, Jr, Mayadas TN. Role of Epac1, an exchange factor for Rap GTPases, in endothelial microtubule dynamics and barrier function. Mol Biol Cell. 2008;19:1261–1270. doi: 10.1091/mbc.E06-10-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins WA, Watrach AM, Schmale JD, Schultz RM, Harris JA. Cultural and antigenic properties of newly established cell strains derived from adenocarcinomas of the human colon and rectum. J Natl Cancer Inst. 1974;52:1101–1110. doi: 10.1093/jnci/52.4.1101. [DOI] [PubMed] [Google Scholar]
- Turner SM, Scott-Tucker A, Cooper LM, Henderson IR. Weapons of mass destruction: virulence factors of the global killer enterotoxigenic Escherichia coli. FEMS Microbiol Lett. 2006;263:10–20. doi: 10.1111/j.1574-6968.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- Vossler MR, Yao H, York RD, Pan MG, Rim CS, Stork PJ. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]