Abstract
In human alcoholics, the cell density is decreased in the prefrontal cortex (PFC) and other brain areas. This may be due to persistent activation of cell death pathways. To address this hypothesis, we examined the status of cell death machinery in the dorsolateral PFC in alcoholics. Protein and mRNA expression levels of several key pro- and anti-apoptotic genes were compared in post-mortem samples of 14 male human alcoholics and 14 male controls. The findings do not support the hypothesis. On the contrary, they show that several components of intrinsic apoptotic pathway are decreased in alcoholics. No differences were evident in the motor cortex, which is less damaged in alcoholics and was analysed for comparison. Thus, cell death mechanisms may be dysregulated by inhibition of intrinsic apoptotic pathway in the PFC in human alcoholics. This inhibition may reflect molecular adaptations that counteract alcohol neurotoxicity in cells that survive after many years of alcohol exposure and withdrawal.
Keywords: Alcoholism, apoptosis, neurotoxicity, post-mortem brain, prefrontal cortex
Introduction
Several brain areas including the prefrontal cortex (PFC) demonstrate physiological and structural alterations in human alcoholics (Chanraud et al., 2007; Dao-Castellana et al., 1998; Fadda and Rossetti, 1998; Goldstein et al., 2004; Sullivan and Pfefferbaum, 2005). Imaging studies show reduced grey- and white-matter volumes, with marked losses in the frontal lobes, while regional alterations are associated with impairment of cognitive functions (Chanraud et al., 2007; Dao-Castellana et al., 1998; Goldstein et al., 2004; Sullivan and Pfefferbaum, 2005). Histopathological analyses demonstrate that neurons and glial cells of alcoholics are abnormal and their density is decreased in the PFC and several other brain areas (Harper et al., 2003; Kril et al., 1997; Miguel-Hidalgo et al., 2002). Cellular and white-matter loss may contribute to cognitive impairment associated with alcoholism. Mechanisms underlying cell loss/neurodegeneration have not yet been identified, although apoptosis or programmed cell death has been implicated (Fadda and Rossetti, 1998; Freund, 1994; Ikegami et al., 2003).
Apoptosis appears to contribute to the pathogenesis of neurodegeneration (Mattson and Magnus, 2006; Okouchi et al., 2007). Protein families that control apoptosis include the caspases with caspase-3 as the effector enzyme and the Bcl-2 group of proteins, of which Bcl-2 and Bcl-XL suppress cell death and Bax induces it. Studies of the developing and adult murine brain demonstrate that acute ethanol intoxication triggers apoptotic neurodegeneration or excitotoxic cell death, which are dependent on activation of caspase-3 and up-regulation of Bax, and is negatively regulated by Bcl-2 (Heaton et al., 1999; Nowoslawski et al., 2005; Rajgopal et al., 2003; Young et al., 2005).
Similarly, persistent activation of cell death pathways by alcohol consumption may contribute to neurodegenerative changes in the brain of human alcoholics. To address this hypothesis, we evaluated the status of the cell death machinery in the PFC of human alcoholics. Expression of several key pro- and anti-apoptotic genes were analysed at protein and mRNA levels in post-mortem samples from alcoholics and control subjects. The motor cortex (MC), which is less damaged in alcoholics (Harper et al., 2003; Ikegami et al., 2003; Kril et al., 1997; Miguel-Hidalgo et al., 2002), was analysed for comparison.
Methods
Case selection
Samples of the dorsolateral PFC (Brodmann area 9) and MC (Brodmann area 4) were collected from 14 alcoholic and 14 control subjects (Table 1), all Caucasian males at the Tissue Resource Center, University of Sydney, Australia (Sheedy et al., 2008), and matched for age at death and post-mortem interval (PMI). Cortical samples were punched from coronally sliced frozen sections, powdered and used for extraction of proteins and RNA. The samples contained all cortical layers with both grey and white matter at approximately equal composition. Alcohol subjects met DSM-IV criteria and National Health and Medical Research Council/World Health Organization criteria as individuals who had consumed >80 g ethanol per day for the majority of their adult lives. Controls had either abstained from alcohol completely or were social drinkers who consumed <20 g ethanol per day on average. Cases with a history of poly-drug abuse (evidence that the individual abused other drugs such as cocaine or heroin) or with medical complications such as liver cirrhosis and the Wernicke–Korsakoff syndrome or alcoholic cases with concomitant diseases were excluded. The main body of the population was smokers including 83% of alcoholics and 75% of control subjects. Samples were taken by qualified pathologists under full ethical clearance from the Sydney South West Area Health Service Human Ethics Committee (X03-0074) and informed written consent was obtained from next of kin.
Table 1.
Demographic data of alcoholics and control subjects
| Subject no. | Age (yr) | PMI (h) | Brain pH | Storage time (months) | Smoking history | Cause of death | Toxicological findings at time of death |
|---|---|---|---|---|---|---|---|
| Controls | |||||||
| 1 | 34 | 20.5 | 6.73 | 52 | Yes | Acute exacerbation of asthma | n.a. |
| 2 | 78 | 6.5 | 6.20 | 30 | No | Dehydration and adenocarcinoma of the lung and rectum with multiple metastases | None |
| 3 | 63 | 72 | 6.90 | 36 | Yes | Coronary artery atherosclerosis | None |
| 4 | 82 | 23.5 | 6.40 | 46 | NA | Sepsis | None |
| 5 | 38 | 13.5 | 6.26 | 127 | Yes | ACSVD | None |
| 6 | 69 | 16 | 6.60 | 34 | Yes | ACSVD | Paracetamol and CO |
| 7 | 56 | 24 | 6.53 | 83 | Yes | Coronary artery atheroma | n.a. |
| 8 | 59 | 20 | 6.56 | 81 | Yes | Coronary thrombosis | None |
| 9 | 56 | 25 | 6.10 | 32 | NA | CAD | Codeine, morphine, naproxen |
| 10 | 56 | 37 | 6.76 | 38 | Yes | LV scarring, hypertension and cardiomegaly | None |
| 11 | 82 | 36 | 6.24 | 55 | No | Myocardial infarction | n.a. |
| 12 | 44 | 50 | 6.60 | 40 | Yes | CAD | None |
| 13 | 61 | 24 | 6.52 | 83 | Yes | CAD | n.a. |
| 14 | 53 | 16 | 6.84 | 50 | No | Dilated cardiomyopathy | Lignocaine, sotalol |
| Mean ± S.E.M. | 59±1 | 27±1 | 6.5±0.02 | 56±2 | Yes | ||
| Alcoholics | |||||||
| 1 | 34 | 8.5 | 6.61 | 98 | Yes | Hanging | Alcohol |
| 2 | 77 | 20 | 6.34 | 97 | Yes | Lobular pneumonia | None |
| 3 | 79 | 48 | 6.34 | 87 | Yes | CAD | Temazepam |
| 4 | 39 | 24 | 6.56 | 78 | Yes | Aortic sclerosis | n.a. |
| 5 | 70 | 33.5 | 6.24 | 74 | Yes | Respiratory failure | None |
| 6 | 56 | 45 | 6.51 | 73 | NA | BOV | Alcohol |
| 7 | 59 | 24 | 6.57 | 71 | No | Cardiomyopathy | None |
| 8 | 56 | 22 | 6.52 | 71 | Yes | CAD and UGIH | None |
| 9 | 56 | 15 | 6.66 | 67 | NA | CAD and emphysema | Nordiazepam |
| 10 | 81 | 36 | 6.44 | 58 | Yes | Sepsis | None |
| 11 | 44 | 15 | 6.48 | 51 | No | CAD | Diazepam, noridazepam |
| 12 | 52 | 45.5 | 6.78 | 45 | Yes | Lobar pneumonia | None |
| 13 | 62 | 49 | 6.49 | 44 | Yes | CAD | Sertraline |
| 14 | 53 | 57 | 6.75 | 40 | Yes | CAL | n.a. |
| Mean ± S.E.M. | 58±1 | 32±1 | 6.5±0.01 | 68±1 | |||
ACSVD, Atherosclerotic cardiovascular disease; BOV, bleeding oesophageal varices; CAD, ischaemic heart disease; CAL, chronic airflow limitation; CO, carbon monoxide; LV, left ventricular; n.a., not available; PMI, post-mortem interval; UGIH, upper gastrointestinal haemorrhage.
Western blotting (Yakovleva et al., 2007)
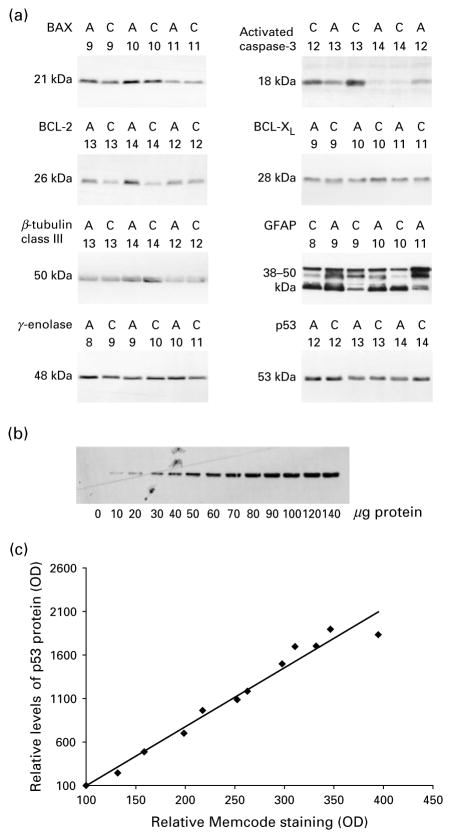
Powdered tissue samples were boiled in pre-warmed SDS extraction buffer [0.45 M Tris–HCl (pH 8.5), 2.5% glycerol, 4% SDS, 0.5 mM DTT] and 5× protease inhibitors for 5 min, sonicated and protein extracts aliquoted and kept at −80 °C until usage. Protein concentration was determined with the DC protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Aliquots of tissue extracts [10–140 μg protein for the calibration curve (see Fig. 1); and 70 μg protein for comparison of alcoholics and controls] were heated for 5 min at 95 °C in the presence of 5 mM β-mercaptoethanol and resolved by SDS–PAGE on 10% tricine gels. Reference samples consisting of pooled cerebellar extracts from control subjects were loaded onto three wells, two at each edge and one in the middle of each gel and their density values used to ascertain reproducibility on each blot and for interblot comparison. Proteins were transferred onto nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany) at 4 °C and stained with Memcode Reversible Protein Stain kit (Pierce, Rockford, IL, USA). Densitometry values of protein load were used for normalization of Western blot data [immunoreactivity optical density (OD)]. After destaining, membranes were incubated with stripping buffer [62.5 mM Tris–HCl, 2% SDS, 100 mM β-mercaptoethanol (pH 6.7)] for 20 min at 55 °C, blocked for 30 min with 5% non-fat dry milk in 50 mM Tris–HCl, 0.15 M NaCl, 0.05% Tween-20 buffer and probed with monoclonal antibodies against BAX (B-9; 1:330; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), BCL-2 (clone 100, 1:500; Upstate Biotechnology, Lake Placid, NY, USA), BCL-XL (H-5, 1:50; Santa Cruz Biotechnology), p53 (PAb 1801, 1:100; Calbiochem, San Diego, CA, USA), β-tubulin class III (MMS-435P, 1:10000; Nordic Biosite AB, Täby, Sweden), γ-enolase (NSE-P2, 1:1000, Santa Cruz Biotechnology) or glial fibrillary acidic protein (GFAP) (cocktail of monoclonal antibodies, 1:1000; BD Transduction Laboratories, Lexington, KY, USA); or with rabbit polyclonal antibodies against activated caspase-3 (PAb CM1, 1:800; BD Transduction Laboratories). Goat anti-mouse and anti-rabbit antibodies conjugated with horseradish peroxidase (Bio-Rad) were used as secondary antibodies. Membranes were developed with the ECL detection system (Amersham, Little Chalfont, UK). Fuji Film image gauge software 3.12 was used for densitometric analysis. Protein levels were calculated as the ratio of OD of protein immunoreactivity to Memcode OD.
Figure 1.
Western blot analysis of proteins in human brain samples. (a) Representative Western blots of proteins in the prefrontal cortex (PFC) of three control subjects (C) and three alcoholics (A). Identification numbers of subjects appear above the images. A total of 70 μg protein extracts were loaded on the gel. Proteins produced a single band with the predicted molecular size of 21 (BAX), 26 (BCL-2), 50 (β-tubulin class III), 48 (γ-enolase) 18 (activated caspase-3), 28 (BCL-XL) and 53 (p53) kDa, or a cluster of bands with the predicted molecular size of 38–50 kDa [glial fibrillary acidic protein (GFAP)]. GFAP levels were analysed as a sum of optical density (OD) of all bands. (b) Representative analysis of p53 protein at serial dilutions of PFC extract. Sample buffer or extract aliquots containing 10–140 μg protein were loaded on the gel and analysed by Western blot. (c) Densitometry assessment of p53 bands shown in panel (b) demonstrates linear dependence (R2=0.97, p=0.0001) between the protein immunoreactivity measured as OD and protein load measured as Memcode OD. OD is presented as relative units minus background determined in the absence of protein load. Immunoreactivity OD and Memcode OD at the 10 μg protein load were taken as 100 relative units.
mRNA analysis by quantitative RT–PCR (Johansson et al., 2007)
RNA was prepared using RNeasy Lipid Tissue Mini kit (Qiagen, Germantown, MD, USA) and was quantified by Nanodrop® (Nanodrop Technologies, Wilmington, DE, USA). RNA quality was controlled using Agilent 2100 Bioanalyzer (Agilent, Palo Alto, CA, USA). RNA with clear ribosomal 18S and 28S RNA was used for further analysis. cDNA synthesis was performed with the High Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA, USA). Analysis of mRNA levels was performed with TaqMan® low density arrays (Applied Biosystems) fabricated according to custom design. A pre-prepared micro fluidic card containing probes and primers for each gene, cDNA and TaqMan® Universal PCR Master Mix (Applied Biosystems) was added in a final concentration of 65 pg cDNA per sample and gene. Every sample was run in duplicate on the same array for each gene. The PCR amplification was performed at 50 °C for 2 min, 94.5 °C for 10 min and 40 cycles of 97 °C for 30 s followed by 59.7 °C for 1 min. To measure the quantity of a given RNA species, the threshold cycles (Ct) were monitored by the Applied Biosystems 7900HT Fast Real-Time PCR system. The levels were normalized using a normalization factor (geometric mean of two reference genes selected by the geNORM program: http://medgen.ugent.be/genorm/) and qBASE program for internal and external calibration and also for easy care of large RT–PCR datasets (http://medgen.ugent.be/qbase/). The β-actin (ACTB) and ribosomal large P0 (RPLP0) genes were chosen for normalization in the PFC, and the 18S and RPLP0 genes in the MC.
Statistical analysis
Data normality was analysed using Shapiro–Wilk’s W test. A general stepwise linear regression model and Student’s t test were used for normally distributed datasets. Datasets that were not normally distributed were analysed by Mann–Whitney U test and Spearman correlations. Each protein/mRNA was treated individually such that no multiple comparison tests were made. Significance was set at p<0.05 and trends considered for p<0.10. Statistica 6.0 package (StatSoft Scandinavia, Uppsala, Sweden) was used for analysis.
Results
Analysis of the demographic characteristics (Table 1) showed no significant differences in age (t28=0.01, p=0.99), PMI (t28=0.85, p=0.40), brain pH (t28=0.11, p=0.91), storage time (t28=2.34, p=0.37) and proportions of smokers and non-smokers (Fisher’s test, p=0.5) between controls and alcoholics.
Levels of proteins of cell suicide (activated caspase-3, BAX and p53) and protection machineries (BCL-2 and BCL-XL), and levels of neuronal (β-tubulin class III and γ-enolase) and glial (GFAP) protein markers were analysed using Western blot (Figures 1 and 2a). Measurements of all proteins were performed within the linear range of detection as evident from experiments with serial dilutions of reference sample (Figure 1b); the correlation coefficient between protein immunoreactivity measured as OD and protein load measured as Memcode OD was ≥0.98 (Figure 1c).
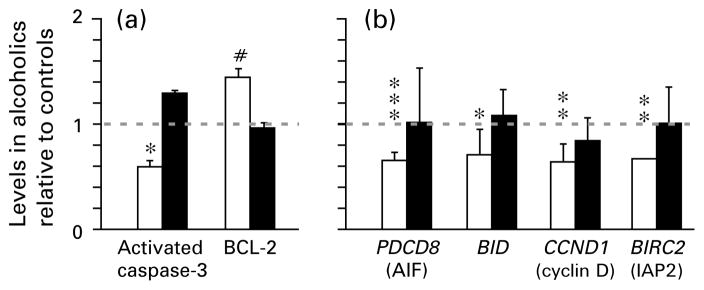
Figure 2.
(a) Protein and (b) mRNA levels of key components of cell death/protection machineries in human alcoholics compared to controls. Data for alcoholics are presented as relative levels (mean±S.E.M.) compared to controls, which are set as 1. Data for prefrontal cortex (□) and motor cortex (■). * p<0.05, ** p<0.01, *** p=0.001.
In the PFC, activated caspase-3 was significantly decreased in alcoholics (1.9-fold ; Mann–Whitney U test, p=0.017) (Figure 2a, Table 2). In contrast, BCL-2 was increased with trend (1.4-fold, Mann–Whitney U test, p=0.073). Levels of other proteins did not differ between controls and alcoholics. No differences were evident in the MC. The absence of differences between the two groups of subjects in neuronal and glial markers suggests that these proteins and total protein content are altered in parallel, and that cell death/neurodegeneration affects both cell types similarly in alcoholics. Alternatively, remaining cells may increase synthesis of the markers.
Table 2.
Levels of proteins/mRNAs of cell death/protection pathways and of neuronal and glial protein markers in alcoholics and control subjects
| Prefrontal cortex
|
Motor cortex
|
|||||
|---|---|---|---|---|---|---|
| Controls (mean±S.E.M.) | Alcoholics (mean±S.E.M.) | p value | Controls (mean±S.E.M.) | Alcoholics (mean±S.E.M.) | p value | |
| Proteins | ||||||
| Activated caspase-3 | 0.73±0.10 | 0.43±0.09 | 0.017c | 0.63±0.14 | 0.81±0.16 | 0.37c |
| BAX | 0.78±0.07 | 1.01±0.12 | 0.11a | 0.92±0.08 | 0.75±0.07 | 0.13a |
| BCL-XL | 0.76±0.04 | 0.70±0.07 | 0.52a | 0.54±0.04 | 0.47±0.03 | 0.18c |
| BCL-2 | 0.51±0.04 | 0.71±0.09 | 0.073c | 0.66±0.04 | 0.63±0.06 | 0.67a |
| p53 | 1.30±0.09 | 1.16±0.06 | 0.19a | 0.96±0.05 | 0.97±0.07 | 0.88a |
| β-tubulin class III | 1.32±0.11 | 1.15±0.11 | 0.23c | 1.39±0.10 | 1.35±0.09 | 0.80a |
| GFAP | 0.76±0.11 | 0.77±0.06 | 0.50c | 0.61±0.06 | 0.74±0.07 | 0.18a |
| γ-enolase | 1.16±0.07 | 1.18±0.06 | 0.65c | 1.41±0.10 | 1.24±0.06 | 0.11a |
| mRNAs | ||||||
| BID | 4.48±0.50 | 3.19±0.23 | 0.031c | 2.25±0.17 | 2.43±0.25 | 0.75a |
| BIRC2 (cIAP2) | 2.33±0.34 | 1.55±0.06 | 0.002c | 3.42±0.37 | 3.45±0.34 | 0.89c |
| PDCD8 (AIF) | 2.17±0.19 | 1.43±0.07 | 0.001a | 4.72±0.37 | 4.79±0.53 | 0.71c |
| CCND1 (cyclin D1) | 3.49±0.56 | 2.21±0.21 | 0.010c | 2.35±0.15 | 1.98±0.24 | 0.10a |
| BAX | 1.52±0.08 | 1.47±0.08 | 0.70a | 2.79±0.21 | 3.07±0.27 | 0.46c |
| BCL-2 | 2.48±0.32 | 1.98±0.14 | 0.25a | 2.55±0.30 | 2.40±0.28 | 0.70a |
| FLIP | 1.81±0.15 | 1.60±0.16 | 0.70b | 1.67±0.13 | 1.52±0.13 | 0.35b |
| GADD45A | 2.52±0.31 | 2.00±0.19 | 0.17a | 2.08±0.20 | 2.04±0.12 | 0.88a |
| TP53 | 2.00±0.15 | 1.75±0.17 | 0.18b | 2.02±0.21 | 2.01±0.22 | 0.79b |
| CDKN1A (p21) | 3.18±0.67 | 3.66±0.66 | 0.43a | 2.94±0.44 | 3.06±0.30 | 0.35b |
Significance of differences between groups was evaluated by
Student’s t test, or
covariance by multiple regression analysis when data were normally distributed, and
Mann–Whitney U test when data were not normally distributed.
mRNA levels of pro-apoptotic BAX, PDCD8, BID, CCND1 and TP53; anti-apoptotic BCL-2, BIRC2, FLIP and CDKN1A; and GADD45A were measured by quantitative RT–PCR (for functions of protein products in cell death pathways, see Discussion). In the PFC, the following mRNAs were significantly decreased in alcoholics; PDCD8 (1.5-fold ; Student’s t test, p=0.001), BID (1.4-fold; Mann–Whitney U test, p=0.031), CCND1 (1.6-fold ; Mann–Whitney U test, p=0.010), and BIRC2 (1.5-fold; Mann–Whitney U test, p=0.002) (Figure 2b, Table 2). Levels of other mRNAs did not differ between the two groups. No differences were found in the MC.
Two-way ANOVA (group and region as independent factors) revealed significant group×region interaction [F(1, 40)=6.62, p<0.01] for activated caspase- 3, indicative of a region-specific alteration in levels of this protein. No interaction was evident for other proteins.
No influence of brain pH, age at death and PMI as covariates on the differences found was observed. Although influence of storage time was found for PDCD8 [F(1, 19)=21.78, p=0.001], use of storage time as a covariate failed to affect significance of differences for this mRNA. Non-parametric Spearman correlation analysis revealed significant association between age and BCL-2 (R=+0.46, p<0.05); age and activated caspase-3 (R=+0.42, p<0.05); and storage time and activated caspase-3 (R=−0.44, p<0.05). Covariate analysis revealed significant influence of smoking on PDCD8 [F(1, 19)=14.68, p=0.001], however, without apparent effects on the significance of differences found.
Discussion
The findings of the present study do not support the hypothesis that chronic alcohol consumption activates cell death pathways that may underlie neurodegenerative changes in chronic alcoholics. On the contrary, down-regulation of activated caspase-3, the key pro-apoptotic protein along with decreases in mRNA levels of both pro-apoptotic PDCD8 (apoptosis-inducing factor, AIF) and BID, and of CCND1 (cyclin D1) in the PFC of alcoholics were found. Previous studies established a critical role of these proteins in neuronal and glial cell death. Thus, AIF released from mitochondria has a causative role in caspase independent death signalling; Bid when cleaved by proteases may induce multiple mitochondrial dysfunctions including the release of the inter-membrane space proteins and generation of reactive oxygen species; while CCND1 plays critical roles in the delayed death component (Heaton et al., 1999; Mattson and Magnus 2006; Nowoslawski et al., 2005; Okouchi et al., 2007; Rajgopal et al., 2003; Sumrejkanchanakij et al., 2003; Young et al., 2005). Down-regulated proteins/mRNAs and BCL-2 belong to the intrinsic apoptotic pathway, or are essential for delayed cell death (CCND1). Interestingly, BIRC2 [an inhibitor of apoptosis protein 2 (cIAP2)], which is not directly involved in apoptotic signalling but may suppress effector caspase activity was also down-regulated. The mRNA (TPp53) and protein levels of the critical regulator of neuronal and glial cell death, the p53 tumour suppressor protein, and also mRNA levels of two p53 transcriptional targets, CDKN1A (cyclin-dependent kinase inhibitor p21waf1), which may protect cells against apoptosis, and GADD45A, which may regulate repair of DNA damaged by chronic alcohol exposure/withdrawal, did not differ between alcoholics and controls. Moreover, no differences were evident in FLIP, a modulator of caspases-8 and -10. Collectively, these findings suggest that in the PFC of alcoholics the cell death machinery is dysregulated by inhibition of several components of the intrinsic cell death pathway.
Some limitations of the present study are that the findings are applicable to only males (no female subjects were analysed); and that toxicology screen was incomplete (data is available only for five cases) and we were unable to make correlations with toxicological parameters. Instead, the five patients who had traces of medications in their blood (two in the control group with traces of paracetamol and codeine; three in the alcoholic group with traces of diazepam) were excluded and the data were re-analysed; the differences between controls and alcoholics remained significant besides those for BID. PMI as covariate does not influence the differences between the two groups albeit inclusion of four controls and seven alcoholics with PMI >25 h is a limitation. Another limitation is that the alterations in the cell death machinery were not localized to a specific cell type, or to grey or white matter.
Several scenarios might explain the findings. First, cell death machinery may be differentially altered in the brain of alcoholics and controls after death. However, Spearman rank correlation failed to reveal interactions of PMI and storage time with pro- or anti-apoptotic proteins/mRNAs. Second, the findings may reflect inherited molecular differences between controls and alcoholics. Third, the observed differences may be the manifestation of molecular adaptations that counteract alcohol neurotoxicity in cells that survive after many years of alcohol exposure and withdrawal. Consistently, up-regulation of several genes involved in cell protection in the PFC in human alcoholics was reported (Flatscher-Bader et al., 2005). Adaptations in cell suicide mechanisms would limit the extent of alcohol-induced brain damage, thus protecting cognitive and other PFC functions in chronic alcoholics. Such adaptations may be developed after initial activation of cell death pathways during the first years of heavy alcohol drinking resulting in gross loss of gray and white matter; this notion is generally supported by animal studies on acute alcohol intoxication (Heaton et al., 1999; Nowoslawski et al., 2005; Rajgopal et al., 2003; Young et al., 2005).
Acknowledgments
This work was supported by grants from the AFA Forsäkring and the Research Foundation of the Swedish Alcohol Retail Monopoly (SRA) to T.J.E., A.K. and G.B., and the Swedish Science Research Council, the Swedish National Drug Policy Coordinator and the Torsten och Ragnar Söderbergs stiftelser to G.B. The Australian Brain Donor Programs NSW Tissue Resource Centre was supported by The University of Sydney, National Health and Medical Research Council of Australia, National Institute of Alcohol Abuse and Alcoholism and NSW Department of Health RO1 AA012725.
Footnotes
Statement of Interest
None.
References
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, Feldman L, Barrucand D, Rancurel G, Féline A, Syrota A. Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychological Medicine. 1998;28:1039–1048. doi: 10.1017/s0033291798006849. [DOI] [PubMed] [Google Scholar]
- Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Progress in Neurobiology. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Flatscher-Bader T, van der Brug M, Hwang JW, Gochee PA, Matsumoto I, Niwa S, Wilce PA. Alcohol-responsive genes in the frontal cortex and nucleus accumbens of human alcoholics. Journal of Neurochemistry. 2005;93:359–370. doi: 10.1111/j.1471-4159.2004.03021.x. [DOI] [PubMed] [Google Scholar]
- Freund G. Apoptosis and gene expression: perspectives on alcohol-induced brain damage. Alcohol. 1994;11:385–387. doi: 10.1016/0741-8329(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42:1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Harper C, Dixon G, Sheedy D, Garrick T. Neuropathological alterations in alcoholic brains. Studies arising from the New South Wales Tissue Resource Centre. Progress in Neuropsychopharmacology and Biological Psychiatry. 2003;27:951–961. doi: 10.1016/S0278-5846(03)00155-6. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Moore DB, Paiva M, Gibbs T, Bernard O. Bcl-2 overexpression protects the neonatal cerebellum from ethanol neurotoxicity. Brain Research. 1999;817:13–18. doi: 10.1016/s0006-8993(98)01173-1. [DOI] [PubMed] [Google Scholar]
- Ikegami Y, Goodenough S, Inoue Y, Dodd PR, Wilce PA, Matsumoto I. Increased TUNEL positive cells in human alcoholic brains. Neuroscience Letters. 2003;349:201–205. doi: 10.1016/s0304-3940(03)00826-7. [DOI] [PubMed] [Google Scholar]
- Johansson S, Fuchs A, Okvist A, Karimi M, Harper C, Garrick T, Sheedy D, Hurd Y, Bakalkin G, Ekstrom TJ. Validation of endogenous controls for quantitative gene expression analysis: application on brain cortices of human chronic alcoholics. Brain Research. 2007;1132:20–28. doi: 10.1016/j.brainres.2006.11.026. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nature Reviews Neuroscience. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Wei J, Andrew M, Overholser JC, Jurjus G, Stockmeier CA, Rajkowska G. Glia pathology in the prefrontal cortex in alcohol dependence with and without depressive symptoms. Biological Psychiatry. 2002;52:1121–1133. doi: 10.1016/s0006-3223(02)01439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowoslawski L, Klocke BJ, Roth KA. Molecular regulation of acute ethanol-induced neuron apoptosis. Journal of Neuropathology and Experimental Neurology. 2005;64:490–497. doi: 10.1093/jnen/64.6.490. [DOI] [PubMed] [Google Scholar]
- Okouchi M, Ekshyyan O, Maracine M, Aw TY. Neuronal apoptosis in neurodegeneration. Antioxidants & Redox Signaling. 2007;9:1059–1096. doi: 10.1089/ars.2007.1511. [DOI] [PubMed] [Google Scholar]
- Rajgopal Y, Chetty CS, Vemuri MC. Differential modulation of apoptosis-associated proteins by ethanol in rat cerebral cortex and cerebellum. European Journal of Pharmacology. 2003;470:117–124. doi: 10.1016/s0014-2999(03)01795-3. [DOI] [PubMed] [Google Scholar]
- Sheedy D, Garrick T, Dedova I, Hunt C, Miller R, Sundqvist N, Harper C. An Australian Brain Bank: a critical investment with a high return! Cell and Tissue Banking. 2008;9:205–216. doi: 10.1007/s10561-008-9076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berlin) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sumrejkanchanakij P, Tamamori-Adachi M, Matsunaga Y, Eto K, Ikeda MA. Role of cyclin D1 cytoplasmic sequestration in the survival of postmitotic neurons. Oncogene. 2003;22:8723–8730. doi: 10.1038/sj.onc.1206870. [DOI] [PubMed] [Google Scholar]
- Yakovleva T, Marinova Z, Kuzmin A, Seidah NG, Haroutunian V, Terenius L, Bakalkin G. Dysregulation of dynorphins in Alzheimer disease. Neurobiology of Aging. 2007;28:1700–1708. doi: 10.1016/j.neurobiolaging.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Young C, Roth KA, Klocke BJ, West T, Holtzman DM, Labruyere J, Qin YQ, Dikranian K, Olney JW. Role of caspase-3 in ethanol-induced developmental neurodegeneration. Neurobiology of Disease. 2005;20:608–614. doi: 10.1016/j.nbd.2005.04.014. [DOI] [PubMed] [Google Scholar]