Abstract
Animal studies demonstrated a role of neuropeptide nociceptin (NC) and its receptor (opiate receptor like-1, OPRL1) in ethanol-induced reward; activation of the OPRL1 by natural or synthetic ligands reduced ethanol self-administration and prevented relapse to ethanol drinking. The endogenous NC may function in neuronal circuits involved in reinforcing or conditioning effects of ethanol as a “brake” to limit ethanol intake (Roberto, M., Siggins, G.R. 2006. Nociceptin/orphanin FQ presynaptically decreases GABAergic transmission and blocks the ethanol-induced increase of GABA release in central amygdala. Proc. Natl. Acad. Sci. USA 103. 9715–9720), whereas repeated ethanol intake may downregulate the endogenous NC/OPRL1 system resulting in activation of ethanol consumption. To address this hypothesis, we evaluated whether expression of the pronociceptin (PNOC) and OPRL1 genes is altered in human alcoholics. mRNAs transcribed from these genes were analyzed by quantitative RT-PCR in the prefrontal and orbitofrontal cortices, central amygdala and hippocampal dentate gyrus, structures controlling alcohol consumption. Reduction in PNOC mRNA (1.7-fold) was found in the hippocampus of alcoholics, whereas OPRL1 mRNA levels were decreased (1.4-fold) in the central amygdala. No changes in expression of these genes in other brain areas analyzed were evident. We hypothesise that chronic ethanol intake downregulates PNOC and OPRL1 gene expression in the hippocampus and amygdala, respectively. The findings may be also interpreted as inherited molecular differences between alcoholics and controls. The PNOC/OPRL1 downregulation may underlie impairment of cognitive control over alcohol seeking in alcoholics. Stimulation of the OPRL1 receptors with synthetic agonists may increase threshold for activation of ethanol-related behaviour by environmental cues, and thus may reduce cue- or stress-primed relapse to ethanol consumption.
Keywords: Alcoholism, Nociceptin, OPRL1, Amygdala, Hippocampus
1. Introduction
The OPRL1 receptor is the G-protein coupled receptor activated by the endogenous neuropeptide NC (Meunier et al., 1995; Reinscheid et al., 1995, 1998). In rats, this receptor demonstrates wide expression throughout the CNS with high levels in areas involved in reward, reinforcement, motivation and learning (Mollereau and Mouledous, 2000). Consistently, in the human brain OPRL1 mRNA is abundant in cortical areas including the prefrontal and cingulate cortices, hippocampal dentate gyrus, striatum, thalamus and hypothalamus (Peluso et al., 1998), whereas ligand binding reveals high levels of functional OPRL1 receptors in the cerebral cortex and hippocampus (Berthele et al., 2003). Significant heterogeneity in the NC levels has been found in human CNS with high concentrations in the central gray matter and locus coeruleus, moderate in the amygdala and low in the cerebral cortex and hippocampus (Witta et al., 2004).
Animal studies propose the pharmacological activation of the OPRL1 receptor as an alternative to the blockade of the classic opioid receptors, e.g. by naltrexone, for the therapy of alcoholism(Ciccocioppo et al., 2000; Martin-Fardon et al., 2000; Kuzmin et al., 2003, 2007). NC appears to act as a functional antagonist of corticotrophin-realising factor (Ciccocioppo et al., 2004) suggesting that the NC/OPRL1 system is involved in stress related relapse to ethanol intake (Martin-Fardon et al., 2000). Complementarily, neurochemical animal studies demonstrated that repeated systemic ethanol administration markedly reduces NC levels measured by radioimmunoassay in several brain regions including the hippocampus and cingulate cortex (Lindholm et al., 2002). Moreover, the brain levels of NC are different in rodent strains differing in preferences for alcohol drinking (Ploj et al., 2000). Collectively, behavioural and neurochemical data support the notion that the endogenous NC functions in neuronal circuits involved in reinforcing or conditioning effects of ethanol as a “brake” to limit ethanol intake (Roberto and Siggins, 2006), and that repeated ethanol intake downregulates the endogenous NC/OPRL1 system.
To our knowledge, the role of the NC/OPRL1 system in ethanol-related behaviour has been evaluated only in animals, while relevant human molecular and behavioural data was lacking. The present study was designed to address the hypothesis whether alcohol dependence is associated with alterations in the NC/OPRL1 system. For this purpose, we compared the expression levels of the pronociceptin (PNOC) and OPRL1 genes between human alcoholics and control subjects by analysis of post-mortem human specimens. Because of the accumulating evidence for the role of NC/OPRL1 in regulation of learning and memory (Sandin et al., 2004; Higgins et al., 2002) and processing of aversive stimuli (Mamiya et al., 2003; Hiramatsu and Inoue, 1999), and the emerging concept of cognition as the critical factor controlling alcohol and drug dependence, in this study we focused on the analysis of the PNOC/OPRL1 system in brain areas involved in neurocognitive processes including the prefrontal (PFC) and orbitofrontal (OFC) cortices, hippocampus, and central amygdala (CeA), which is also critical for aversive processes. Notably, neurocognitive circuits of the PFC, OFC and amygdala have recently received increasing attention regarding their functions in the development and maintenance of ethanol-taking behaviour (Koob, 2003; Bechara and Van Der Linden, 2005; Mitchell et al., 2005, Boettiger et al., 2007).
2. Results
The demographic characteristics of control and alcoholic subjects are given in Table 1 (for more detail, see Table 1 in Johansson et al., 2009). We found no significant differences in age (P = 0.96), post-mortem interval (PMI; P = 0.96), storage time (P = 0.17) and proportions of smokers and non-smokers (Fisher test, P = 0.5) between the two groups of subjects. Brain pH was determined to assess the agonal state; mean brain pH values were not significantly different (P = 0.31) between controls and alcoholics.
Table 1.
Sample demographics.
| Characteristics | Control subjects | Alcoholics |
|---|---|---|
| N | 15 | 15 |
| Age (years) | 59±15 | 58±14 |
| PMI (hours) | 27±16 | 32±16 |
| Brain pH | 6.5±0.2 | 6.5±0.2 |
| Storage time (months)a | 56±28 | 68±19 |
| Smoking history | 25% NS, 75% S | 17% NS, 83% S |
PMI, post-mortem interval; NS, non smoker; S, smoker. Mean values±S.D. are shown.
N=13 in each group; smoking histories for four subjects including two control subjects and two alcoholics were not available. For details, see Table 1 in Johansson et al., 2009.
Levels of PNOC and OPRL1 mRNAs were quantified by RTPCR in samples of the PFC (Brodmann area 9), OFC (Brodmann area 11), hippocampus (dentate gyrus) and CeA (Fig. 1; Table 2). The motor cortex (MC; Brodmann area 4) not involved in reward and processing of cognitive information in the same extent was analyzed for comparison.
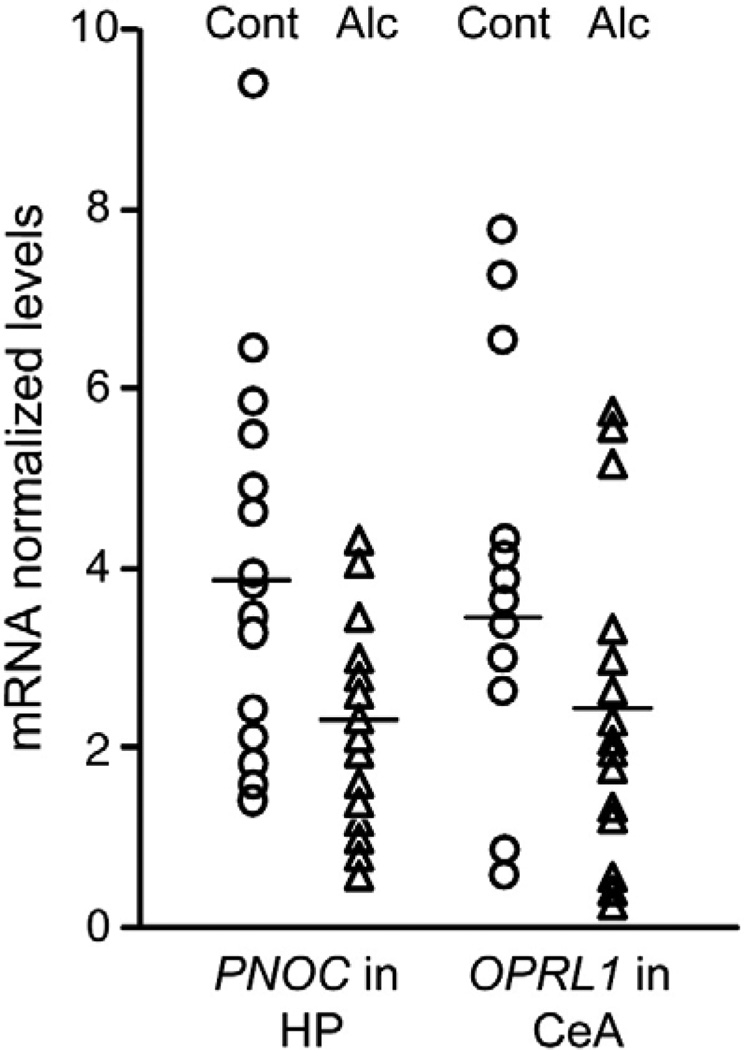
Fig. 1.
Levels of PNOC and OPRL1 mRNAs in the hippocampal dentate gyrus (HP) and central amygdala (CeA). mRNA levels are presented as normalized to reference genes. Open circles and triangles represent individual levels of mRNAs for control and alcoholic subjects, respectively. Mean levels for the groups are shown by horizontal lines.
Table 2.
Levels of PNOC and ORL1 mRNAs in alcoholics and control subjects.
| mRNA | Control subjects | Alcoholics | Relative levels, % of control |
P | |||
|---|---|---|---|---|---|---|---|
| Mean±S.D. | Number of subjects a |
Mean±S.D. | Number of subjects a |
||||
| HP | PNOC | 3.9±2.2 | 14 | 2.3±0.9 | 15 | 60 | 0.03 |
| OPRL1 | 10.4±3.2 | 14 | 8.4±2.9 | 15 | 81 | 0.07 | |
| CeA | PNOC | 0.5±0.2 | 7 | 0.5±0.2 | 10 | 107 | 1.00 |
| OPRL1 | 3.5±0.5 | 7 | 2.4±0.5 | 9 | 61 | 0.005 | |
| PFC | PNOC | 76.8±41.7 | 14 | 84.0±64.8 | 12 | 109 | 0.73 |
| OPRL1 | 5.5±4.8 | 14 | 4.9±2.9 | 12 | 88 | 0.68 | |
| OFC | PNOC | 17.8±12.7 | 15 | 15.8±11.8 | 15 | 89 | 0.66 |
| OPRL1 | 1.2±0.4 | 15 | 1.0±0.3 | 15 | 90 | 0.35 | |
| MC | PNOC | 8.5±5.7 | 14 | 7.7±6.1 | 12 | 91 | 0.73 |
| OPRL1 | 4.2±3.1 | 14 | 3.7±1.8 | 12 | 89 | 0.62 | |
HP (hippocampal dentate gyrus), CeA (central amygdala), PFC (prefrontal cortex; Brodmann area 9), OFC (orbitofrontal cortex; Brodmann area 11), MC (motor cortex; Brodmann area 4).
A number of subjects used for comparison between two groups; molecular analysis was performed for all 15 cases in each group and outliers were excluded on the basis of 95% confidence interval. Three CeA samples from control group did not produce reliable data in RT-PCR and were not included in final calculations. Significance of differences was evaluated using Student's t-test.
The PNOC mRNA levels were substantially lower (on 40%; P < 0.05) in the hippocampus of alcoholics (n = 15) compared to control subjects (n = 14) whereas no differences in expression of this gene in other structures analyzed were evident (Fig. 1 and Table 2). Data for the hippocampus, PFC and MC for one control subject were not included in the final calculations because experimental values exceeded 95% confidence interval. For the same reason, samples of the PFC and MC of three alcoholics were excluded from statistical analysis.
mRNA prepared from the CeA tissue of three control subjects did not give a signal in RT-PCR and these samples were omitted form analysis. The remaining CeA samples exhibited high variability in both the PNOC and OPRL1 mRNA levels (Fig. 1), probably, due to cellular heterogeneity in this brain area. When outliers were excluded on the basis of 95% confidence interval, significantly lower levels (on 31%; P < 0.01) of OPRL1 mRNA were detected in alcoholics (n = 9) compared to control subjects (n = 7; Fig. 1 and Table 2).
No influence of brain pH, age at death, storage time and PMI as covariates on the differences found was observed. Covariate analysis revealed no significant influence of smoking on the observed results. The sample toxicology screen was focused primarily on benzodiazepines, and the three alcoholics who had traces of this class of medications in their blood at the time of death were excluded and the data were reanalyzed. Again, essentially the same results were obtained as when all subjects were included into the analysis; PNOC mRNA levels were lower on 45% in the hippocampus of alcoholics compared to controls (t-test, p< 0.05; n = 14 for controls, and n = 12 for alcoholics), while the OPRL1 gene was expressed at 30% lower levels in the CeA of alcoholics relative to controls (t-test, p < 0.05; n = 7 for controls, and n = 8 for alcoholics).
3. Discussion
In animal experiments, repeated ethanol treatment decreased NC concentration in the hippocampus and cingulate cortex but not in the mesolimbic system (Lindholmet al., 2002). In the present port-mortem human study, the substantially lower levels of PNOC mRNA were found in the hippocampus of human alcoholics compared to control subjects that corroborate the animal data.
Several animal studies imply a role of NC and OPRL1 in neurocognitive processes. Thus, the activation of the hippocampal OPRL1 receptor has been shown to inhibit formation of long-term memory (Goeldner et al., 2008). Consistently, functional antagonism between the NC/OPRL1 system and glutamatergic mechanisms, which facilitate learning and memory formation, has been proposed as a factor modulating cognitive processes (Goeldner et al., 2009). Taking into account that the OPRL1 stimulation blocks cue and stress-induced relapse to ethanol-taking behaviour (Martin-Fardon et al., 2000; Kuzmin et al., 2007), it is tempting to speculate that the decrease in activation of the OPRL1 receptor in the hippocampus of alcoholics may enhance a glutamatergic signal and facilitate the association between environmental cues and ethanol-taking behaviour. This notion is indirectly supported by the observation that hippocampal formation is involved in modulation of learning behaviour related to drug abuse (Torres-Reveron et al., 2009).
Amygdala involved in processing of aversive stimuli (LeDoux, 2000) is considered as a brain region regulating alcohol consumption (Koob, 2003). NC injected into the CeA inhibited ethanol self-administration in the alcohol-preferring animals (Economidou et al., 2008). In the CeA of ethanol-dependent rats, the enhanced sensitivity to NC effects has been described while this peptide blocked the ethanol-induced augmentation of inhibitory postsynaptic currents (Roberto and Siggins, 2006). The present study suggests that the OPRL1 receptor expression may be downregulated in the CeA of alcoholics. If animal findings by Roberto and Siggins (2006), were correct for human alcoholism, the increased sensitivity to the nociceptin effects may be developed to counteract the downregulation of the OPRL1 receptor.
The limitations of the present study are a) that the findings are applicable to only males because no female subjects were analyzed; and b) that these findings are not correlated with toxicological parameters because the toxicology screen is incomplete (data is available only for five cases). Another limitation is a low number of CeA samples used in statistical analysis; a number of outliers excluded from the analysis were greater in the CeA than in other brain areas possibly because of the substantial CeA cellular heterogeneity. Nevertheless, the differences between alcoholics and controls in the hippocampus and CeA point out to the importance of plasticity in the NC/OPRL1 system in these brain areas for alcohol dependence.
Our results support the concept postulating that chronic ethanol consumption and withdrawal downregulate the PNOC/OPRL1 system, which critically controls alcohol intake. However, the findings may be also interpreted as inherited molecular differences between alcoholics and control subjects. Two human genetic studies on the association of the PNOC and OPRL1 genes with alcohol dependence do not shed light on this issue due to contradicting results reported (Huang et al., 2008; Xuei et al., 2008). In the first study, 10 SNPs covering OPRL1 and the adjacent area and 15 SNPs covering PNOC in European American subjects were examined resulting in no convincing association between alcohol dependence and OPRL1 and PNOC polymorphisms (Xuei et al., 2008). The second study demonstrated the association of one of 18 OPRL1 SNPs analyzed with alcohol dependence in Scandinavian population (Huang et al., 2008). Relationship between polymorphisms of the OPRL1 and PNOC genes and levels of expression of these genes in the human brain has not yet been investigated. Thus, it is still unclear whether the genetics approach may be used to distinguish the two aforementioned mechanisms of the downregulation of the PNOC/OPRL1 system in human alcoholics.
In conclusion, the downregulation of the PNOC/OPRL1 system associated with alcohol dependence may affect neurotransmission in neuronal circuits involved in the coupling of external information and internal emotional status modified by alcohol intake, and, as a result may weaken the inhibitory control over the conditional or stress-induced relapse to alcohol seeking and taking behaviour. Stimulation of the OPRL1 by synthetic agonists might produce “antirelapse” effects by elevation threshold levels for activation of these brain circuits.
4. Experimental procedures
4.1. Human samples/case selection
Tissues were collected at the New South Wales Tissue Resource Centre (TRC), University of Sydney, Australia (http://www.pathology.usyd.edu.au/trc.htm). All subjects were male Caucasians. Alcoholics met criteria for Diagnostic and Statistical Manual for Mental Disorders, 4th edition and also National Health and Medical Research Council/World Health Organization criteria, and consumed greater than 80 g of ethanol per day for the majority of their adult lives. Controls had either abstained from alcohol completely or were social drinkers who consumed less than 20 g of ethanol per day on average. Control cases were matched to alcoholic cases by sex, age, race and post-mortem interval. Cases with a history of poly drug abuse (with evidence that the individual abused other drugs such as cocaine or heroin) or with medical complications such as liver cirrhosis and the Wernicke–Korsakoff syndrome or alcoholic cases with concomitant diseases were excluded. Cases with a prolonged agonal life support, or cases with a history of cerebral infarction, head injury, or neurodegenerative disease (e.g., Alzheimer's disease), were also excluded. The main body of the population was smokers including 83% of alcoholics and 75% of control subjects. Samples were taken by qualified pathologists under full ethical clearance from the Sydney South West Area Health Service Human Ethics Committee (X03-0074) and informed written consent from the next of kin. The study was approved by the local ethical committee of the Karolinska Institutet.
4.2. mRNA expression levels analysis by quantitative Real-time PCR using TaqMan® low density array
RNA preparation was performed using RNeasy Lipid Tissue Mini Kit (QIAGEN, Maryland, USA). RNA was quantified by Nanodrop® and Agilent 2100 Bioanalyzer (Agilent, Palo Alto, CA) was used to control RNA quality. Only RNA with clear ribosomal RNA, 18S and 28S, was used for further analysis. cDNA was synthesized with the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). mRNA levels were quantified by TaqMan® Low Density Arrays (Applied Biosystems, Foster City, CA). In a pre-prepared micro fluidic card containing probes and primers for each gene, cDNA and TaqMan® Universal PCR Master Mix (Applied Biosystems) was added in a final concentration of 65 pg cDNA per sample and gene. Every sample was run in triplicate (PFC, MC, CeA and hippocampus) or quadruplicate (OFC) on the same array for each gene. The PCR amplification was performed at 50 °C for 2 min, 94. 5 °C for 10 min and 40 cycles of 97 °C for 30 s followed by 59.7 °C for 1 min. To measure the quantity of a given RNA species, the threshold cycles (Ct) were monitored by the Applied Biosystems 7900HT Fast Real-Time PCR System. Each mRNA expression was calculated by relative quantification using a normalization factor (geometric mean of two reference genes selected by geNORM program, http://medgen.ugent.be/genorm/) (Vandesompele et al., 2002) and qBASE program for internal and external calibration and also for easy care of large RT-PCR datasets (http://medgen.ugent.be/qbase/). According to our analysis of reference genes (Johansson et al., 2007, 2009), the beta-actin (ACTB) and ribosomal large P0 (RPLP0) genes for the PFC, 18S rRNA and TATA box binding protein (TBP) genes for CeA, the peptidylprolyl isomerase A (cyclophilin A) (PPIA) and phosphoglycerate kinase 1 (PGK1) genes for OFC, polymerase (RNA) II (DNA directed) polypeptide A (POLR2A) and ubiquitin C (UBC) for hippocampus, and 18S and RPLP0 genes for MC were chosen for normalization.
4.3. Statistical analysis
Statistical analysis was carried out using the Statistica 8.0 package (StatSoft Scandinavia, Uppsala, Sweden). Normality of data distribution was analyzed using Shapiro–Wilk's Wtest. If the data does not show normal distribution, it was normalized by exclusion of the subjects exceeding 95% confidence interval. A general stepwise linear regression model was used to evaluate group differences and identify covariates (age, brain pH and PMI). Student's t-test was used to assess the differences between groups when no covariates were found. The main body of the population was smokers (Table 1). To assess the influence of nicotine abuse on the mRNA levels, ANCOVA analysis was performed with smoking as a covariant. A significance level of P < 0.05 was accepted as statistically significant and all tests were two-tailed.
Acknowledgments
This work was supported by grants from the AFA Forsäkring and the Research Foundation of the Swedish Alcohol Retail Monopoly (SRA) to AK and GB, and the Swedish Science Research Council, the Swedish National Drug Policy Coordinator and the Torsten och Ragnar Söderbergs stiftelser to GB. The Australian Brain Donor Programs NSW Tissue Resource Centre was supported by The University of Sydney, National Health and Medical Research Council of Australia, National Institute of Alcohol Abuse and Alcoholism and NSW Department of Health.
Role of the funding source: All the funding sources mentioned above had no involvement in study design, in the collection, analysis, interpretation of data or in the decision to submit the manuscript.
Footnotes
Conflicts of interest: All authors declare that they have no conflicts of interest. The authors declare that, except for income received from the primary employer, no financial support or compensation for research or professional service has been received from any individual or corporate entity over the past three years and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
REFERENCES
- Bechara A, Van Der Linden M. Decision-making and impulse control after frontal lobe injuries. Curr. Opin. Neurol. 2005;18:734–739. doi: 10.1097/01.wco.0000194141.56429.3c. [DOI] [PubMed] [Google Scholar]
- Berthele A, Platzer S, Dworzak D, Schadrack J, Mahal B, Büttner A, Assmus HP, Wurster K, Zieglgänsberger W, Conrad B, Tölle TR. [3H]-nociceptin ligand-binding and nociceptin opioid receptor mRNA expression in the human brain. Neuroscience. 2003;121:629–640. doi: 10.1016/s0306-4522(03)00484-6. [DOI] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D'Esposito M, Fields HL. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J. Neurosci. 2007;27:14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Sanna PP, Weiss F, Massi M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur. J.Pharmacol. 2000;404:153–159. doi: 10.1016/s0014-2999(00)00590-2. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, Massi M. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl) 2004;172:170–178. doi: 10.1007/s00213-003-1645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, Fedeli A, Martin-Fardon R, Massi M, Ciccocioppo R, Heilig M. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol. Psychiatry. 2008;64:211–218. doi: 10.1016/j.biopsych.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeldner C, Reiss D, Wichmann J, Meziane H, Kieffer BL, Ouagazzal AM. Nociceptin receptor impairs recognition memory via interaction with NMDA receptor-dependent mitogen-activated protein kinase/extracellular signal-regulated kinase signaling in the hippocampus. J. Neurosci. 2008;28:2190–2198. doi: 10.1523/JNEUROSCI.3711-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeldner C, Reiss D, Wichmann J, Kieffer BL, Ouagazzal AM. Activation of nociceptin opioid peptide (NOP) receptor impairs contextual fear learning in mice through glutamatergic mechanisms. Neurobiol. Learn. Mem. 2009;91:393–401. doi: 10.1016/j.nlm.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Kew JN, Richards JG, Takeshima H, Jenck F, Adam G, Wichmann J, Kemp JA, Grottick AJ. A combined pharmacological and genetic approach to investigate the role of orphanin FQ in learning and memory. Eur. J. Neurosci. 2002;15:911–922. doi: 10.1046/j.1460-9568.2002.01926.x. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M, Inoue K. Effects of nocistatin on nociceptin-induced impairment of learning and memory in mice. Eur. J. Pharmacol. 1999;367:151–155. doi: 10.1016/s0014-2999(99)00003-5. [DOI] [PubMed] [Google Scholar]
- Johansson S, Fuchs A, Okvist A, Karimi M, Harper C, Garrick T, Sheedy D, Hurd Y, Bakalkin G, Ekstrom TJ. Validation of endogenous controls for quantitative gene expression analysis: application on brain cortices of human chronic alcoholics. Brain Res. 2007;1132:20–28. doi: 10.1016/j.brainres.2006.11.026. [DOI] [PubMed] [Google Scholar]
- Johansson S, Ekström TJ, Marinova Z, Okvist A, Sheedy D, Garrick T, Harper C, Kuzmin A, Yakovleva T, Bakalkin G. Dysregulation of cell death machinery in the prefrontal cortex of human alcoholics. Int. J. Neuropsychopharmacol. 2009;12:109–115. doi: 10.1017/S1461145708009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur. Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J. Pharmacol. Exp. Ther. 2003;304:310–318. doi: 10.1124/jpet.102.041350. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Kreek MJ, Bakalkin G, Liljequist S. The nociceptin/orphanin FQ receptor agonist Ro 64–6198 reduces alcohol self-administration and prevents relapse-like alcohol drinking. Neuropsychopharmacology. 2007;32:902–910. doi: 10.1038/sj.npp.1301169. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lindholm S, Ploj K, Franck J, Nylander I. Nociceptin/orphanin FQ tissue concentration in the rat brain. Effects of repeated ethanol administration at various post-treatment intervals. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26:303–306. doi: 10.1016/s0278-5846(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Mamiya T, Yamada K, et al. Neuronal mechanism of nociceptin-induced modulation of learning and memory: involvement of N-methyl-d-aspartate receptors. Mol. Psychiatry. 2003;8:752–765. doi: 10.1038/sj.mp.4001313. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F. Nociceptin prevents stress-induced ethanol — but not cocaine-seeking behavior in rats. NeuroReport. 2000;11:1939–1943. doi: 10.1097/00001756-200006260-00026. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, Mazarguil H, Vassart G, Parmentier M, Costentin J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. General and specific contributions of the medial prefrontal cortex to knowledge about mental states. NeuroImage. 2005;28:757–762. doi: 10.1016/j.neuroimage.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Mouledous L. Tissue distribution of the opioid receptor-like (ORL1) receptor. Peptides. 2000;21:907–917. doi: 10.1016/s0196-9781(00)00227-8. [DOI] [PubMed] [Google Scholar]
- Peluso J, LaForge KS, Matthes HW, Kreek MJ, Kieffer BL, Gavériaux-Ruff C. Distribution of nociceptin/orphanin FQ receptor transcript in human central nervous system and immune cells. J. Neuroimmunol. 1998;81:184–192. doi: 10.1016/s0165-5728(97)00178-1. [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Gustavsson L, Nylander I. Basal levels and alcohol-induced changes in nociceptin/orphanin FQ, dynorphin, and enkephalin levels in C57BL/6J mice. Brain Res. Bull. 2000;53:219–226. doi: 10.1016/s0361-9230(00)00328-2. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G proteincoupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Higelin J, Henningsen RA, Monsma FJ, Jr, Civelli O. Structures that delineate orphanin FQ and dynorphin A pharmacological selectivities. J. Biol. Chem. 1998;273:1490–1495. doi: 10.1074/jbc.273.3.1490. [DOI] [PubMed] [Google Scholar]
- Roberto M, Siggins GR. Nociceptin/orphanin FQ presynaptically decreases GABAergic transmission and blocks the ethanol-induced increase of GABA release in central amygdala. Proc. Natl. Acad. Sci. U.S.A. 2006;103:9715–9720. doi: 10.1073/pnas.0601899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin J, Ogren SO, Terenius L. Nociceptin/orphanin FQ modulates spatial learning via ORL-1 receptors in the dorsal hippocampus of the rat. Brain Res. 2004;997:222–233. doi: 10.1016/j.brainres.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Torres-Reveron A, Khalid S, Williams TJ, Waters EM, Jacome L, Luine VN, Drake CT, McEwen BS, Milner TA. Hippocampal dynorphin immunoreactivity increases in response to gonadal steroids and is positioned for direct modulation by ovarian steroid receptors. Neuroscience. 2009;159:204–216. doi: 10.1016/j.neuroscience.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witta J, Palkovits M, Rosenberger J, Cox BM. Distribution of nociceptin/orphanin FQ in adult human brain. Brain Res. 2004;997:24–29. doi: 10.1016/j.brainres.2003.08.066. [DOI] [PubMed] [Google Scholar]
- Huang J, Young B, Pletcher MT, Heilig M, Wahlestedt C. Association between the nociceptin receptor gene (OPRL1) single nucleotide polymorphisms and alcohol dependence. Addict. Biol. 2008;13:88–94. doi: 10.1111/j.1369-1600.2007.00089.x. [DOI] [PubMed] [Google Scholar]
- Xuei X, Flury-Wetherill L, Almasy L, Bierut L, Tischfield J, Schuckit M, Nurnberger JI, Jr, Foroud T, Edenberg HJ. Association analysis of genes encoding the nociceptin receptor (OPRL1) and its endogenous ligand (PNOC) with alcohol or illicit drug dependence. Addict. Biol. 2008;13:80–87. doi: 10.1111/j.1369-1600.2007.00082.x. [DOI] [PubMed] [Google Scholar]