Abstract
Research into neuropsychiatric disorders, including alcohol-related problems, is limited in part by the lack of appropriate animal models. However, the development of new technologies in pathology and molecular biology means that many more questions can be addressed using appropriately stored human brain tissues. The New South Wales Tissue Resource Centre (TRC) in the University of Sydney (Australia) is a human brain bank that can provide tissues to the neuroscience research community studying alcohol-related brain disorders, schizophrenia, depression and bipolar disorders. Carefully standardised operational protocols and integrated information systems means that the TRC can provide high quality, accurately characterised, tissues for research. A recent initiative, the pre-mortem donor program called “Using our Brains”, encourages individuals without neuropsychiatric illness to register as control donors, a critical group for all research. Community support for this program is strong with over 2,000 people registering their interest. Discussed herein are the protocols pertaining to this multifaceted facility and the benefits of investment, both scientific and financial, to neuroscience researchers and the community at large.
Keywords: Alcohol, Australia, Bipolar disorder, Brain bank, Brain damage, Donor program, Neuropathology, Research, Schizophrenia
Introduction
The New South Wales Tissue Resource Centre (TRC) is an Australian brain bank located in Pathology at The University of Sydney, Australia. The first autopsy brain specimens were collected specifically for intramural research projects that focused on the structural changes in the brain in alcohol-related disorders. Subsequently, with appropriate protocols, ethics approval and additional funding, this unique resource has evolved into a brain bank for researchers studying alcohol-related brain damage, dependence and tolerance and the pathogenesis of schizophrenia, depression and bipolar disorders. TRC staff review and collect the tissues from coronial and non-coronial cases and process the tissues. Cases are stored at −80°C for molecular studies and in formalin for pathological studies. Staff co-ordinate two pre-mortem donor programs and perform neuropsychological assessments on live donors. Review of relevant medical and lifestyle information is critical for the accurate characterisation of cases and is done by trained clinical nurses and psychologists. There is a complex, interactive database for the TRC that allows us to select appropriate tissues for both intramural and extramural research groups and provide them with anonymous details of each case. It also facilitates the preparation of reports and promotional material for stakeholders. There is an independent management committee who oversee the TRC and makes decisions about the allocation of tissues based on scientific merit and feasibility of the studies and the track record of requesting researchers.
In recent years, the reliance on accessing donors through forensic and hospital institutions has changed because of the decline in autopsy rates (Jeganathan et al. 2006; Ward et al. 2001), a worldwide phenomenon (Burton and Underwood 2003; Nemetz et al. 2006; Sinard 2001). Pre-mortem donor programs have now become an important part of the TRC as they provide us with a comprehensive, longitudinal profile of the health and lifestyle of donors. These programs also have an educational benefit as they highlight the need for brain tissue banking to the community. The two pre-mortem donor programs coordinated by the TRC are the “Gift of Hope” for those with a psychiatric illness and their relatives and “Using our Brains” for healthy individuals who register as control donors, a critical group for research.
Recent advances in high throughput technologies like genomics and proteomics are being used commonly in neuroscience research and this has led to an increased demand for frozen tissue. Researchers require comprehensive clinical and pathological information pertaining to the tissues. Each researcher using TRC material is required to submit a report annually. These reports enable us to modify our TRC protocols and procedures to adjust to the needs of researcher. Those conducting molecular techniques are asked to forward their results on the tissue integrity such as RIN. The annual report also enables us to summarise outcomes from the research such as publication of manuscripts and presentations at conferences.
The TRC is now part of the recently developed Australian Brain Donor Program and the Australian Brain Bank Network. These affiliations allow us to communicate regularly with brain banks in other States in Australia, develop similar protocols, share data, and combine resources to provide tissues to researchers. Together, we are able to seek infrastructure funding and reach a wider public for promotion of brain banking for medical research. Each bank tends to have specific disease categories that are different from each other. This review of the TRC outlines our current protocols and procedures and provides details of outcomes.
Methods
There are two main pathways that the TRC uses to access human brain tissue for research. Firstly, through forensics institutes and secondly pre-mortem donor programs. The TRC has ethics approval from Sydney South West Area Health Service (Protocol number: X07-0074) and The University of Sydney (Ref No. 555). Currently, most of the cases are collected from the Departments of Forensic Medicine (DOFM) in Sydney. TRC staff contact the next-of-kin (NOK) on the day of autopsy and ask them to consider donating the brain of the deceased to medical research. Cases are also collected through two pre-mortem donor programs, “Using our Brains” (www.braindonors.org) and “Gift of Hope” (www.schizophreniaresearch.org.au) as described below. There is a tissue retrieval team on call 24 h/7 days a week for these programs to aid in minimising the post mortem interval times.
The TRC selection criteria for DOFM cases prescribes that potential donors are over 17 years of age and have no history of developmental or neurological disorder. They have no infectious diseases such as HIV or Hepatitis C, no history of substance dependence/abuse (except for alcohol and/or nicotine) and they did not die of a significant head injury. Potential donors who have undergone assisted ventilation for more than 24 h prior to death, or with poor agonal status due to other causes, are also excluded from the collection.
Donor programs
The ‘Using our Brains’ program encourages people without neuropsychiatric illness to register as ‘control’ donors. The ‘Gift of Hope’ program invites people with a major psychiatric illness to consider donating their brain to medical research. People can indicate their interest in the programs by telephone or registering “on-line” (www.braindonors.org). Those who register are sent a “consent kit” that includes a questionnaire covering life-style and general health issues (previous illnesses, diet, alcohol intake, smoking, drug therapy etc.) and consent forms. Donors are asked to participate in an interview (about 1 h) that assesses memory, language and other aspects of thinking. Assessments measure psychiatric illness and drug/alcohol use (Diagnostic Instrument for Psychosis—DIP), premorbid intellectual functioning (National Adult Reading Test—NART), cognitive functioning including immediate/delayed memory, visuospatial/constructional ability, language and attention (Repeatable Battery for the Assessment of Neuropsychological Status—RBANS), verbal fluency (Controlled Oral Word Association Test—COWAT), working memory (Letter-Number Sequencing—LNS), abstract reasoning and problem solving (Wisconsin Card Sort 64—WCST64). Donors are also invited to have an MRI scan. All donors are contacted annually and assessments are repeated every 1–6 years, depending on age and previous performance. Donors can withdraw from the program at any time.
Brain dissection procedure
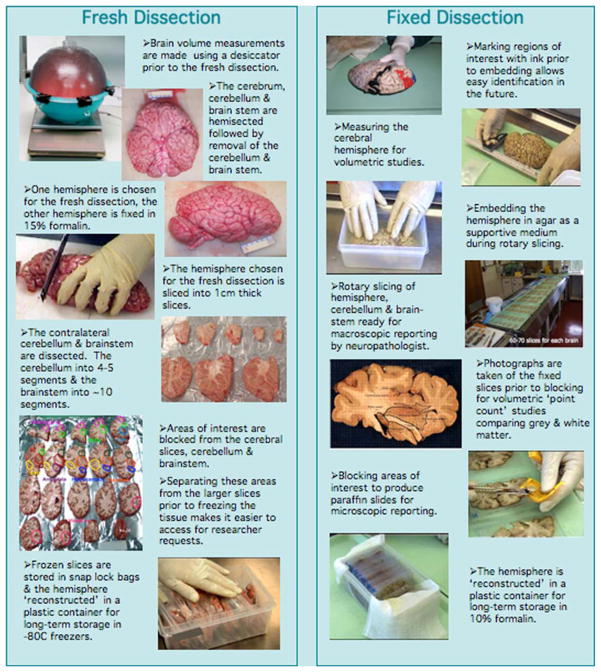
At autopsy, the brain weight and volume are determined (Harper et al. 1988b) and the brain is photographed prior to dissection. The cerebrum, cerebellum and brainstem are divided in the sagittal plane. The cerebellar hemisphere and brainstem are removed from each cerebral hemisphere by transversely sectioning the brainstem through the rostral midbrain at the level of the superior colliculi. Each cerebral hemisphere and its contralateral cerebellar hemisphere and brainstem are assigned a “fresh” or “fixed” status on a random basis. The fresh hemisphere is cut into 1 cm coronal slices and several areas are dissected (prefrontal cortex, anterior cingulate cortex, posterior cingulate cortex, caudate putamen, basal forebrain, amygdala, thalamus, hippocampus, visual cortex, superior temporal cortex and motor frontal cortex) and digitally photographed. The dissected tissue blocks and remaining slices are frozen on pre-cooled trays at a temperature of −80°C. Once frozen the slices and dissected areas are bagged into clip-lock plastic bags that are sized to reduce the air volume surrounding the tissue.
The other cerebral hemisphere is fixed in 15% buffered formalin for 3 weeks, and then embedded in an agar block (Sarris et al. 2002). The agar facilitates serial slicing of the hemisphere at 3 mm intervals in the coronal plane. Each slice is photographed digitally for documentation and regional volume analysis.
A number of regions are dissected from the fixed hemisphere for detailed neuropathological examination. Blocks from the following regions, frontal, parietal, occipital, temporal, ammons horn, basal ganglia, thalamus, mamillary body, cingulate cortex, cerebellum, vermis, midbrain, pons and medulla are taken for paraffin embedding. These are sectioned at 7 μm and stained with a standard hematoxylin and eosin protocol. At the discretion of the neuropathologist, additional stains can be performed including cresyl violet, Luxol fast blue and Bodian silver with Congo red and immunoperoxidase preparations such as tau, ubiquitin, alpha-synuclein and beta-amyloid. These stains help to diagnose dementias such as Alzheimer’s disease. The remainder of the fixed material is stored indefinitely in 10% formalin until required for research purposes. Figure 1 gives an overview the dissection processes.
Fig. 1.
Over view of the fresh and fixed dissection methods employed by the TRC
Autopsy reports
Information available from the autopsy reports includes age at death, sex, post mortem interval, cause of death and relevant pathological findings. Liver pathology is categorised into: normal, congestion, steatosis (fatty liver) and cirrhosis. The DOFM Analytical Laboratories perform a toxicological screen on blood samples taken at the time of autopsy. These provide vital information on blood alcohol levels and therapeutic drug toxicology including neuroleptics, paracetamol, and anti-depressants. The results of macroscopic and microscopic neuropathological examinations are also obtained.
Daily alcohol consumption and life time consumption
Daily alcohol consumption is assessed after review of all available medical records and information from the NOK. Consumption rates are recorded as grams of ethanol per day, and calculated based on numbers of standard drinks per day. Lifetime consumption is reported as kilograms of 100% alcohol. This is calculated from numbers of years of drinking × 365 (days) × mean consumption rate. The age of commencement of drinking is assumed to be 25 years of age in all calculations except where other ages were specified. Any period(s) of known abstinence that are greater than 6 months are subtracted from the drinking years (Harper et al. 1988a).
Smoking history
Smoking history is collated after review of all available medical records and information from the NOK. This information is categorised into the following groups: non-smoker, moderate (less than one packet per day), and heavy (≥1 packet per day) and those who were ex smokers. This information is included in the dataset for researchers.
Type and dose of antipsychotic medication
If cases have been prescribed an antipsychotic medication during their lifetime, chlorpromazine equivalent dosages are calculated based on available information. Antipsychotic medication use is always an estimate, and is accompanied by the qualifier that medication compliance varies. Donors for whom a single chlorpromazine equivalence estimate is recorded (rather than a range), were prescribed either a single, or a static dose of antipsychotic medication during their lifetime. In addition, information regarding the predominant type of antipsychotic medication use (typical vs. atypical) is also recorded, and is based on a review of available medication use throughout a donor’s lifetime.
Determination of case inclusion
Both tissue integrity and accurate case classification are critical to ensure the reliability of the material for research. A number of assessments are performed before a case is included in the TRC for researchers. These include, diagnostic characterisation, review of agonal status, measurement of brain tissue pH, histological assessment of post-mortem autolysis and a full neuropathological examination. Cases that are excluded from the research bank, either due to inability to confirm a DSM-IV psychiatric diagnosis or for neuropathological reasons, can still be used for developing and testing new methodologies and techniques.
Diagnostic characterization of DOFM cases
The post-mortem clinical diagnosis of each case is determined primarily through extensive review of medical records by one of two independent clinicians. Where available, data from pathology reports and neuropsychological tests is also collected. Information relating to the donor’s psychiatric history, developmental history, family history of mental illness, drug, alcohol and medical treatment history is collated into a formatted, structured treatment summary, as previously described (Sarris et al. 2002). If required, further information is obtained from telephone interviews with the NOK, general practitioners and specialist health personnel.
The Diagnostic Instrument for Brain Studies (DIBS) is then applied to the clinical summary described above, to generate a DSM-IV diagnosis (American Psychiatric Association 1994). The DIBS is a semi-structured instrument designed for post-mortem psychiatric assessment using medical records and informants where possible. This instrument enables diagnosis at a sub-syndrome and symptom-based level, providing comprehensive information for prospective research, while increasing the reliability of clinical diagnosis (Garrick et al. 2006; Keks et al. 1999).
For substance use (alcohol cases), additional operational criteria may be used. The clinical diagnosis of Wernicke-Korsakoff Syndrome (WKS) is based on criteria that were established by Caine and her colleagues. These were developed after reviewing the clinical histories and neurological examinations of 4,000 post-mortem cases (Caine et al. 1997). Postmortem and neuropathology reports are also crucial in establishing a diagnosis of WKS. The diagnosis is based on classical histopathological changes in the mamillary bodies (Harper 1979).
Brain tissue pH
To assess tissue quality (integrity) approximately 3 g of lateral cerebellar hemisphere is taken and frozen at −80°C at the time of brain dissection. This region has cerebellar cortex, granular cell layer and white matter and has been shown to be representative of the whole brain for pH measurement (Kingsbury et al. 1995; Stan et al. 2006). Brain tissue pH is later measured using one gram of this cerebellar tissue homogenized in 10 volumes of distilled water at neutral pH (Harrison et al. 1995). Measurements are taken in duplicate using a temperature compensating Mettler Toledo MP220 pH meter (Mettler USA).
Histological assessment of autolytic change of cerebellar granule cell layer
The cerebellum is dissected fresh into sagittal slices 1–2 cm thick approximately and fixed in 15% buffered formalin for 2–3 weeks. Fixed tissues are embedded in paraffin and 7 μm sections are cut and stained with routine hematoxylin and eosin. The granular cell layer of the cerebellum is assessed microscopically to determine the degree of autolytic degradation by an experienced neuropathologist. Degradation is graded as nil, mild, moderate or severe as previously described by Albrechtsen and Ogata et al. (Albrechtsen 1977; Ogata et al. 1986). They defined mild (slight) as a definite decrease in the number of the nuclei stained by hematoxylin, moderate when there is a diffuse decrease in the number of nuclei and severe as complete loss of nuclei. Figure 2 shows the different degrees of cerebellar granule cell layer degradation.
Fig. 2.
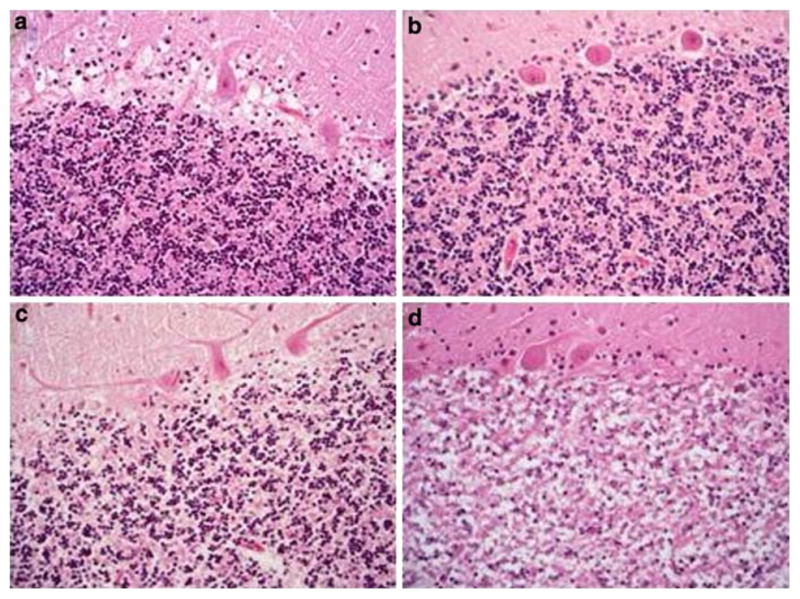
Histological findings of autolysis of the cerebellar granule cell layer. Slides were Hematoxylin and Eosin stained and photographed at magnification 40×. (a) Normal, (b) Mild, (c) Moderate and (d) Severe
Brain volume measurement
This bank performs volumetric analysis on those cases that meet a DSM-IV classification and the control cases. Estimates of the volumes of regions of interest are calculated by application of the Cavalieri principle (Howard and Reed 2005).
where V, estimate of volume; T, slice thickness; A, area associated with each point on the grid
Calculation of the volumes of cortical grey matter, deep grey matter and white matter for each case is based on photograph images of a sagittally sliced fixed hemisphere at 3 mm. The overlay grid is divided at 0.6 cm intervals giving an area of 0.36 cm2 between points.
Tissue requests
The TRC provides frozen and/or fixed post-mortem human brain tissue to Australian and international research groups. In order to access this resource a researcher must first submit a tissue request. The Tissue Request Application form can be obtained from the website (http://www.nnf.com.au or http://www.pathology.usyd.edu.au/trc.htm) or via email (trc@med.usyd.edu.au). This form has been developed in line with current New South Wales legislation and meets ethical and privacy requirements. A principal investigator must provide his/her biographic sketch, a list of co-investigators and a detailed research proposal. A current Ethics Committee approval for the research from their host institution is mandatory. The Scientific Advisory Committee of the Australian Brain Donor Program, made up of TRC members and independent neuroscientists, reviews each research proposal. A Material Transfer Agreement must be signed by both parties before tissues are sent to the researcher. All unused tissues must be returned to the TRC after completion of the project. Researchers must complete an Annual Evaluation Form this helps us assess the quality of tissue that they received and provides us with information concerning presentations and publications arising from their research. They are also invited to comment on the overall performance of the TRC.
Information system
The current system comprises twelve inter-related databases supported by Filemaker Pro Advanced 8.5 and Filemaker Server (Filemaker Inc, USA). These cover each of the main activities of the TRC. Security and confidentiality is maintained through internal and institutional protection mechanisms. Access to the databases is defined for each staff member based upon position within the TRC. There is a log for data entry and backup protocols include offsite storage.
Demographic, clinical, laboratory, radiological and pathological data is collated in a standardize manner. Data relating to the donor programs includes a comprehensive overview of lifestyle and neuropsychiatric assessment information. Data sets are provided for all research projects and include the general demographics, neuropathology diagnosis, DSM-IV, brain pH, and post mortem interval. The researcher may request other information depending on project and analysis.
The information system supports databases that store data on the journal publications and conference presentations arising from research projects facilitated by the TRC and include a pdf version of article. Information is collected on presentations given by staff to various community groups. Generation of reports for the stakeholders is streamlined with the use of specific devised scripts.
Statistics
Statistical analysis was performed using JMP 6.0 (SAS Institute Inc). Correlation analyses were performed using the variables of liver pathology and autolysis grading. One-way ANOVA and student t-tests were applied. Current demographic data relating to the donor programs, and outcomes from the TRC were compared to data collated previously for the period 1994–1999.
Results
A comparison of the initial 5 years of the brain bank to now has shown a sustained growth in reported outcomes. Between 1994 and 1999 there were 143 cases collected and 34 research projects facilitated. Currently there are 535 cases within the TRC collection, of these 46 cases are from the pre-mortem donor programs. To date there have been 7% of the total number of cases not meeting the inclusion criteria to research bank, of which 5% were due to insufficient clinical information to determine the DSM-IV classification and 2% for neuropathology reasons. Cases not meeting the inclusion criteria to the main bank are useful as trial tissue for technique work up.
The percentage of TRC cases available to researchers comprise of the following diagnostic groups; Controls 27%, substance abuse (alcohol) 26%, schizophrenia and allied disorders 20% neurodegenerative 21% and other (brain tumour, rare neuropathology) 6%.
The range of post mortem interval of all the TRC cases is 4.5 to 72 h with the mean of 27.7 ± 14.5 h and the median of 24.5 h. For the cases collected through the donor programs the post mortem interval range was 5–70 h with the mean as 16.5 ± 14 h and median of 12.5 h. Our donors are located throughout metropolitan and rural areas and transport to our facility may take as long as 8 h. Even so an improvement is seen in the mean and median post mortem intervals of donor collections.
The pre-mortem donor programs have 1,220 donors who have progressed through the consenting process. Fifty-six percent of the donors are male and 44% are female. These are similar percentages to the first year of the program. However, there is now a change in the age distribution within each gender group. In 2002, 48% of males and 79% of females were less than 60 years of age and 52% of males and 21% of females were more than 60 years. Currently (2007), 45% of males and 56% of females are less than 60 years of age and 55% of males and 44% of females are more than 60 years. Figure 3 shows the current percentage distribution of age groups for males and females. There have been 838 assessments performed, 670 first assessments and 168 follow-up assessments.
Fig. 3.
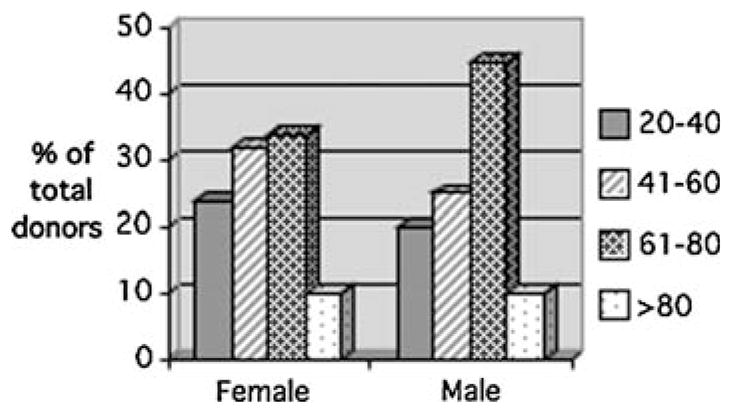
The age distribution of male and female consented through the pre-mortem donor program
Information regarding the promotion of the donor programs and how the donor found out about the program is useful in the allocation of future resources. The three main avenues of recruitment are radio (28%), television (30%) and staff presentations to stakeholders (31%).
Our protocols and procedures have been modified in accordance with new technologies and to optimize tissue quality and availability. In recent years the amendments to the dissection procedure for freshly frozen brain tissue were significant. An average of 11 anatomical regions were blocked during fresh dissections in 1998 as compared to 49 regions in 2006. This assists with the retrieval of tissue for requests and reduces the freeze thaw cycle that is detrimental to the tissue integrity.
Over the past decade the TRC has provided tissue to 22 Australian and 25 International Institutions with 110 researchers accessing this facility. The TRC has to date supported 276 different tissue requests. There has been a continued growth in journal and conference publications, 160 peer reviewed journals and over 200 conference abstracts have now been presented. In the period of 1994/99 there were 26 peer reviewed journal publications.
Presently there are approximately 35 research projects supported by the TRC annually, which is equal to the total number of research projects supported in the first 5 years of the TRC. Over 70% of tissue requests utilise fresh frozen brain tissue dictated by the most popular research methodologies—genomics and proteomics. This is different to the 1994/99 period where 85% of tissue requests were for neuropathological studies (fixed tissues). The vast majority of researchers were satisfied with the integrity of provided samples and with the practices of the TRC.
According to researchers’ feedback, the information on medication type, alcohol consumption, illicit drug usage and smoking are of growing interest for research purposes. This information is now included in the data set together with the current clinical characterization, information on family history of illness and other medical and medication histories. Liver pathology is included in the data set for those studying the effects of alcohol on the brain.
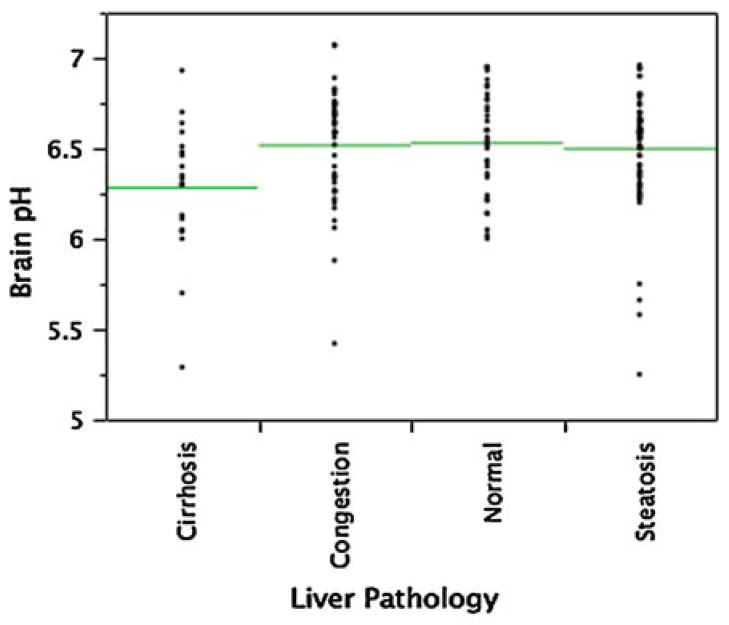
The influence of liver pathology on the brain pH has been assessed for all cases. The mean brain pH for the four liver pathology categories: normal, steatosis, congestion and cirrhosis were correlated independent of DSM-IV classification. One way ANOVA and a student’s T test was applied. Cases of cirrhosis showed significance to all other groups (normal 0.006, congestion 0.007, and steatosis 0.008). Figure 4 shows the distribution and means for each liver pathology group.
Fig. 4.
The mean brain pH (line) of each liver pathology classification
A review of the histological findings of the cerebellar granular cell layer in all cases enabled them to be allocated into normal, mild, moderate or severe groupings. These groups were then correlated to the brain pH and showed a positive correlation to brain tissue pH independent of DSM-IV classification. One way ANOVA followed by a student’s T test showed significance in the severe (P > 0.0001), moderate (P = 0.006) and mild (P = 0.009) group as compared to the normal. The mean brain pH for each group is shown below (Fig. 5).
Fig. 5.
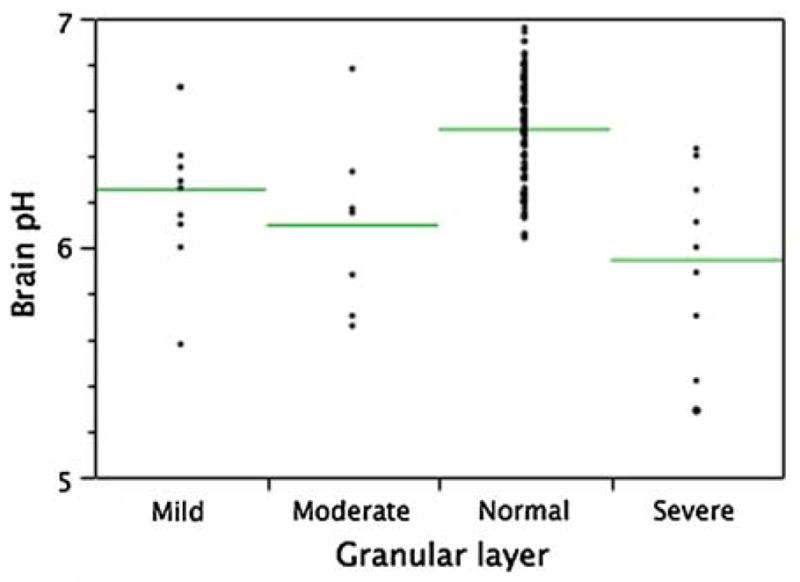
The mean brain pH (line) for the degree of cerebellar granule cell layer autolysis
The database system has developed from a single separate database to the current twelve inter-related system. The system handles over 3,000 records relating to the pre mortem donor programs and 1,000 records relating to the tissue based activities. The system now allows the selection of cases for tissue requests on an increased number of variables. Feedback from researchers has shown a requested increase in the amount of data available on each case and the requirement for data storage capacity.
Discussion
The adherence to regulations on establishing, maintaining and the use of human postmortem tissue is reflected in the management system of the TRC. These regulations have changed over time in response to social and cultural challenges and require the building of trust and acceptance from all stakeholders (Gottweis and Zatloukal 2007). Coupled with regulations and administrative requirements is the need to provide a data management platform that allows the selection of appropriate cohorts of cases for studies based on search criteria across epidemiological, clinical and pathological domains. Standard operational procedures allow the utilisation of the expertise in multiple research centres to analyse the same samples using various methodologies such as histology, morphometry, genomics, proteomics, and bioinformatics.
Recent discussions on the planning of biobanks identify that a multidisciplinary team with clinicians, pathologists, molecular biologists, statisticians, bioinformaticians and tissue resource managers is ideal (Riegman et al. 2007). In addition, as with our donor programs, other personnel are required including a donor coordinator who also acts as a community liaison person and a psychologist to perform the neuropsychiatric assessments for longitudinal documentation. This long-term investment in infrastructure and personnel is important for the success of brain banking and ensures a high standard of information on each case that improves translational research.
Donor programs are long-term enterprises that play a crucial role in supporting the TRC by providing additional cases that have a complete medical and lifestyle history and short post-mortem interval. UoB donors have participated in studies that have examined the main motivating factors that influenced them in their decision to become brain donors. These studies indicate that the most common reasons for being involved were; to benefit science, to benefit medicine and to benefit the community. These are similar to motivating factors that are reported in the transplant literature (Garrick et al. 2006).
Other research that has arisen from the UoB program is the establishment of demographically adjusted normative data for the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in an Australian context. In 1998 Randolph (Randolph 1998) devised this brief, highly portable test based on 540 healthy individuals from North America, who were between the ages of 20–89 years. It has the dual purpose of identifying and characterizing abnormal cognitive decline in the older adult and as a neuropsychological screening battery for younger patients (Randolph 1998). Australian donors performed above expectation on all RBANS summary scores relative to the normative group mean, with the largest difference found on language based tasks. Results were more variable for individual RBANS subtests. In general, Australian participants performed slightly better on all verbal memory tasks except the list recognition subtest. They also performed better for line orientation, picture naming, semantic fluency and digit span subtests. The largest difference between the two groups occurred on the semantic fluency test where Australian participants scored up to 8 points higher (Green et al. 2008).
The influence of recently developed research technologies demands the integrity of tissue, accurate and correct diagnostic classification of cases each of which must have a minimum data set including demographics and health and lifestyle data such as drinking and smoking habits (Ferrer et al. 2007; Schmitt et al. 2007; Stan et al. 2006; Webster 2006). The validity of postmortem human brain research relies upon accurate clinical and psychopathological diagnosis. Our recent findings in comparing the predominant ante-mortem psychiatric diagnosis to the corresponding post-mortem diagnosis of the TRC cases indicates a moderate to excellent inter-rater reliability (Sundqvist et al. 2008).
Review of all available medical and lifestyle histories and pre and post-mortem conditions contribute to the accuracy of case classification and to the value of data analysis (Webster 2006). For example, it has been shown that the clinical diagnosis for almost 80% of Wernicke Korsakoff Syndrome cases that are diagnosed at autopsy appear to be missed in major teaching hospitals (Harper et al. 1986; Thomson et al. 2008). Adherence to the diagnostic criteria as described above and collecting information on pre-mortem variables aids in the accuracy of diagnosis and allocation of tissue for research projects.
Medical conditions seen in cases that have long-term alcohol use disorders include cirrhosis of the liver, cardiovascular and pulmonary episodes and seizures. These conditions can have a significant impact on the metabolic energy cycle which effects brain structure and function and can modify brain pH. Patients with cirrhosis usually have a poor nutritional reserve due to anorexia, poor diet, malabsorption, gastrointestinal bleeding and altered metabolic state. The normal liver converts ammonia to urea in periportal hepatocytes, and to glutamine in perivenous hepatocytes, but if the liver is diseased ammonia may reach toxic levels within the portal vein. Neurotoxins like ammonia and manganese have been studied by Kaila and colleagues who have shown that these effect the homeostasis of brain pH (Kaila and Ransom 1998). Studies of our cases show that those with cirrhosis have the lowest brain pH. This might be explained by the metabolic dysregulation of pH during severe ammonia or manganese intoxication as the cases do not all have prolonged agonal states or long PMIs; the latter are the most common causes of low pH. Nevertheless, feedback from researchers who have used tissues from the cirrhotic cases has shown the RNA quality to be of good integrity (Flatscher-Bader et al. 2006; Guerrini et al. 2005; Okvist et al. 2007).
Review of the histopathological changes of the cerebellar granular cell layer is performed during the neuropathology reporting. As shown here, there is a correlation between the severe necrosis and lower mean brain tissue pH. Each case must meet the standard criteria of the neuropathology review to be suitable for the research collection. This measure helps in the assessment of the agonal state of cases. This has recently been discussed by Weiss and colleagues in assessing quality control for microarray analysis (Weis et al. 2007). The assessment of the cerebellar granule cell layer as a quality measure may assist banks that do not have facilities to perform the extraction and RNA Integrity Number (RIN). The TRC researchers using molecular techniques are asked to provide feedback and forward the results of tissue integrity for each assigned case and this information is entered into the database.
As previously mentioned, feedback from researchers and donors provides valuable information for changes in protocols and ultimately, the future management of the TRC. A review of researcher evaluations lets us know if other data is required and protocols and procedures are upgraded to include these where possible. This information, together with lay descriptions of outcome of projects, is useful in preparing reports for stakeholders. The progress of any tissue bank can be analysed statistically by reviewing outcomes such as manuscripts arising from the research, conferences presentations and higher degrees awarded to undergraduate and postgraduate students. This information is important when applying for funding and justifying budgets. A comprehensive information management system is therefore crucial to enable the progress of any tissue bank to be monitored. It is also essential for the application of future bioinformatic approaches that may arise.
The linkage with the National Brain Bank Network has been instrumental in the development of quality control analyses of procedures between the centres. A review is currently in progress and the outcomes will be reported in the near future.
Conclusion
Brain banks are an important resource that will ensure progress in neuroscience research. Studies on human tissue are particularly relevant for disorders that have inappropriate or no animal models. Compliance to ethical, pathological and clinical standards and an understanding of pre-mortem variables that affect the quality of the tissues enhance the value of research findings. Building and maintaining relationships with stakeholders is an important consideration for continued growth and support of these types of infrastructure research facilities.
Acknowledgments
On behalf of the current members of the TRC we would like to acknowledge the efforts of previous staff, DOFM staff and the kind support of the donor families. The NSW TRC is supported by The University of Sydney, Schizophrenia Research Institute (SRI), National Institutes on Alcohol Abuse and Alcoholism (NIAAA—grant no: R01AAA01272508), Sydney South Western Area Health Service (SSWAHS), National Health and Medical Research Council (NHMRC).
Abbreviations
- TRC
New South Wales Tissue Resource Centre
- DOFM
Department of Forensic Medicine
- NOK
Next-of-Kin
- UoB
Using our Brains Donor Program
- DIBS
Diagnostic Instrument for Brain Studies
- DIP
Diagnostic Instrument for Psychosis
- RBANS
Repeatable Battery for the Assessment of Neuropsychological Status
- WKS
Wernicke-Korsakoff’s Syndrome
Contributor Information
D. Sheedy, Email: donnas@med.usyd.edu.au, Discipline of Pathology, University of Sydney, Blackburn Building, D06, Sydney, NSW 2006, Australia
T. Garrick, Discipline of Pathology, University of Sydney, Blackburn Building, D06, Sydney, NSW 2006, Australia. Schizophrenia Research Institute of Australia, 384 Victoria Street, Darlinghurst, Sydney, NSW 2010, Australia
I. Dedova, Discipline of Pathology, University of Sydney, Blackburn Building, D06, Sydney, NSW 2006, Australia. Schizophrenia Research Institute of Australia, 384 Victoria Street, Darlinghurst, Sydney, NSW 2010, Australia
C. Hunt, Discipline of Pathology, University of Sydney, Blackburn Building, D06, Sydney, NSW 2006, Australia
R. Miller, Discipline of Pathology, University of Sydney, Blackburn Building, D06, Sydney, NSW 2006, Australia
N. Sundqvist, Schizophrenia Research Institute of Australia, 384 Victoria Street, Darlinghurst, Sydney, NSW 2010, Australia
C. Harper, Discipline of Pathology, University of Sydney, Blackburn Building, D06, Sydney, NSW 2006, Australia. Royal Prince Alfred Hospital, Sydney, NSW, Australia
References
- Albrechtsen R. The pathogenesis of acute selective necrosis of the granular layer of the human cerebellar cortex. Acta Neuropathol. 1977;37(1):31–34. doi: 10.1007/BF00684537. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Burton JL, Underwood JC. Necropsy practice after the “organ retention scandal”: requests, performance, and tissue retention. J Clin Pathol. 2003;56(7):537–541. doi: 10.1136/jcp. 56.7.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Santpere G, Arzberger T, Bell J, Blanco R, Boluda S, et al. Brain protein preservation largely depends on the postmortem storage temperature: implications for study of proteins in human neurologic diseases and management of brain banks: a BrainNet Europe Study. J Neuropathol Exp Neurol. 2007;66(1):35–46. doi: 10.1097/nen.0b013e31802c3e7d. [DOI] [PubMed] [Google Scholar]
- Flatscher-Bader T, Landis N, van der Brug M, Wilce PA. Comparative gene expression in brain regions of human alcoholics. Genes Brain Behav. 2006;5(Suppl 1):78–84. doi: 10.1111/j.1601-183X.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Garrick T, Howell S, Terwee P, Redenbach J, Blake H, Harper C. Brain donation for research: who donates and why? J Clin Neurosci. 2006;13(5):524–528. doi: 10.1016/j. jocn.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Gottweis H, Zatloukal K. Biobank governance: trends and perspectives. Pathobiology. 2007;74(4):206–211. doi: 10.1159/000104446. [DOI] [PubMed] [Google Scholar]
- Green A, Garrick T, Sheedy D, Blake H, Shores A, Harper C. Repeatable Battery for the Assessment of Neuro-psychological Status (RBANS): Preliminary Australian normative data. Aust J Psychol. 2008 doi: 10.1080/000495 30701656257. [DOI] [Google Scholar]
- Guerrini I, Thomson AD, Cook CC, McQuillin A, Sharma V, Kopelman M, Reynolds G, Jauhar P, Harper C, Gurling HM. Direct genomic PCR sequencing of the high affinity thiamine transporter (SLC19A2) gene identifies three genetic variants in Wernicke Korsakoff syndrome (WKS) Am J Med Genet B, Neuropsychiatr Genet. 2005;137(1):17–19. doi: 10.1002/ajmg.b.30194. [DOI] [PubMed] [Google Scholar]
- Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49:341–345. doi: 10.1136/jnnp.49.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Kril J, Daly J. Does a “moderate” alcohol intake damage the brain? J Neurol Neurosurg Psychiatry. 1988a;51(7):909–913. doi: 10.1136/jnnp.51.7.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Kril JJ, Daly JM. Brain shrinkage in alcoholics is not caused by changes in hydration: a pathological study. J Neurol Neurosurg Psychiatry. 1988b;51:124–127. doi: 10.1136/jnnp.51.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Heath PR, Eastwood SL, Burnet PW, McDonald B, Pearson RC. The relative importance of premortem acidosis and postmortem interval for human brain gene expression studies: selective mRNA vulnerability and comparison with their encoded proteins. Neurosci Lett. 1995;200(3):151–154. doi: 10.1016/0304-3940(95)12102-A. [DOI] [PubMed] [Google Scholar]
- Howard CV, Reed MG. Unbiased stereology: three-dimensional measurement in microscopy. Bios Scientific; Oxford: 2005. [Google Scholar]
- Jeganathan VS, Walker SR, Lawrence C. Resuscitating the autopsy in Australian hospitals. ANZ J Surg. 2006;76(4):205–207. doi: 10.1111/j.1445-2197.2006.03703.x. [DOI] [PubMed] [Google Scholar]
- Kaila K, Ransom B. pH and brain function. 1. John Wiley and Sons; New York: 1998. [Google Scholar]
- Keks NA, Hill C, Opeskin K, Copolov DL, Dean B. Psychiatric diagnosis after death: the problems of accurate diagnosis from case history review and relative interviews. In: Dean B, Kleinmann JE, Hyde TM, editors. Using CNS tissue in psychiatric research. Harwood Academic Publishers; Amsterdam: 1999. [Google Scholar]
- Kingsbury AE, Foster OJ, Nisbet AP, Cairns N, Bray L, Eve DJ, et al. Tissue pH as an indicator of mRNA preservation in human post-mortem brain. Brain Res Mol Brain Res. 1995;28(2):311–318. doi: 10.1016/0169-328X(94)00219-5. [DOI] [PubMed] [Google Scholar]
- Nemetz PN, Tanglos E, Sands LP, Fisher WP, Jr, Newman WP, III, Burton EC. Attitudes toward the autopsy – an 8-state survey. MedGenMed. 2006;8(3):80. [PMC free article] [PubMed] [Google Scholar]
- Ogata J, Yutani C, Imakita M, Ueda H, Waki R, Ogawa M, et al. Autolysis of the granular layer of the cerebellar cortex in brain death. Acta Neuropathol. 1986;70(1):75–78. doi: 10.1007/BF00689517. [DOI] [PubMed] [Google Scholar]
- Okvist A, Johansson S, Kuzmin A, Bazov I, Merino-Martinez R, Ponomarev I, et al. Neuroadaptations in human chronic alcoholics: dysregulation of the NF-kappaB system. PLoS ONE. 2007;2(9):e930. doi: 10.1371/journal.pone. 0000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C. Repeatable battery for the assessment of neuropsychological status manual. The Psychological Corporation; San Antonio, TX: 1998. [Google Scholar]
- Riegman PH, Dinjens WN, Oosterhuis JW. Biobanking for interdisciplinary clinical research. Pathobiology. 2007;74(4):239–244. doi: 10.1159/000104451. [DOI] [PubMed] [Google Scholar]
- Sarris M, Garrick TM, Sheedy D, Harper CG. Banking for the future: an Australian experience in brain banking. Pathology. 2002;34(3):225–229. doi: 10.1080/00313020220131260. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Bauer M, Heinsen H, Feiden W, Falkai P, Alafuzoff I, et al. How a neuropsychiatric brain bank should be run: a consensus paper of Brainnet Europe II. J Neural Transm. 2007;114(5):527–537. doi: 10.1007/s00702-006-0601-8. [DOI] [PubMed] [Google Scholar]
- Sinard JH. Factors affecting autopsy rates, autopsy request rates, and autopsy findings at a large academic medical center. Exp Mol Pathol. 2001;70(3):333–343. doi: 10.1006/exmp.2001.2371. [DOI] [PubMed] [Google Scholar]
- Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, et al. Human postmortem tissue: what quality markers matter? Brain Res. 2006;1123(1):1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist N, Garrick T, Bishop I, Harper C. Reliability of post-mortem psychiatric diagnosis for neuroscience research. Aust N Z J Psychiatry. 2008;42(3):221–227. doi: 10.1080/00048670701827242. [DOI] [PubMed] [Google Scholar]
- Thomson AD, Cook CC, Guerrini I, Sheedy D, Harper C, Marshall EJ. Wernicke’s encephalopathy: ‘Plus ça change, plus c’est la mème chose’. Alcohol Alcohol. 2008;43(2):180–186. doi: 10.1093/alcalc/agm149. [DOI] [PubMed] [Google Scholar]
- Ward HE, Clarke BE, Zimmerman PV, Cleary MI. The decline in hospital autopsy rates in 2001. Med J Aust. 2001;176(2):91. doi: 10.5694/j.1326-5377.2002.tb04307.x. [DOI] [PubMed] [Google Scholar]
- Webster MJ. Tissue preparation and banking. Prog Brain Res. 2006;158:3–14. doi: 10.1016/S0079-6123(06)58001-X. [DOI] [PubMed] [Google Scholar]
- Weis S, Llenos IC, Dulay JR, Elashoff M, Martinez-Murillo F, Miller CL. Quality control for microarray analysis of human brain samples: the impact of postmortem factors, RNA characteristics, and histopathology. J Neurosci Methods. 2007;165(2):198–209. doi: 10.1016/j.jneumeth.2007.06.001. [DOI] [PubMed] [Google Scholar]