Abstract
The hematogenous dissemination of cancer and development of distant metastases is the cause of nearly all cancer deaths. Detection of circulating tumor cells (CTCs) as a surrogate biomarker of metastases has gained increasing interest. There is accumulating evidence on development of novel technologies for CTC detection, their prognostic relevance and their use in therapeutic response monitoring. Many clinical trials in the early and metastatic cancer setting, particularly in breast cancer, are including CTCs in their translational research programs and as secondary end points. We summarize the progress of detection methods in the context of their clinical importance and speculate on the possibilities of wider implementation of CTCs as a diagnostic oncology tool, the likelihood that CTCs will be used as a useful biomarker, especially to monitor therapeutic response, and what may be expected from the future improvements in technologies.
Keywords: cancer stem cells, characterization, circulating tumor cells, CTC, detection, DTC, enrichment, epithelial mesenchymal transition, metastasis
Major progress has been achieved in the treatment of various cancers over the last decade. Nevertheless, metastatic cancer remains incurable. It is generally believed that distant metastases spread from the primary tumor through invasion into circulation and finally distant sites, where they may reinitiate growth, depending on the microenvironment. One of the surrogates of hematological spread are disseminated tumor cells (DTC) as detected in bone marrow aspirations by Redding et al. in one of the first studies of its kind, using epithelial membrane antigen to identify DTC in bone marrow at the time of primary surgery in women with no overt metastases [1], and later by our group using a combination of epithelial cell surface antigens and cytokeratins to identify DTC in early stage breast carcinoma [2,3]. The prognostic significance of the presence of DTC in bone marrow aspirates has been established for several cancers, with most evidence related to breast cancer [4]. Owing to the invasive procedure, limited value of prognostic information and, as recently shown, low number of positive patients [5], bone marrow aspirations are not routinely performed. Many attempts have been made to optimize the detection of circulating tumor cells (CTCs) from peripheral blood. Blood would be the ideal source because of the potential of serial cost–effectiveness and relative low invasiveness of the procedure. Since a blood draw is frequently performed in patients undergoing treatment, in the majority of cases only additional tubes need to be obtained from patients. Despite the progress in recent year, demonstrating not only the prognostic value, but also the use of CTCs in monitoring tumor responses to systemic therapy in breast and other cancers [6–8], the detection of CTCs is far from widely used in the clinical management of cancer patients. Here we review enrichment and detection techniques, and summarize the evidence on the clinical value of CTC detection and the possibility and impediments to more widespread use of CTC detection in the clinic. Finally, we speculate on the potential development of the field in the next few years.
Techniques for enrichment & detection of CTCs
The greatest challenge in detection of CTCs is their rarity in the blood. Human blood consists of white blood cells (5–10 × 106/ml), red blood cells (5–9 × 109/ml) and platelets (2.5–4 × 108/ml); very few CTCs will be present even in patients with known metastatic disease, often less than one CTC per ml of blood. Owing to their rarity, CTCs have to be enriched prior to their detection. A variety of techniques have been used for enrichment and detection of CTCs, and both we and others have described them in detail previously [9,10]. Here we provide only a brief overview.
Enrichment techniques
Techniques for enrichment of CTCs are based on biological and/or physical properties that distinguish CTCs from normal blood cells. Affinity-based enrichment is by far the most commonly employed strategy to separate CTCs from blood cells. Affinity techniques take advantage of distinctive antigens expressed either by CTCs but not blood cells (e.g., EpCAM), or by blood cells but not CTCs (e.g., CD45). Affinity methods are restricted to CTCs that show differential antigen expression, for example, the EpCAM-expressing epithelial tumor cells. Enrichment of CTCs is either based on positive selection of tumor cells by targeting the expression of epithelial markers (e.g., EpCAM), or on depletion of nontumor background, where the most typical example is depletion of lymphocytes that express CD45 [11]. EpCAM is expressed to various degrees by many epithelial cancers and is also known as HEA and BerEp4 [12,13]. However, EpCAM is not expressed by all epithelial cancers (e.g., renal cell cancer), and is heterogeneously expressed even by highly expressing tumors (e.g., breast cancer). EpCAM is not expressed by nonepithelial cancers, such as melanoma. This represents a major drawback of affinity-based methods. The most common strategy for affinity-based enrichment is immunomagnetic separation, which uses magnetic beads functionalized with antibodies that bind to CTCs (positive enrichment) or blood cells (negative enrichment). Immunomagnetic enrichment currently exists on the market in various formats including columns, beads and cartridges for automation of the process, and is the most well-developed enrichment technique. More recently, microchip-based affinity methods have been developed, which are described fully below.
An alternative and older approach is enrichment of cells by their buoyant density, a physical property of the cell. An example of such a method is Ficoll–Hypaque [14], which separates red blood cells from nucleated cells in the blood or bone marrow, including tumor cells. This method was used for most of the studies that demonstrated the clinical significance of detecting DTC in the bone marrow of early stage breast cancer patients. However, recovery of tumor cells by this method is low, and enrichment is poor, thus the need for better enrichment methods became clear.
Another property of tumor cells that is useful for enrichment is their size [15]. Tumor cells, particularly those derived from solid tumors, are larger than most cells present in blood. While these differences are small, they are consistent. An advantage of this approach is that a broader range of tumors are potentially amenable to size-based separation, and heterogeneity of antigen expression is not an impediment. The idea of using size for filtering CTCs from peripheral blood is an old one [16], however, it was not pursued until recently. Several techniques based on size-based separation of tumor cells are now either commercially available or under development [17,18]. We have developed a size-based microfilter for enrichment and detection of CTCs [19], which appears to be highly efficient and much faster compared with affinity-based separation techniques. Furthermore, our platform can be used for detailed molecular characterization [20], which is described more fully below.
In recent meetings on CTCs, many novel technologies have been presented with capability of single cell detection, molecular profiling and definition of the biological potential. Many of these systems currently lack clinical data, therefore, their clinical utility is yet to be determined. Most systems rely on affinity separation. After a brief summary of existing CTC detection methods, the most promising novel technologies with clinical evidence will be briefly discussed in the next section.
Detection methods
Although CTC enrichment methods increase the ratio of target (CTC) to background cells, no method results in a pure population of tumor cells. Thus, all separation techniques require a method to distinguish CTCs from the nonspecifically captured cells. This is generally performed by cytomorphological characterization of CTCs, immunocytochemical detection of tumor-specific antigens or various real-time PCR (RT-PCR) approaches. Cytomorphological characterization relies on classification of tumor cells based on their distinct morphological features [18]. Immunocytochemical detection of CTCs employs antibodies specific for epithelial cells; the most commonly used antibodies are those to a wide range of cytokeratins (which are broadly expressed by epithelial tumor cells), including both low- and high-molecular weight cytokeratins [21]. Figure 1 demonstrates detection of a CTC based on immunofluorescent detection of cytokeratins. Multimarker approaches have been developed, enabling simultaneous visualization of multiple markers on a single cell [22,23]. Detection of CTCs by immunocytochemistry has one major drawback: the potential to miss cells not expressing the intended target antigens. As recently shown, this may occur due to epithelial–mesenchymal transition (EMT) and expression of mesenchymal markers by epithelial CTCs [24]. This issue also represents a potential major limitation both for affinity-based enrichment and detection of CTCs.
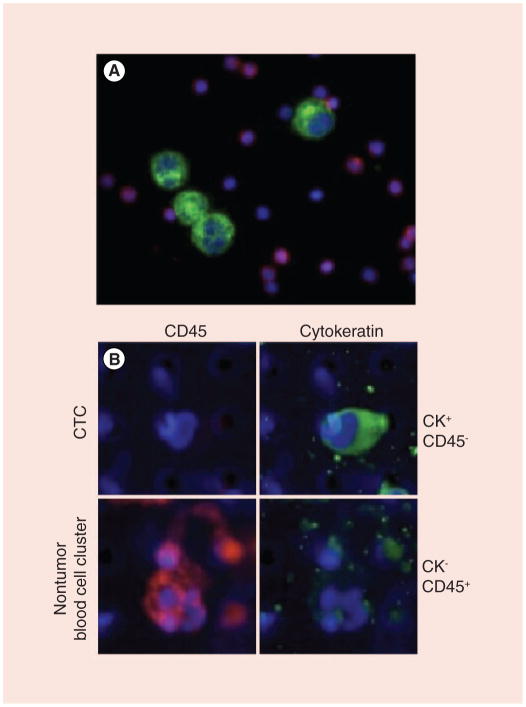
Figure 1. Circulating tumor cells are identified on the membrane microfilter device using immunofluorescence and morphologic criteria consistent with a tumor cell.
Rabbit polyclonal antihuman pan-cytokeratin conjugated with goat antirabbit Alexa Fluor 594 fluorescent secondary antibody (pseudo-colored green) and mouse monoclonal antihuman CD45 conjugated with goat antimouse Alexa Fluor 488 fluorescent secondary antibody (pseudo-colored red) are used for the positive selection of CTCs and negative selection of nontumor blood cells, respectively. Samples are labeled with 4′,6-diamidino-2-phenylindole (pseudo-colored blue) for nuclear identification. (A) positive control where a cytospin slide was prepared where T24 bladder cancer cells and peripheral blood mononuclear cells from a normal healthy donor were combined and stained as described above. (B, top row) A CTC from an advanced colorectal cancer patient is identified as CK+/CD45− by the microfilter device; (B, bottom row) nontumor blood cells captured by the microfilter device in the same patient are identified as CK−/CD45+. Note the differences in size between tumor cells in the model system and CTCs, compared with peripheral blood mononuclear cells and nontumor blood cells. CTC: Circulating tumor cell.
Several RT-PCR protocols have been developed, striving for quantification and better sensitivity [25–28]. However, it has been previously demonstrated that the detection of CTCs by RT-PCR-based methods has limited specificity, as many of the transcripts used to identify CTCs have basal expression levels in nontumor blood cells [29]. In addition, the detection of CTCs by RT-PCR requires the preparation of lysates to recover total RNA, eliminating the ability to perform morphologic analysis on cells of interest.
All these methods are time consuming and cost intensive. Recent progress in technology focuses either on lowering the costs or facilitating molecular characterization of CTCs, or ideally both.
CellSearch®
The unique position of the CellSearch® System by Veridex/Johnson and Johnson (NJ, USA) as the only US FDA-approved method for detection of CTCs in breast, colon and prostate cancer is the reason why this method is separately described. None of the other methods has yet undergone such careful preclinical and clinical validation. The CellSearch method has been shown in multiple studies to identify patients with metastatic cancer who will have a shorter survival, and more importantly, can identify patients that are responding, or not responding, to systemic therapy, often after the first treatment, based on the number of tumor cells identified in 7.5 ml of blood. The FDA approval for CellSearch is for prognostic evaluation and therapeutic-response monitoring in patients with metastatic breast, prostate or colon cancer [6,30–35]. CellSearch is a fully automated system for detection of CTCs and has been shown to be reproducible across different independent testing sites. Briefly, the immunomagnetic enrichment is based on positive selection using EpCAM-labeled iron oxide nanoparticles, and subsequent detection of cytokeratin-positive CTCs. CellSearch adds an additional step to identifing cells that express CD45: cells that express cytokeratins, but lack the expression of CD45, and that have the cytomorphologic characteristics of tumor cells (appropriate size, presence of a nucleus and appropriate nuclear to cytoplasmic ratio), are counted as tumor cells. After enrichment and labeling, positive events are sorted, and are reviewed by trained personnel for the above characteristics. A 7.5-ml sample of blood is thus evaluated for the presence, and number, of CTCs. The system possesses the capability of additional detection channels for HER2/neu; however, the ability to interrogate the CTCs is limited. In a study comparing different enrichment methods we demonstrated that CellSearch outperformed three other methods: another immunomagnetic positive enrichment method, depletion of CD45-positive cells (negative enrichment); and density-based enrichment by OncoQuick® [36]. A series of clinical studies evaluating the clinical significance of the presence of CTCs were performed employing CellSearch for the detection of CTCs and will be discussed more fully below.
Novel microchip platforms for enrichment & detection of CTCs
Affinity-based microchips
Several microchip-based CTC-capture platforms, which capture cells based on antigen expression, have been described [37]. One of the first reported microchips was an affinity-based microfluidic chip developed for CTC enrichment by creating an array of microposts coated with EpCAM antibodies [38,39]. The reported capture efficiency of the device was >60%. When the microchip was used in clinical samples, CTCs could be isolated from each blood sample in all patients tested, including patients with meta-static lung, prostate, pancreatic, breast and colon cancers. With this method, monitoring of CTCs was demonstrated for meta-static non-small-cell lung cancer patients, with the correlation of CTC count with tumor response [40]. This chip was also capable of capturing tumor cells from which DNA could be extracted for EGF-receptor mutational analysis [36]. In addition to the caveats described for affinity-based methods, these chips need to have a very slow flow of blood, often taking more than 10 h to capture tumor cells in a single 7.5 ml sample of blood.
Size-based microchips
The size of tumor cells has been increasingly used as a property for their enrichment. Several platforms using size as the capture method have been described [15]. Our group has developed a simple size-based filter for isolation of CTCs with the potential for integrated downstream RNA, DNA and multimarker protein characterization. The ability to fabricate high-density pores enabled us to enhance the enrichment factor and recovery rate. In the initial studies we showed recovery rates of >90% for capturing cultured cancer cells spiked in peripheral blood. The filtration of a 7.5 ml blood sample required less than 3 min for processing. In a comparison with CellSearch we were able to detect CTCs in 51 out of 57 patients with metastatic prostate, colon, breast or bladder cancer using the microdevice, compared with only 26 patients with the CellSearch method. Furthermore, on average, 5.5-times more CTCs were recovered by the microfilter device than by CellSearch [19]. Recently, a report on a comparison of isolation by size of epithelial tumor cell assay with CellSearch was published [41]. Again, discordant results were obtained by both methods, indicating the limitations of EpCAM-based enrichment. More recently, the ScreenCell® device (Paris, France) has been developed, employing similar principles as the isolation by size of epithelial tumor cell platform, where CTCs are enriched in a size-based fashion. Although commercially available, no significant clinical data has yet been reported using this technology. A major difference between the filter technology developed by our group compared with virtually all other size-based separation platforms is the density and regularity of the pores. Most filters are track-etched and produced by ionizing radiation. This results in an irregular pore distribution, with a low density of pores and significant overlap of pores resulting in holes large enough to allow CTCs to pass through. By contrast, our filter uses advanced lithography techniques, resulting in a highly regular and dense pore pattern and the ability to precisely vary the size and shape of the pores, an advantage not shared by track-etched filters. In addition, we have recently described an alternative design of our filters, which allows for the capture of live cells [42]. Live cell capture may become increasingly important as understanding the functional status of CTCs becomes critical in discovery and management of cancer.
Alternative microchip methods
Another microfluidic device was developed based on deterministic hydrodynamic flow and size-based separation [43–45]. This device is composed of a micropost array inside a microfluidic chamber. The diameter of the circular micropost, the distance between the micropost in individual rows and the row-to-row shift determine the performance of this microdevice. It can be used for separation of: plasma from blood cells; different types of blood cells from each other; and DNA fragments of different size. The limiting factor is the time needed to process higher volumes of blood sample.
Finally, dielectrophoresis is being used for separation of target cells. Dielectrophoretic forces are applied through the micro-electrode arrays onto the field. Cells with similar properties find their position at a specific distance from electrodes [46,47]. Cells of different types are separated, without interfering with their viability, according to their dielectric and hydrodynamic flow properties [48,49]. This technique has demonstrated >90% separation efficiency when defined numbers of cultured cells were spiked in peripheral blood [50]. One limitation of this technique is the requirement of a small sample volume. Furthermore, for dielectrophoretic forces to work properly, the blood samples need to be suspended in isotonic working medium with specified low conductivity. Recent papers published utilizing dielectrophoresis for CTC isolation either dilute the blood sample (10×) with working medium or resuspend blood cells in working medium, which results in a high processing volume (70 ml) with a slow processing rate (0.5 ml/h) [49] or significant loss of CTCs with the added density gradient step [47,50], respectively.
Characterization of CTCs
Taking into account that early dissemination is the basis of adjuvant treatment, targeted therapies should ideally be based on characteristics identified on CTCs. Based on the heterogeneity of methods for enrichment and detection/characterization of the CTC, it is obvious that different methods may potentially identify heterogenous cells and have different abilities to characterize CTCs. Here, we address a few clinically important aspects of CTC characterization.
There is accumulating evidence that solid tumors consist of heterogeneous cell populations, and only subpopulations of cells, termed cancer stem cells or cancer-initiating cells, have tumorigenic properties [51]. These cells have been identified in many hematological malignancies and solid tumors. For breast cancer, the putative breast cancer stem cell phenotype was identified as CD44+CD24−/low [52] and independently associated with aldehyde dehydrogenase activity [53]. The ability of cancer stem cells to undergo self-renewal and thus retain the pool of cancer stem cells, is a major challenge for current adjuvant and palliative treatment strategies.
In a retrospective analysis of disseminated tumor cells detected in bone marrow aspirates of Stage 1 and 2 breast cancer patients, we have shown that the putative breast cancer stem cell phenotype of disseminated tumor cells is common and the majority of detected cells had this phenotype [54]. Similar studies on CTCs in metastatic breast cancer patients followed [55–57].
Although performed in metastatic breast cancer patients and employing different methods and markers, these studies have all confirmed the presence of stem cell markers in CTCs. With novel studies it has become increasingly important to refine the technologies used for detection and characterization, taking into account the heterogeneity of the disease [58].
Advances in molecular technology are now enabling molecular characterization at single cell level [59,60]. There are preliminary results on DNA [60,61] and RNA profiling of CTCs [62]. Single cell evaluation is now possible and will simplify direct target identification.
For example, studies have revealed that HER2/neu status of primary tumor is not always concordant with the HER2/neu status of CTCs [63]. In fact, up to 30% of discordance was demonstrated between primary tumors and CTCs [63,64]. This fact has lead to the first clinical studies randomizing patients with HER2/neu-negative tumors and HER2/neu-positive CTCs to treatment with trastuzumab controlled to no HER2/neu-targeted treatment. Evidence for the biological instability of CTCs with respect to recognized biomarkers of breast cancer (e.g., estrogen and progesterone receptors and HER2/neu status) is accumulating [65,66], and the need for a ‘real-time’ biopsy to better optimize the treatment of breast cancer patients with progressive disease is becoming increasingly apparent. Thus, the role of CTC detection and characterization may become increasingly relevant in order to manage patients being treated in the adjuvant setting or for metastatic disease. CTC capture and characterization may offer a true opportunity for performing repeated ‘liquid biopsies’ in patients undergoing treatment, and thus monitor not only tumor number, but important aspects of tumor biology (such as the cancer stem cell phenotype) and changes in therapeutic targets over the course of treatment. A relevant example, mentioned previously, is the ability to determine the EGF-receptor mutation status of CTCs in patients with non-small-cell lung cancer [38]. This approach may offer both the possibility for selection of patients who are suitable for treatment with a specific drug and monitoring efficacy during the course of treatment. Another recent study has demonstrated the likelihood of detection of discordant ER and HER2/neu status between primary tumor or metastatic tumor sites and CTCs in metastatic breast cancer. Although preliminary, these data are intriguing and needs further clinical validation [67]. Also, KRAS mutation status was successfully derived from CTCs [68,69]. These are only a few examples with concrete data suggesting that the molecular characterization of CTCs, in addition to the enumeration of CTCs, can contribute to improvements in cancer patient management, which will be discussed in detail in the following section.
Clinical evidence on the prognostic & predictive significance of CTCs
The prognostic significance of the presence of CTCs in peripheral blood has been established for several types of cancers either by the use of immunohistochemistry [6,7,32,33] or by a variety of RT-PCR methods [70,71]. The cost–effectiveness of the prognostic and predictive significance of CTCs in several cancers have led to the US FDA approval of the CellSearch method. Table 1 provides a summary of the studies that have demonstrated the prognostic and predictive significance of CTCs in various cancer types.
Table 1.
Summary of clinical studies that have demonstrated prognostic significance of circulating tumor cells.
| Studies performed (year) | Method (cutoff value) | Patients (n) | Median overall survival CTC+ (months) | Median overall survival CTC− (months) | n patients with CTCs > cutoff (%) | n patients with CTCs < cutoff (%) | p-value | Ref. |
|---|---|---|---|---|---|---|---|---|
| Breast cancer | ||||||||
| Cristofanilli et al. (2004) | CellSearch (5 CTCs) | 177 | 10.1 | 18 | 88 (49) | 89 (51) | p = <0.001 | [6] |
| Nole et al. (2008) | CellSearch (5 CTCs) | 80 | NA | NA | 49 (61) | 31 (39) | p = 0.014 | [72] |
| Gaforio et al. (2003) | MACS + enrichment | 90 | NA | NA | 56 (62) | 34 (38) | p = 0.003 | [77] |
| Bauernhofer et al. (2005) | MACS + enrichment | 32 | 4 | 13 | 8 (25) | 24 (75) | p = <0.001 | [7] |
| Ignatiadis et al. (2008) | Real-time PCR (1 Marker+) | 175 | NA | NA | 77 (44) | 98 (56) | p = 0.044 | [28] |
| Colorectal cancer | ||||||||
| Cohen et al. (2008) | CellSearch (3 CTCs) | 413 | 9.4 | 18.5 | 107 (26) | 306 (74) | p = <0.0001 | [33] |
| Sato et al. (2011) | PCR based | 42 | NA | NA | 27 (64) | 15 (36) | p = 0.03 | [78] |
| Iinuma et al. (2011) | Real time PCR (3 Marker+) | 735 | NA | NA | 181 (25) | 554 (75) | p = 0.001 | [70] |
| Prostate cancer | ||||||||
| De Bono et al. (2008) | CellSearch (5 CTCs) | 231 | 11.5 | 21.7 | 132 (57) | 99 (43) | p = <0.0001 | [73] |
| Scher et al. (2009) | CellSearch (5 CTCs) | 156 | NA | NA | 85 (54) | 71 (46) | p = <0.0001 | [34] |
| Lung cancer | ||||||||
| Krebs et al. (2011) | CellSearch (5 CTCs) | 60 | 4.3 | 8.1 | 15 (25) | 45 (75) | p = <0.001 | [74] |
CTC: Circulating tumor cell; MACS: Magnetic-activated cell sorting column; NA: Not available.
CellSearch & prognostic/predictive significance of CTCs
In a landmark study performed by Cristofanilli et al., the prognostic significance of enumerating CTCs using a cutoff level of 5 cells in 7.5 ml of blood from metastatic breast cancer patients was demonstrated [6]. Furthermore, the predictive significance of changes in CTC levels during treatment was also established. In a follow-up study, Hayes and Cristofanilli, with colleagues, have shown that CTCs detected at each follow-up visit during the treatment of metastatic breast cancer were measures predictive for outcome and response, or lack of response, to therapy [31]. The prognostic significance of CTCs detected by CellSearch was validated in an independent study performed by Nole et al. in 80 metastatic breast cancer patients [72]. The authors also used a threshold of five cells [72]. Analogous studies for other tumor types followed, such as prostate and colorectal cancer, allowing for expansion of FDA approval of the method. For colorectal cancer patients a threshold of three cells was used for determination of prognosis [33]. Over 400 patients were evaluated and 26% of patients had more than three CTCs/7.5 ml blood at baseline. Their detection was associated with unfavorable outcome. In castration-resistant prostate cancer the threshold of five CTCs and categorization of patients in groups with favorable versus unfavorable prognosis distinguished between two groups with more than 10 months difference in overall survival [73]. Further studies have confirmed the prognostic relevance of CTCs in castration-resistant prostate cancer [32,34,73]. Finally, Krebs et al. analyzed peripheral blood of 101 Stage 3 and 4 non-small-cell lung cancer patients [74]. Generally, higher numbers of CTCs were associated with higher stage of disease, with levels up to 146 cells/7.5 ml blood in Stage 4 patients. With the threshold of five cells, both progression free and overall survival were significantly favorable for patients with CTC counts below the threshold [74]. Based mostly on these studies, it was suggested that serial determination of CTCs should be performed in addition to conventional imaging in order to provide the most information on prognosis of the patient and their response to therapy. In a comparison of CTCs detection and imaging for prediction of outcome in metastatic breast cancer, Budd et al. have indicated that CTCs may provide pertinent data much earlier [75]. Indeed, the predictive value of CTC counts often become clear after the first round of therapy [31] and may be helpful in determining the modality of the first-line treatment [76]. Based on a greater inter-reader variability for evaluation of computed tomography than CTC counts, the authors concluded that determination of CTCs is also a more reproducible way for predicting the outcome [75]. The potential to use CTC evaluation to monitor therapy will be discussed further below.
Confirming evidence of prognostic relevance of CTCs by alternative methods
The prognostic significance of CTCs in the peripheral blood of cancer patients was demonstrated not only with the CellSearch method but also with other methods. As early as 2003, Gaforio et al. demonstrated that 62% (56 out of 90) of a mixed population of untreated breast cancer patients (both early stage and metastatic breast cancer patients) had detectable CTCs in peripheral blood, and their presence was associated with shorter overall survival [77]. All patients where no CTCs were detected were still alive at the end of the study, whereas 11 patients with CTCs in their blood died. Gaforio et al. have used the Carcinoma Cell Enrichment and Detection Kit by Miltenyi Biotec (Bergisch Gladbach, Germany) and separation on a magnetic-activated cell-sorting column. In a similar analysis, we have demonstrated prognostic significance of CTCs in 32 metastatic breast cancer patients. Detection of CTCs was associated with significantly shorter overall survival (4 vs 13 months; p > 0.001) [7]. In patients with colorectal cancer, Sato et al. evaluated a PCR-based technique for detection of CTCs, and compared it with CellSearch [78]. The sensitivity and the prognostic information were comparable with the results generated with CellSearch.
In early stage breast cancer, few studies have shown the prognostic impact of detection of CTCs. Stathopholou et al. demonstrated that detection of CK-19 by RT-PCR in the peripheral blood of patients with Stages 1 and 2 breast cancer before initiation of adjuvant therapy had independent prognostic value [79]. Using RT and nested PCR, Ignatiadis et al. have detected CTCs in 41% of breast cancer patients before the start of adjuvant treatment and their presence was associated with shorter disease-free and overall survival [28]. Molloy et al. defined detection of CTCs as a predictor of shorter relapse-free survival [80] and also breast cancer-specific survival in early stage breast cancer [81]. Iinuma et al. demonstrated the utility of RT-PCR for detection of CTCs in early stage colon cancer based on CEA, CK and CD133, a stem cell marker of colon cancer, and were able to show the prognostic impact of their detection [70]. Matsutsaka et al. have evaluated the significance of CTC detection and their utilisation for monitoring of therapeutic response in a Japanese population of colorectal cancer patients and have come to similar conclusions, both regarding the prognostic significance and the clinical utility for response monitoring [82]. Papavasiliou et al. analyzed the presence of CTCs preoperatively and during follow-up in patients with colorectal cancer and liver metastases. They indicated an association of CTCs with decreased overall survival [83]. In a meta-analysis on more than 3000 colorectal cancer patients Rahbari et al. confirmed the prognostic significance of CTCs [84].
Helo et al. compared the utility of CTC detection in localized and metastatic prostate cancer by RT-PCR for kalikrein-related peptidase 3, prostate-specific antigen and kalikrein-related peptidase 2. In a comparison with CellSearch, detection of CTCs was highly concordant with both methods and their detection was associated with shorter overall survival [85].
All these methods have one common characteristic: evaluation for signals of epithelial and tumor-specific markers in peripheral blood where these are usually not detected in a healthy population. In recent years, it has become evident that EMT plays an important role in pathogenesis of cancer, stemness and metastasis. As cells undergoing EMT may lose differentiation markers, such as epithelial specific antigens, this may hold challenges for detecting CTCs using these markers and may be in part responsible for lack of detection of CTCs in certain instances [86,87]. Several studies have examined the expression of EMT markers in peripheral blood [24,88]. The investigation of EMT, and the role of EMT in the CTC population will definitely become an important component of further research.
Expert commentary: implications of CTC detection for cancer management
As is clear from the above discussion, there is growing evidence that the detection of CTCs has clinical utility in prognostic assessment and, in particular, in monitoring efficacy of therapy in patients with cancer. Indeed, there has been increasing use of the FDA-approved CellSearch technology to assess therapeutic response in patients with metastatic breast, prostate and colon cancer. There is increasing acceptance by insurers to pay for the test, and there are efforts to create reimbursement codes specifically for CTC detection. Furthermore, the detection of CTCs is being used in clinical trials assessing new therapies. However, detection of CTCs has not been widely adopted for the management of patients. There are several possible reasons for the lack of general use and acceptance of CTC detection technologies. First, there is great variability in the currently available technologies to detect CTCs; a great deal of preanalytical validation is required and few technologies have achieved the degree of validation and quality control as the FDA-approved CellSearch. Furthermore, affinity-based enrichment methods have the drawbacks indicated above and approximately 50% of patients with metastatic cancers that are the targets of the technology do not have detectable CTCs in their blood samples. Importantly, the use of the CTC technology would require a change from the usual practice of oncologists, that is, to deliver a treatment through to completion as indicated in the protocol and then to assess response. A major question has been, if the CTC technology indicates lack of response, what should the clinician do? In the case of metastatic breast cancer, evidence that second-line treatment is effective is sparse; however, the majority of patients who fail first-line therapy go on to second-line therapy (and beyond) [89]. While it is possible that changing therapy earlier may be of benefit, there is currently little evidence of this, as studies in support on this issue have only recently begun. Current standard clinical guidelines have not yet included CTC evaluation in the management of patients with cancer [90,91]. Currently, the SWOG 0500 clinical trial is investigating the benefit of early changes in therapy based on CTC testing by CellSearch. If this approach proves useful, then it may become a unique opportunity to evaluate the response at an early time point. In this setting, detection of CTCs may become clinically useful for early decision making in patient treatment; this study may indeed change the clinical practice. However, a major issue will be how to interpret the results if the study fails; will this invalidate the use of CTCs to manage patients, or will it be the failure of the therapeutic approach used?
One aspect of CTC evaluation that may find early traction in patient management is the use of CTCs as a virtual biopsy. CTCs can be used to assess therapeutic targets, including ER, PR, HER2, EGF receptor, and others. The ability to assess targets without an invasive biopsy may hold particular advantages. Furthermore, if CTCs are actually representative of the population of cancer cells capable of forming metastatic deposits, evaluation of CTCs may be particularly desirable.
The cost of CTC evaluation is another drawback. However, these costs are minimal compared with the cost of even the least expensive therapies. Avoiding even a fraction of failed therapies sooner would not only save on the costs of the drugs, but also the expense of managing the inevitable side effects that result from treatment with anticancer therapies. If CTC evaluation were to be used to determine effective therapies, there is little doubt that it would be highly cost effective. Clearly, the potential to influence clinical decisions based on the information gained with CTC detection and thus possibly save the costs of further nonbeneficial treatment, and deliver beneficial therapy earlier in the course of disease, would facilitate its wider implementation in the clinic. There continue to be numerous clinical studies involving CTCs as an end point currently being performed, which will provide further evidence on the clinical relevance of CTCs. The numerous technologies for CTC enrichment and assessment that continue to be developed and evaluated further attest to the robust interest and belief that CTC detection and assessment is a clinically relevant tool.
Five-year view: future development of CTC clinical utility
Currently, there is great interest to develop new methods for CTC enrichment and evaluation, and the next few years will see the emergence and investigation of several novel platforms. These platforms will need to be validated and compared, in particular, to the current standard FDA-approved CellSearch technology. The relative merits of these new technologies will largely influence their potential adoption in clinical trials. If, with the novel technologies, sensitivity increases further, new thresholds will have to be defined in order to determine the level of impact CTCs have on the prognosis of patients.
In the next few years the focus of research will be more on molecular characterization of CTCs and definition of biomarkers for therapeutic strategies. Technical progress promises a very exciting era of new biologic insights into the process of metastasis. Better understanding of the biology of cancer and potential of cells or a subpopulation of cells to undergo EMT, and other transformations from cancer stem cells to differentiated cells, and the likelihood to dedifferentiate again are some of the interesting current hypothesis that will be further studied and validated. Novel technologies will increasingly enable evaluation of these processes at a single cell level. This may lead to identification of novel therapeutic targets and more powerful treatment strategies for cancer patients with various cancer types and provide even more compelling reasons to study CTCs.
Key issues.
Circulating tumor cells (CTCs) have been shown to be of prognostic significance in patients with various cancers.
The longitudinal determination of CTC counts have predictive value for evaluating therapeutic response.
The CellSearch method is the only US FDA-approved method for detection of CTCs as an aid for monitoring therapeutic response in cancer patients.
Despite a growing body of supporting evidence and approval by the FDA, the detection and molecular characterization of CTCs to aid in prognosis and monitoring of therapeutic response has not yet reached widespread clinical use.
Novel technologies, which are currently in development, may facilitate molecular characterization of CTCs at a single cell level.
Characterization of CTCs for clinically validated biomarkers, such as ER, PR, HER2/neu in breast cancer, KRAS mutation status in colorectal cancer patients and EGF-receptor status in non-small-cell lung cancer patients may prove clinically useful.
SWOG 0500 has a potential to change the clinical utility of CTC evaluation.
CTCs and their characterization with novel techniques is one of the most promising research areas today, holding enormous potential for changing the way we deliver and evaluate therapy to patients with cancer.
Footnotes
Financial & competing interests disclosure
RJ Cote, RH Datar and H Lin are among the inventors of the patented parylene microfilter technology, which has been licensed to a company Filtini Inc. (CA, USA). As inventors, they stand to gain from royalties arising out of commercialization of the technology. Also, RJ Cote owns stocks in Filtini Inc., while RH Datar serves as a scientific advisor to Filtini Inc. and has stock options. This work was supported by NCI grant CA-123027 (RJC). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
For reprint orders, please contact reprints@expert-reviews.com
References
- 1.Redding WH, Monaghan P, Imrie SF. Detection of micrometastases in patients with primary breast cancer. Lancet. 1983;322(8362):1271–1274. doi: 10.1016/s0140-6736(83)91150-9. [DOI] [PubMed] [Google Scholar]
- 2.Cote RJ, Rosen PP, Lesser ML, Old LJ, Osborne MP. Prediction of early relapse in patients with operable breast cancer by detection of occult bone marrow micrometastases. J Clin Oncol. 1991;9(10):1749–1756. doi: 10.1200/JCO.1991.9.10.1749. [DOI] [PubMed] [Google Scholar]
- 3.Balic M. Disseminated tumor cells as biomarkers for breast cancer. Biomark Med. 2009;3(3):215–217. doi: 10.2217/bmm.09.17. [DOI] [PubMed] [Google Scholar]
- 4.Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353(8):793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 5.Giuliano AE, Hawes D, Ballman KV, et al. Association of occult metastases in sentinel lymph nodes and bone marrow with survival among women with early-stage invasive breast cancer. JAMA. 2011;306(4):385–393. doi: 10.1001/jama.2011.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 7.Bauernhofer T, Zenahlik S, Hofmann G, et al. Association of disease progression and poor overall survival with detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer. Oncol Rep. 2005;13(2):179–184. [PubMed] [Google Scholar]
- 8.Miller MC, Doyle GV, Terstappen LW. Significance of circulating tumor cells detected by the cellsearch system in patients with metastatic breast colorectal and prostate cancer. J Oncol. 2010:617421. doi: 10.1155/2010/617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin H, Balic M, Zheng S, Datar R, Cote RJ. Disseminated and circulating tumor cells: role in effective cancer management. Crit Rev Oncol Hematol. 2011;77(1):1–11. doi: 10.1016/j.critrevonc.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Lianidou ES, Markou A. Circulating tumor cells in breast cancer: detection systems, molecular characterization, and future challenges. Clin Chem. 2011;57(9):1242–1255. doi: 10.1373/clinchem.2011.165068. [DOI] [PubMed] [Google Scholar]
- 11.Iinuma H, Okinaga K, Adachi M, et al. Detection of tumor cells in blood using CD45 magnetic cell separation followed by nested mutant allele-specific amplification of p53 and K-ras genes in patients with colorectal cancer. Int J Cancer. 2000;89(4):337–344. doi: 10.1002/1097-0215(20000720)89:4<337::aid-ijc4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 12.Trzpis M, Mclaughlin PM, De Leij LM, Harmsen MC. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol. 2007;171(2):386–395. doi: 10.2353/ajpath.2007.070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Went PT, Lugli A, Meier S, et al. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004;35(1):122–128. doi: 10.1016/j.humpath.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg R, Gertler R, Friederichs J, et al. Comparison of two density gradient centrifugation systems for the enrichment of disseminated tumor cells in blood. Cytometry. 2002;49(4):150–158. doi: 10.1002/cyto.10161. [DOI] [PubMed] [Google Scholar]
- 15.Vona G, Sabile A, Louha M, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol. 2000;156(1):57–63. doi: 10.1016/S0002-9440(10)64706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleischer RL. Cancer filter deja vu. Science. 2007;318(5858):1864. doi: 10.1126/science.318.5858.1864b. [DOI] [PubMed] [Google Scholar]
- 17.Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett. 2007;253(2):180–204. doi: 10.1016/j.canlet.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Vona G, Estepa L, Beroud C, et al. Impact of cytomorphological detection of circulating tumor cells in patients with liver cancer. Hepatology. 2004;39(3):792–797. doi: 10.1002/hep.20091. [DOI] [PubMed] [Google Scholar]
- 19.Lin HK, Zheng S, Williams AJ, et al. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin Cancer Res. 2010;16(20):5011–5018. doi: 10.1158/1078-0432.CCR-10-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng S, Lin H, Liu JQ, et al. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J Chromatogr A. 2007;1162(2):154–161. doi: 10.1016/j.chroma.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 21.Cote RJ, Beattie EJ, Chaiwun B, et al. Detection of occult bone marrow micrometastases in patients with operable lung carcinoma. Ann Surg. 1995;222(4):415–423. doi: 10.1097/00000658-199522240-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao X, Yang L, Petros JA, Marshall FF, Simons JW, Nie S. In vivo molecular and cellular imaging with quantum dots. Curr Opin Biotechnol. 2005;16(1):63–72. doi: 10.1016/j.copbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Balic M, Rapp N, Stanzer S, et al. Novel immunofluorescence protocol for multimarker assessment of putative disseminating breast cancer stem cells. Appl Immunohistochem Mol Morphol. 2011;19(1):33–40. doi: 10.1097/PAI.0b013e3181ebf4e8. [DOI] [PubMed] [Google Scholar]
- 24.Mego M, Mani SA, Lee BN, et al. Expression of epithelial–mesenchymal transition-inducing transcription factors in primary breast cancer: the effect of neoadjuvant therapy. Int J Cancer. 2011;130(4):808–816. doi: 10.1002/ijc.26037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith B, Selby P, Southgate J, Pittman K, Bradley C, Blair GE. Detection of melanoma cells in peripheral blood by means of reverse transcriptase and polymerase chain reaction. Lancet. 1991;338(8777):1227–1229. doi: 10.1016/0140-6736(91)92100-g. [DOI] [PubMed] [Google Scholar]
- 26.Stathopoulou A, Gizi A, Perraki M, et al. Real-time quantification of CK-19 mRNA-positive cells in peripheral blood of breast cancer patients using the lightcycler system. Clin Cancer Res. 2003;9(14):5145–5151. [PubMed] [Google Scholar]
- 27.Stathopoulou A, Ntoulia M, Perraki M, et al. A highly specific real-time RT-PCR method for the quantitative determination of CK-19 mRNA positive cells in peripheral blood of patients with operable breast cancer. Int J Cancer. 2006;119(7):1654–1659. doi: 10.1002/ijc.22017. [DOI] [PubMed] [Google Scholar]
- 28.Ignatiadis M, Kallergi G, Ntoulia M, et al. Prognostic value of the molecular detection of circulating tumor cells using a multimarker reverse transcription-PCR assay for cytokeratin 19, mammaglobin A, and HER2 in early breast cancer. Clin Cancer Res. 2008;14(9):2593–2600. doi: 10.1158/1078-0432.CCR-07-4758. [DOI] [PubMed] [Google Scholar]
- 29.Bostick PJ, Chatterjee S, Chi DD, et al. Limitations of specific reverse-transcriptase polymerase chain reaction markers in the detection of metastases in the lymph nodes and blood of breast cancer patients. J Clin Oncol. 1998;16(8):2632–2640. doi: 10.1200/JCO.1998.16.8.2632. [DOI] [PubMed] [Google Scholar]
- 30.Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23(7):1420–1430. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- 31.Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12(14 Pt 1):4218–4224. doi: 10.1158/1078-0432.CCR-05-2821. [DOI] [PubMed] [Google Scholar]
- 32.Danila DC, Heller G, Gignac GA, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13(23):7053–7058. doi: 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- 33.Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 34.Scher HI, Jia X, De Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10(3):233–239. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.US FDA. FDA Approval document Nr K050245. Mar 15th, 2005. [Google Scholar]
- 36.Balic M, Dandachi N, Hofmann G, et al. Comparison of two methods for enumerating circulating tumor cells in carcinoma patients. Cytometry B Clin Cytom. 2005;68(1):25–30. doi: 10.1002/cyto.b.20065. [DOI] [PubMed] [Google Scholar]
- 37.Saliba AE, Saias L, Psychari E, et al. Microfluidic sorting and multimodal typing of cancer cells in self-assembled magnetic arrays. Proc Natl Acad Sci USA. 2010;107(33):14524–14529. doi: 10.1073/pnas.1001515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359(4):366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450(7173):1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sequist LV, Nagrath S, Toner M, Haber DA, Lynch TJ. The CTC-chip: an exciting new tool to detect circulating tumor cells in lung cancer patients. J Thorac Oncol. 2009;4(3):281–283. doi: 10.1097/JTO.0b013e3181989565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farace F, Massard C, Vimond N, et al. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br J Cancer. 2011;105(6):847–853. doi: 10.1038/bjc.2011.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng S, Lin HK, Lu B, et al. 3D microfilter device for viable circulating tumor cell (CTC) enrichment from blood. Biomed Microdevices. 2011;13(1):203–213. doi: 10.1007/s10544-010-9485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis JA, Inglis DW, Morton KJ, et al. Deterministic hydrodynamics: taking blood apart. Proc Natl Acad Sci USA. 2006;103(40):14779–14784. doi: 10.1073/pnas.0605967103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang LR, Cox EC, Austin RH, Sturm JC. Continuous particle separation through deterministic lateral displacement. Science. 2004;304(5673):987–990. doi: 10.1126/science.1094567. [DOI] [PubMed] [Google Scholar]
- 45.Inglis DW, Davis JA, Austin RH, Sturm JC. Critical particle size for fractionation by deterministic lateral displacement. Lab Chip. 2006;6(5):655–658. doi: 10.1039/b515371a. [DOI] [PubMed] [Google Scholar]
- 46.Wang XB, Yang J, Huang Y, Vykoukal J, Becker FF, Gascoyne PR. Cell separation by dielectrophoretic field-flow-fractionation. Anal Chem. 2000;72(4):832–839. doi: 10.1021/ac990922o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moon HS, Kwon K, Kim SI, et al. Continuous separation of breast cancer cells from blood samples using multi-orifice flow fractionation (MOFF) and dielectrophoresis (DEP) Lab Chip. 2011;11(6):1118–1125. doi: 10.1039/c0lc00345j. [DOI] [PubMed] [Google Scholar]
- 48.Becker FF, Wang XB, Huang Y, Pethig R, Vykoukal J, Gascoyne PR. Separation of human breast cancer cells from blood by differential dielectric affinity. Proc Natl Acad Sci USA. 1995;92(3):860–864. doi: 10.1073/pnas.92.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alazzam A, Stiharu I, Bhat R, Meguerditchian AN. Interdigitated comb-like electrodes for continuous separation of malignant cells from blood using dielectrophoresis. Electrophoresis. 2011;32(11):1327–1336. doi: 10.1002/elps.201000625. [DOI] [PubMed] [Google Scholar]
- 50.Gascoyne PR, Noshari J, Anderson TJ, Becker FF. Isolation of rare cells from cell mixtures by dielectrophoresis. Electrophoresis. 2009;30(8):1388–1398. doi: 10.1002/elps.200800373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea – a paradigm shift. Cancer Res. 2006;66(4):1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 52.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balic M, Lin H, Young L, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12(19):5615–5621. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 55.Theodoropoulos PA, Polioudaki H, Agelaki S, et al. Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett. 2010;288(1):99–106. doi: 10.1016/j.canlet.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 56.Gradilone A, Naso G, Raimondi C, et al. Circulating tumor cells (CTCs) in metastatic breast cancer (MBC): prognosis, drug resistance and phenotypic characterization. Ann Oncol. 2011;22(1):86–92. doi: 10.1093/annonc/mdq323. [DOI] [PubMed] [Google Scholar]
- 57.Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S. Stem cell and epithelial–mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009;11(4):R46. doi: 10.1186/bcr2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wicha MS, Hayes DF. Circulating tumor cells: not all detected cells are bad and not all bad cells are detected. J Clin Oncol. 2011;29(12):1508–1511. doi: 10.1200/JCO.2010.34.0026. [DOI] [PubMed] [Google Scholar]
- 59.Geigl JB, Speicher MR. Single-cell isolation from cell suspensions and whole genome amplification from single cells to provide templates for CGH analysis. Nat Protoc. 2007;2(12):3173–3184. doi: 10.1038/nprot.2007.476. [DOI] [PubMed] [Google Scholar]
- 60.Mathiesen RR, Fjelldal R, Liestol K, et al. High resolution cost–effectives of copy number changes in disseminated tumor cells of patients with breast cancer. Int J Cancer. 2011 doi: 10.1002/ijc.26444. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 61.Chimonidou M, Strati A, Tzitzira A, et al. DNA methylation of tumor suppressor and metastasis suppressor genes in circulating tumor cells. Clin Chem. 2011;57(8):1169–1177. doi: 10.1373/clinchem.2011.165902. [DOI] [PubMed] [Google Scholar]
- 62.Markou A, Strati A, Malamos N, Georgoulias V, Lianidou ES. Molecular characterization of circulating tumor cells in breast cancer by a liquid bead array hybridization assay. Clin Chem. 2011;57(3):421–430. doi: 10.1373/clinchem.2010.154328. [DOI] [PubMed] [Google Scholar]
- 63.Fehm T, Becker S, Duerr-Stoerzer S, et al. Determination of HER2 status using both serum HER2 levels and circulating tumor cells in patients with recurrent breast cancer whose primary tumor was HER2 negative or of unknown HER2 status. Breast Cancer Res. 2007;9(5):R74. doi: 10.1186/bcr1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Munzone E, Nole F, Goldhirsch A, et al. Changes of HER2 status in circulating tumor cells compared with the primary tumor during treatment for advanced breast cancer. Clin Breast Cancer. 2010;10(5):392–397. doi: 10.3816/CBC.2010.n.052. [DOI] [PubMed] [Google Scholar]
- 65.Idirisinghe PK, Thike AA, Cheok PY, et al. Hormone receptor and c-ERBB2 status in distant metastatic and locally recurrent breast cancer. Pathologic correlations and clinical significance. Am J Clin Pathol. 2010;133(3):416–429. doi: 10.1309/AJCPJ57FLLJRXKPV. [DOI] [PubMed] [Google Scholar]
- 66.Wilking U, Karlsson E, Skoog L, et al. HER2 status in a population-derived breast cancer cohort: discordances during tumor progression. Breast Cancer Res Treat. 2011;125(2):553–561. doi: 10.1007/s10549-010-1029-2. [DOI] [PubMed] [Google Scholar]
- 67.Somlo G, Lau SK, Frankel P, et al. Multiple biomarker expression on circulating tumor cells in comparison to tumor tissues from primary and metastatic sites in patients with locally advanced/inflammatory, and Stage 4 breast cancer, using a novel detection technology. Breast Cancer Res Treat. 2011;128(1):155–163. doi: 10.1007/s10549-011-1508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dharmasiri U, Njoroge SK, Witek MA, et al. High-throughput selection, enumeration, electrokinetic manipulation, and molecular profiling of low-abundance circulating tumor cells using a microfluidic system. Anal Chem. 2011;83(6):2301–2309. doi: 10.1021/ac103172y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang MJ, Chiu HH, Wang HM, et al. Enhancing detection of circulating tumor cells with activating KRAS oncogene in patients with colorectal cancer by weighted chemiluminescent membrane array method. Ann Surg Oncol. 2010;17(2):624–633. doi: 10.1245/s10434-009-0831-8. [DOI] [PubMed] [Google Scholar]
- 70.Iinuma H, Watanabe T, Mimori K, et al. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes’ stage B and C colorectal cancer. J Clin Oncol. 2011;29(12):1547–1555. doi: 10.1200/JCO.2010.30.5151. [DOI] [PubMed] [Google Scholar]
- 71.Reinholz MM, Kitzmann KA, Tenner KS, et al. Cytokeratin-19 and mammaglobin gene expression in circulating tumor cells from metastatic breast cancer patients enrolled in North central cancer treatment group (NCCTG) trials, N0234/336/436/437. Clin Cancer Res. 2011;17(22):7183–7193. doi: 10.1158/1078-0432.CCR-11-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nole F, Munzone E, Zorzino L, et al. Variation of circulating tumor cell levels during treatment of metastatic breast cancer: prognostic and therapeutic implications. Ann Oncol. 2008;19(5):891–897. doi: 10.1093/annonc/mdm558. [DOI] [PubMed] [Google Scholar]
- 73.De Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14(19):6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 74.Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol. 2011;29(12):1556–1563. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- 75.Budd GT, Cristofanilli M, Ellis MJ, et al. Circulating tumor cells versus imaging – predicting overall survival in metastatic breast cancer. Clin Cancer Res. 2006;12(21):6403–6409. doi: 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- 76.Giuliano M, Giordano A, Jackson S, et al. Circulating tumor cells as prognostic and predictive markers in metastatic breast cancer patients receiving first-line systemic treatment. Breast Cancer Res. 2011;13(3):R67. doi: 10.1186/bcr2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gaforio JJ, Serrano MJ, Sanchez-Rovira P, et al. Detection of breast cancer cells in the peripheral blood is positively correlated with estrogen-receptor status and predicts for poor prognosis. Int J Cancer. 2003;107(6):984–990. doi: 10.1002/ijc.11479. [DOI] [PubMed] [Google Scholar]
- 78.Sato N, Hayashi N, Imamura Y, et al. Usefulness of transcription-reverse transcription concerted reaction method for detecting circulating tumor cells in patients with colorectal cancer. Ann Surg Oncol. 2011 doi: 10.1245/s10434-011-1889-7. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 79.Stathopoulou A, Vlachonikolis I, Mavroudis D, et al. Molecular detection of cytokeratin-19-positive cells in the peripheral blood of patients with operable breast cancer: evaluation of their prognostic significance. J Clin Oncol. 2002;20(16):3404–3412. doi: 10.1200/JCO.2002.08.135. [DOI] [PubMed] [Google Scholar]
- 80.Molloy TJ, Bosma AJ, Baumbusch LO, et al. The prognostic significance of tumour cell detection in the peripheral blood versus the bone marrow in 733 early-stage breast cancer patients. Breast Cancer Res. 2011;13(3):R61. doi: 10.1186/bcr2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Molloy TJ, Devriese LA, Helgason HH, et al. A multimarker QPCR-based platform for the detection of circulating tumour cells in patients with early-stage breast cancer. Br J Cancer. 2011;104(12):1913–1919. doi: 10.1038/bjc.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsusaka S, Suenaga M, Mishima Y, et al. Circulating tumor cells as a surrogate marker for determining response to chemotherapy in Japanese patients with metastatic colorectal cancer. Cancer Sci. 2011;102(6):1188–1192. doi: 10.1111/j.1349-7006.2011.01926.x. [DOI] [PubMed] [Google Scholar]
- 83.Papavasiliou P, Fisher T, Kuhn J, Nemunaitis J, Lamont J. Circulating tumor cells in patients undergoing surgery for hepatic metastases from colorectal cancer. Proc (Bayl Univ Med Cent) 2010;23(1):11–14. doi: 10.1080/08998280.2010.11928572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rahbari NN, Aigner M, Thorlund K, et al. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology. 2010;138(5):1714–1726. doi: 10.1053/j.gastro.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 85.Helo P, Cronin AM, Danila DC, et al. Circulating prostate tumor cells detected by reverse transcription-PCR in men with localized or castration-refractory prostate cancer: concordance with CellSearch assay and association with bone metastases and with survival. Clin Chem. 2009;55(4):765–773. doi: 10.1373/clinchem.2008.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 87.Scheel C, Weinberg RA. Phenotypic plasticity and epithelial–mesenchymal transitions in cancer and normal stem cells? Int J Cancer. 2011;129(10):2310–2314. doi: 10.1002/ijc.26311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raimondi C, Gradilone A, Naso G, et al. Epithelial–mesenchymal transition and stemness features in circulating tumor cells from breast cancer patients. Breast Cancer Res Treat. 2011;130(2):449–455. doi: 10.1007/s10549-011-1373-x. [DOI] [PubMed] [Google Scholar]
- 89.Roche H, Vahdat LT. Treatment of metastatic breast cancer: second line and beyond. Ann Oncol. 2011;22(5):1000–1010. doi: 10.1093/annonc/mdq429. [DOI] [PubMed] [Google Scholar]
- 90.Harris L, Fritsche H, Mennel R, et al. American society of clinical oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 91.Cardoso F, Fallowfield L, Costa A, Castiglione M, Senkus E. Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi25–vi30. doi: 10.1093/annonc/mdr372. [DOI] [PubMed] [Google Scholar]