Abstract
Alzheimer disease (AD) is one of the most common forms of dementia in the elderly. In AD patients, β-amyloid peptide (Aβ) plaques and neurofibrillary tangles are common features observed in the central nervous system. Aβ deposition results in the production of reactive oxygen species (ROS) leading to the hyperphosphorylation of tau that are associated with neuronal damage. Cholinesterase inhibitors (ChEI) and a partial NMDA receptor antagonist (memantine) have been identified as potential treatment options for AD. However, clinical studies have found that these drugs fail to prevent the disease progression. From ancient times, garlic (Allium sativum) has been used to treat several diseases. By “aging” of garlic, some adverse reactions of garlic can be eliminated. Recent findings suggest that “Aged Garlic Extract” (AGE) may be a therapeutic agent for AD due to its antioxidant and Aβ lowering properties. To date, the molecular properties of AGE have been sparsely studied in vitro or in vivo. The present study tested specific biochemical and molecular effects of AGE in neuronal and AD rodent models. Furthermore, we identified SAC as one of the most active chemicals responsible for the AGE-mediated effect(s). We observed significant neuroprotective and neurorescue properties of AGE and one of its ingredients, S-allyl-L- cysteine (SAC), from ROS (H2O2)-mediated insults to neuronal cells. Treatment of AGE and SAC were found to protect neuronal cells when they were independently co-treated with ROS. Furthermore, a novel neuropreservation effect of AGE was detected in that pre-treatment with AGE alone protected ~80% neuronal cells from ROS-mediated damage. AGE was also found to preserve pre-synaptic proteins SNAP25 from ROS-mediated insult. For example, treatment with 2% AGE and SAC (20mg/kg) independently increased (~70%) levels of SNAP25 and synaptophysin in Alzheimer's APP-Tg mice, of which the latter was significantly decreased in AD. Taken together, the neuroprotective, including preservation of pre-synaptic proteins by AGE and SAC can be utilized in future drug development in AD.
Keywords: Alzheimer's disease (AD), neuronal cells, pre-synaptic proteins, reactive oxygen species (ROS), transgenic mice
Alzheimer disease (AD), a progressive neurological disorder, is the most common form of dementia in the elderly and also the 7th major cause of death in US alone (A.A 2009). The development of AD is influenced by multiple factors including both genetic and epigenetic (environmental) factors. Environmental factors such as dietary, lifestyle, education and exposure to heavy metals and pestichemicals play major roles in the pathogenesis of sporadic cases of AD (Lahiri et al. 2009, Lahiri & Maloney 2010). Neurochemically, AD is characterized by deposition of amyloid β (Aβ)- containing neuritic plaques in the brain parenchyma followed by neuronal loss (Hardy & Selkoe 2002). Additionally, AD is associated with hyperphosphorylated tau, a neuronal microtubule stabilizing protein (Schmidt et al. 2001), which also leads to neuronal damage. Aβ, a 40-42 amino acid residue, is a product of transmembrane amyloid precursor protein (APP) that is cleaved by enzymatic actions of β secretase (BACE-1) and γ secretase (Vassar et al. 2009). The major species of Aβ, a forty amino acids residue (Aβ1-40), is less pathogenic for the development of AD (Selkoe 2001). However, Aβ with 42 amino acids residue (Aβ1-42) has been shown to fibrillate faster than that of Aβ40, and hence is considered pathognomonic in AD (Walsh & Selkoe 2007). Deposited Aβ in the brain activates microglia, and this interaction between microglia and Aβ generates reactive oxygen species (ROS) and cytochemokines leading to severe neuronal damage (El Khoury et al. 1996, Coraci et al. 2002). Furthermore, generated ROS is directly involved in lipid peroxidation of the cell membranes (Behl & Sagara 1997). ROS such as hydrogen peroxide (H2O2) can also be generated by interaction of Aβ and other metal ions like Cu++, Zn++ and Fe+++ (Opazo et al. 2002).
Apart from a widely used culinary component, garlic (Allium sativum) has been utilized to treat a wide variety of diseases, including cancer. The efficacy of garlic has been attributed to its potent antioxidant properties (Durak et al. 2004a, Durak et al. 2004b, Ichikawa et al. 2002). However, the consumption of raw garlic can cause various side effects including anemia, growth retardation and destruction of gut microflora and alteration of serum protein levels (Nakagawa et al. 1980, Shashikanth et al. 1984). To overcome the adverse effects, aged garlic extract (AGE) has been prepared by soaking sliced garlic in water-ethanol mixture for 20 months, which removes several irritant sulfur containing compounds and also stabilizes some unstable compounds such as allicin (Morihara et al. 2006, Borek 2006). Interestingly, the antioxidant property of AGE is greater than those of raw garlic (Park et al. 2009, Imai et al. 1994). Details of the procedure of making AGE, its quality control and related scientific references can be obtained from Kyolic® [website http://www.kyolic.com/category/truth-about-garlic]
In this study, we have examined the effect(s) of AGE and an active and most abundant ingredient S-allyl-L-cysteine (SAC) in protecting neuronal cells from free radicals (ROS)-mediated damage. We also evaluated AGE and SAC's effect in preserving pre-synaptic proteins in Alzheimer's APP transgenic (Tg) mice, which over produce Aβ peptides. In the cell culture based study, we have observed a significant neuroprotective role of AGE and SAC from ROS (H2O2)-mediated insults to the neuronal cells. We also characterized pre-synaptic markers, such as synaptosomal associated protein of 25kDa (SNAP25). SNAP25 is a member of soluble N-ethylmaleimide sensitive factor attachment protein receptors (SNARE), and plays an important role in pre-synaptic vesicle fusion and exocytosis (Sollner et al. 1993). Our findings also indicate that AGE treatment preserves SNAP25 in neuronally differentiated PC12 cells when they were co-treated with ROS versus cells only treated with ROS. In the context of ROS mediated damage to the neuronal cells, a novel neuropreservation property of AGE was also observed in this study under different ‘challenge conditions’.
For instance, two days pre-treatment with AGE significantly increased cellular viability of differentiated PC12 cells when they were post-treated with ROS alone for 24 hr versus the cells not pretreated with AGE but treated with ROS alone for 24 hours. In both experiments, under the first condition, when the neuronal cells were co-treated with the vehicle, AGE and ROS, and under the second condition when the cells were pre-treated with AGE and post-treated with ROS, AGE treatment preserved neuron like morphology, which was completely lost in non-AGE treated (vehicle) but ROS challenged cells. Furthermore, 4 months independent treatment of AGE and SAC to APP-Tg mice yielded an abundant amount of Aβ and significant increase in levels of the pre-synaptic proteins synaptophysin and SNAP25 versus untreated APP-Tg mice. In humans, both synaptophysin and SNAP25 are significantly decreased in AD versus control cases (Lassmann et al. 1992, Greber et al. 1999). Furthermore, hippocampal-based memory was found to be impaired in AP-Tg mice (Sadowski et al. 2004). Likewise, our findings demonstrate a significant improvement in the hippocampal-based memory as measured by Y-maze spontaneous exploration in SAC treated Tg mice.
Taken together, our findings demonstrate strong evidences that AGE and SAC possess potential neuroprotective and neuropreservative properties against ROS mediated damage, and also preserve pre-synaptic proteins both in cellular and animal models of AD.
Materials and methods
Cell culture
PC12 cells were obtained from American Type Culture Collection (ATCC) and cultured as described previously (Ray & Lahiri 2009). When the cells became 75% confluent, the regular RPMI medium was replaced by low serum (1% FBS) containing RPMI supplemented with 30ng/ml nerve growth factor (NGF, Roche, Indianapolis, IN, USA) to achieve neuronal differentiation. NGF treatment transforms PC12 cell to sympathetic neurons (Ray & Lahiri 2009, Ghosh & Lahiri 1999). Differentiation of PC12 cells with NGF was carried out for 12 days, counted by Trypan blue exclusion method and plated onto a 24 well cell culture plate (Corning, NY, USA) at 150,000 cells/ well in low serum RPMI with NGF. The cells were allowed to settle down for two days prior to the treatment. Drugs were added on 15th day of the differentiation of PC12 cells with NGF.
Drug treatment
AGE (Kyolic™) was manufactured by Wakunaga of America Co. Ltd. (Mission Viejo, CA, USA). Briefly, the raw garlic is aged for 20 months, and the process of aging converts fat soluble sulfur compounds into water soluble compounds, mainly SAC and S-allylmercaptocysteine (Borek 2006). Commercially obtained aqueous aged garlic extract (Kyolic™) contains 300mg/ml of AGE. AGE exerted potential pharmacological effects in cell culture based studies at concentrations between 0.1% to 1.0% v/v (Berginc et al. 2009, Li et al. 2009). We have also tested different doses of AGE and SAC in neuronally differentiated PC12 cells with/without ROS and observed an optimum efficacy of AGE between the dose of 0.2% to 1.0% v/v and that for SAC between the dose of 1μM to 3 μM (Supplemental 1A &1B). In our study, we have used 0.3% and 1.0% v/v doses of AGE in the cell culture experiments. To evaluate whether SAC produces similar results of AGE or in other word, whether AGE's neuroprotective properties are predominantly due to SAC, neuronal cells were also treated with SAC. SAC was obtained as a gift from Wakunaga of America Co. Ltd. and was dissolved in sterile PBS. In cell culture experiments, we have used 1μM and 2μM doses of SAC. Apart from our results, previous study revealed potent physiological activities of SAC in cell culture models between the doses of 1-2μM (Kosuge et al. 2003).
Co-treatment experimental condition
Prior to start the experiment, 1 ml of AGE liquid was passed through a 0.22μM filter (Millipore Corporation, Billerica, MA, USA) and filtered AGE was mixed with RPMI containing 1% FBS, 1x antibiotic mixture and NGF (30ng/ml). The culture medium from each well of the 24 well plate was aspirated out and either medium alone, 0.3% or 1.0% AGE (dissolved in the medium) was added to the respective wells (n=6 for each treatment group). Both the treatment groups were also co-treated with 200μM of H2O2 (Sigma, St. Louis, MO, USA). Differentiated PC12 cells, which were treated with RPMI LSM+ NGF with/ without H2O2, were served as ‘vehicles with ROS’ and ‘no ROS vehicle’ respectively. In the same experiment, differentiated PC12 cells were also treated with 1μM or 2μM of SAC in the presence of ROS. The duration of the treatment was 48 hours.
Pre-treatment experimental condition
In a separate experiment, 15 days differentiated PC12 cells were treated with 0.3% and 1.0% AGE without the presence of H2O2 for 48 hours. After 48 hours, AGE containing medium was gently aspirated out from the wells and RPMI (LSM) + NGF + 200μM H2O2 was added to the wells. Post treatment of cells with H2O2 was carried out for 24 hours. Cells, which were not pre-treated with AGE, but post-treated with H2O2, were served as ‘ROS post-treated vehicle’.
Harvesting of the treated or untreated cells
After the period of treatment, 500μl of the conditioned medium was taken out from each well and stored at -20°C for LDH assay. The remaining conditioned medium from each well was aspirated out, and cells in each wells were rinsed with ice cold 1x DPBS. Cells were lysed by addition of 100μl of M-PER buffer (Pierce) supplemented with protease inhibitor cocktail (Roche, Indianapolis, IN, USA). Cell lysates (CL) were collected and 30μl of CL sample from each of the treated and untreated well were used to determine cell viability (CTG assay) just after lysing the cells, and the remaining CL samples were stored at -20°C freezer for the Western immunoblotting analyses.
Cell viability (CTG) assay
An equal volume (30μl) of the cell lysate samples were taken into each well of a white 96-well plate (Corning), and 30μl of the Cell Titer Glo reagent (CTG, Promega) was added to each well. CTG measures cellular viability by quantifying mitochondrial ATP concentration in the CL, which correlates with the cell number in the lysate (Ray et al. 2009). CTG assay measurement was performed in ‘Varitas’ microplate luminometer (Turner Biosystems, Sunnyvale, CA, USA).
Cellular toxicity (LDH) assay
Cell membrane damage leading to cellular death was measured by actual elevation of lactate dehydrogenase (LDH) in the medium sample. For this, an equal volume of CM samples (15μM) from each treatment group was used to determine cellular toxicity by LDH assay as mentioned previously (Alley et al. 2009). To determine the exact amount of LDH present in the CM, a standard curve with known amounts of rabbit muscle LDH was also performed (not shown).
Treatment of transgenic mice with AGE and SAC
Tg2576 transgenic mice modeling familial Alzheimer's disease harbor Human APP695 double Swedish mutation (KM 670/671 NL), originally produced by Karen Ashe Hsiao (Hsiao et al. 1996), bred on B6SJLF1 background. These mice are now commercially available from Taconic Farms (Taconic Farms, Inc.). Tg2576 mice begin to accumulate Aβ–40/42 peptides beginning ~5+ months of age developing Aβ plaques, oxidative lipid and glycoxidative damages by 9+ months of age, concomitant with cognitive deficits.
Tg2576 original transgenic breeder males obtained from Taconic Farms (German Town, NY), were crossed with the background strain (C57BL/6J x SJL) females obtained from Jackson Labs (Bar Harbor, ME) to produce Tg2576 progeny. At the end of weaning, transgenic (Tgs/Tg2576) and non-transgenic littermates (Lts) were identified by polymerase chain reaction (PCR) amplification of tail genomic DNA using specific primer pairs as established(Chauhan & Siegel 2003b) (Chauhan & Siegel 2003a).
Mice are reported to consume 150 g of food/kg body weight (Jacoby & Fox 1999). Therefore, a diet containing 2% AGE (2 g AGE/100 g Chow), an optimized dose in our laboratory (Chauhan 2003) will result in the intake of 3 g of AGE per mouse. Since 1 g of AGE is known to contain 1mg of SAC, one mouse will consume 3mg of SAC per day, which will translate into 20 mg SAC/kg diet. Based on this best known estimation, concentration of SAC was determined to be 20 mg/kg.
Special rodent diets containing 2% aged garlic extract (Kyolic) and 20 mg SAC/kg (Sigma–Aldrich) were commercially prepared (Research Diets, Inc., New Brunswick, NJ), in which the test ingredients (AGE or SAC) were incorporated into an AIN-76A based standard purified diet [kcal% of proteins (20.8), carbohydrates (67.7), fat (11.5), casein (8.0), corn starch (60.0) and vitamin mix (4.0)]. A control isocaloric diet delivering equivalent amounts of protein, fat and carbohydrates and containing equivalent calories per gram weight also was commercially prepared (Research Diets, Inc., New Brunswick, NJ). Each batch of special diet was prepared in sufficient quantity (50 kg/batch/diet) that would last for the whole experiment in order to avoid batch-variability. 10-month-old transgenic mice or transgenic littermate controls were fed with respective special diet(s) for 4 months. All animal experiments including transgenic breeding, dietary interventions, behavioral studies and brain tissue preparation, were performed in accordance with the National Institute of Health Guide for the Ethics, Care and Use of Laboratory Animals, and as approved by the local institutional Bioethics, Animal Care and Use Committee at the University of Illinois at Chicago and Jesse Brown VA Medical Center.
At the end of treatment, 14 month old mice were evaluated for behavior and then euthanized with ketamine (100mg/kg, i.p.) and xylazine (10mg/kg, i.p.), and perfused with normal sterile saline. Brains were dissected out and cut into two longitudinal bilateral halves. One half of the brain was utilized for biochemical studies and the other half was fixed in 4% buffered paraformaldehyde. Each half of the brain contained all brain regions including cortex, hippocampus, and cerebellum.
Spontaneous Exploratory Working Memory Test
Spontaneous alterations and exploratory behavior in Y maze is a hippocampal-based task that measures working memory. Spontaneous alteration and exploratory deficits were evaluated using Y maze. Y maze apparatus consisted of dark grey acrylic with 3 arms, each of 21 cms long, 4 cms wide and 40 cms tall, placed at an angle of 33°C with a central triangular entry zone. Each animal was subjected to a single trial of 5 min duration for evaluating spontaneous alterations and exploration by introducing the animal in the central entry zone and allowed to explore freely in all 3 arms. Frequent exploration in all 3 arms and less time spent in each arm between the alterations indicated normal exploratory behavior. By contrast, restricted arm entries and reduced explorations with more time spent in each arm indicated deficits in spontaneous exploration. Total number of arm entries and alterations between the arms were registered with the use of CCD camera and Accuscan software. Individual values were averaged to obtain a group mean, and group means were subjected to statistical analysis to derive significance.
Behavioral testing was performed only once at the end of treatment period i.e. on the last day of the 16th week of treatment period.
Preparing mice brain lysate
At the end of the treatment brains were dissected out, washed with sterile saline, weighed and immediately frozen in dry ice. Frozen brains were homogenized in Tris-HCl buffer, pH 7.6 [140 mM NaCl, 3 mM KCl, 25 mM Tris-HCl (pH 7.6), 5 mM EDTA, 2mM Phenanthroline, 0.5% SDS, EDTA-free protease inhibitor cocktail (Roche)] and centrifuged at 30,000 RPM (100,000g) in the ultracentrifuge (SW 55 Ti rotor) to obtain TBS soluble fraction of the brain. This fraction was subjected to protein estimation (Bradford assay), and a defined volume of supernatant containing a fixed amount of protein was analyzed for Western immunoblotting.
Western immunoblotting
Protein content of CL samples was measured by Bradford protein assay as described previously (Bradford 1976). Equal proteins from CL and brain denatured lysates were loaded in two separate 10% Bis-Tris ‘Criterion’ polyacrylamide gels (BioRad) and separated at 180 V for 1.2 hours. Proteins were electrophoretically transferred onto a PVDF membrane (BioRad) using the chilled transfer buffer (25mM Tris, 200mM Glycine and 20% Methanol) at 30V for 6 hours at 4°C. After transfer, the membrane was blocked in 5% nonfat milk in Tris buffered saline, (pH 7.4) containing 0.05% Tween-20 (TBST) at room temperature (RT) for 1 hr. Afterwards, the blot was probed with monoclonal anti-SNAP25 antibody (Clone: SP14; Millipore). The antibody was diluted to 1:3000 in 5% Milk dissolved in TBST and probed overnight at 4°C with gentle rocking. The membrane was washed three times with TBST, and subsequently probed with the secondary antibody, which was HRP-conjugated anti mouse goat IgG (Pierce, Rockford, IL, USA) for 1 hr at RT followed by three times wash with TBST. Detection of protein band signals was achieved by adding chemoluminescent buffer (GE, Buckinghamshire, UK) to the blot, which was immediately photographed using GE chemoluminescent detection film. A specific strip of the membrane was also probed with monoclonal anti-β actin antibody (1:100,000 dilutions, Sigma) for normalization purpose, and β-actin band signals were obtained in the same way as mentioned above.
Immunocytochemistry of differentiated PC12 cells after treatment
In addition to studying the effects of AGE at the molecular level, we also examined the effects at the cellular level. For this, PC12 cells from three wells from each treatment groups were fixed by adding 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) dissolved in phosphate buffer (pH 7.4). Paraformaldehyde fixed cells were washed , permeabilized with 0.12% Triton X-100 (Sigma) and blocked with 10% normal horse serum (Sigma) dissolved in DPBS, followed by incubation with the primary antibody [monoclonal anti α-tubulin (Sigma) diluted 1: 50,000 in 1% horse serum]. After overnight incubation, the cells were washed thoroughly with DPBS and incubated in biotinylated horse anti-mouse secondary antibody (Vector laboratories, Burlingame CA, USA) at 1: 250 dilutions for one hr. After washing, the cells were incubated with a mixture of fluorescein (DTAF) conjugated streptavidin (Jackson Immunoresearch Laboratories, PA, USA) at 1: 300 dilutions for one hr. For nuclear staining, 4', 6-diamidino-2-phenylindole (DAPI, Sigma, diluted to 1.5μg/ml in water) was added to the wells and examined under Leica DMIL HC inverted fluorescent microscope (Leica Microsystems GmbH, Wetzler, Germany) using the following sets of filters: for FITC, excitation of 480-520 nm, band pass of 505 nm and emission of 535-585 nm and for DAPI excitation of 350-400 nm, band pass of 400nm and emission of 460-510 nm. Combination of these filters resulted in minimum crosstalk between the signals from individual fluorochromes and minimized the ‘bleed-through’ between any of these three signals. Images were captures with a SPOT RT-SE digital camera (Diagnostic Instruments, Sterling Heights MI, USA) and superimposed with the SPOT basic software (Diagnostic Instruments). The cells stained with α-tubulin exhibited green fluorescence and nuclei stained with DAPI appeared as blue under their respective filter sets.
Quantification of Western immunoblot bands
Densitometric analyses of Western immunoblot bands were performed by using ‘Image J’ software (NIH) using gel ‘analysis tool’, which measures ‘integrated pixel density’ across the region of interest.
Statistical analyses
Statistical analyses were carried out using “GraphPad Prism; 4.0” (GraphPad Software Inc.; La Jolla, CA, USA). All data are presented as mean± SD. ANOVA for more than two groups and ‘T test’ analyses for less than three groups were carried out. For ANOVA, post-hoc comparisons were assessed after performing Bonferroni correction. ‘P values’ less than 0.05 were considered significant.
Results
AGE treatment protects neuronally differentiated PC12 cells from ROS mediated damage
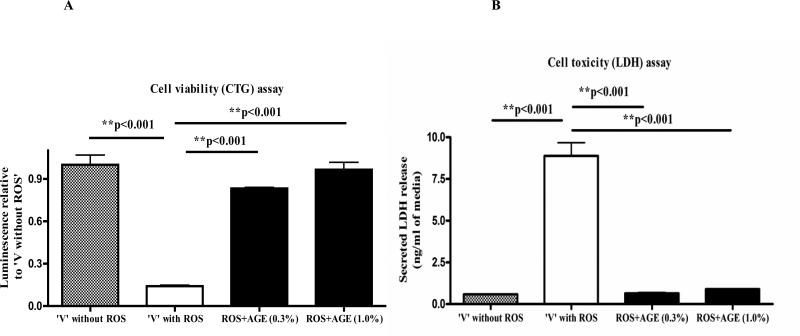
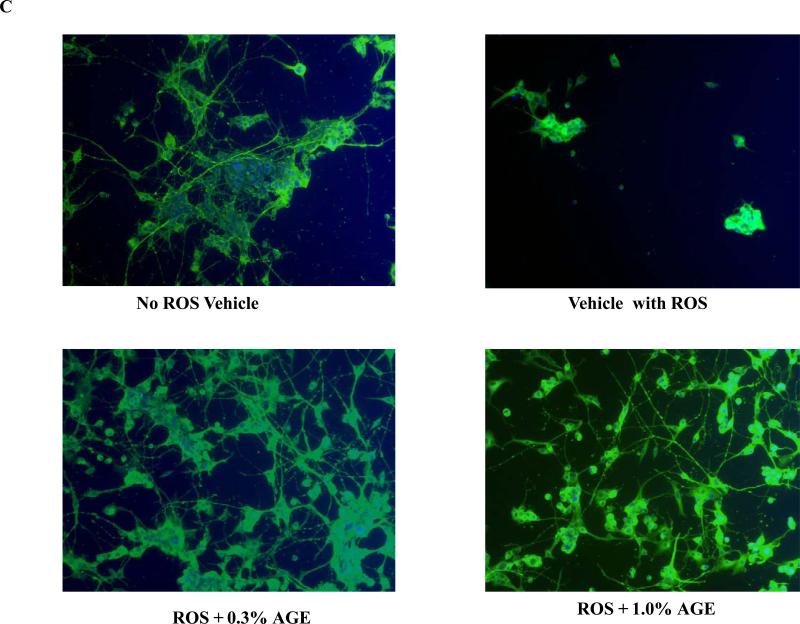
For the neuroprotection experiment, three different assays were employed. First, the CTG assay indicated that H2O2 treatment alone caused a significant decrease (~80%) in the viability of differentiated PC12 cells compared with ‘no ROS vehicle’. We observed a significant increase in the viability of H2O2 treated neuronal PC12 cells when they were co-treated with either 0.3% or 1.0% of AGE (Fig.1A). The increase in the cell viability was approximate 60% and 70% as compared to ‘vehicle with ROS’ with 0.3% and 1.0% AGE co-treatment, respectively. Second, the LDH assay also revealed approximately 80% increase in the secreted LDH in ‘vehicle with ROS’ versus ‘no ROS vehicle’ indicating severe membrane damage and toxicity caused by 200μM H2O2. Interestingly, both 0.3% and 1.0% AGE independently prevented the efflux of secreted LDH in the CM of H2O2-treated PC12 cells. Indeed, the co-treatment of AGE restored the membrane integrity of the H2O2 treated cells as reflected from the similar low level of LDH release in both the AGE treated and ‘no-ROS vehicle’(Fig.1B). Third, cellular morphology studies by immunocytochemistry revealed a substantial loss of cell numbers and neurites in ‘vehicle with ROS’ group versus ‘no ROS vehicle’. Both 0.3% and 1.0% AGE with ROS co-treatment prevented cell death and preserved intercellular connections between cells, which is similar to the ‘no ROS vehicle’ group (Fig. 1C). Thus these biochemical and morphological techniques demonstrate potent neuroprotection properties of AGE against ROS mediated damage.
Fig. 1A. Differentiated PC12 cell co-treated with ROS and AGE: Cell viability assay.
Differentiated PC12 cells (150,000/well) were co-treated with AGE (0.3% and 1.0%) and 200μM H2O2 for 48 hours. After harvest, the viability of cells of different treatment groups was measured by CTG assay. The numbers in the ‘X axis’ represent ‘relative luminescence unit’ and plotted relative to ‘vehicle without ROS’. Results showed a significant loss of viable cells with ROS (H2O2) treatment alone but increase in the viability of cells, which were co-treated with AGE and H2O2 versus ‘vehicle with ROS’.
Fig. 1B: Differentiated PC12 cell co-treated with ROS and AGE: LDH assay Cellular toxicity was measured in the previous experiment with equal volume (15μl) of conditioned media samples. Secreted LDH level was measured and plotted. Results indicate that LDH release was significantly increased (damage) with ROS (H2O2) treatment but lowered in the AGE and H2O2 co-treatment groups versus ‘vehicle with ROS’. (In both Fig. 2A and 2B, the error bars represent mean and standard deviation of the experiment performed in triplicate).
Fig. 1C: Differentiated PC12 cell co-treated with ROS and AGE: Cellular morphology Visualization of the morphology of the cells in the previous treatment group by immunocytochemistry (ICC) technique using α-tubulin (green) as a cytoskeleton marker, and DAPI (blue) as nuclear marker. Data revealed a significant loss of cells and altered cellular morphology in ‘vehicle with ROS’ group. Notably, AGE co-treatment with H2O2 increased the cell number and maintained the intercellular connections between the cells.
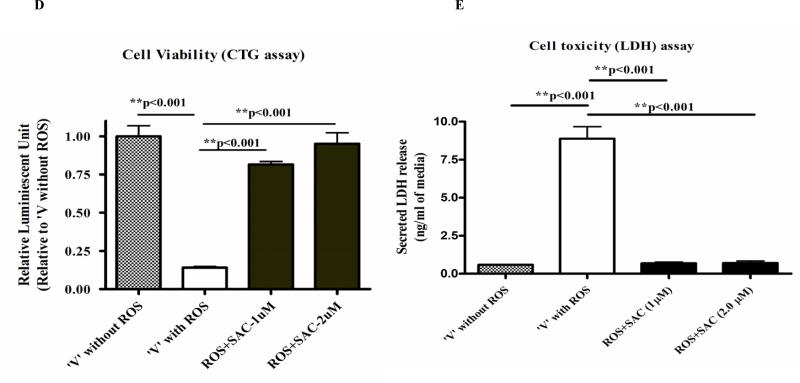
Fig. 1D: Differentiated PC12 cell co-treated with ROS and SAC: Cell viability assay Differentiated PC12 cells (150,000/well) were co-treated with SAC (1μM and 2μM) and 200μM H2O2 for 48 hours as mentioned in Fig 1. After treatment, the viability of the cells of the treatment groups was measured by CTG assay Results showed a significant loss of viable cells with ROS (H2O2) treatment alone but increase in the viability of cells, which were co-treated with SAC and H2O2 versus ‘vehicle with ROS’.
Fig. 1E: Differentiated PC12 cell co-treated with ROS and SAC: LDH assay Cellular toxicity (LDH assay) was measured with equal volume (15μl) of conditioned media samples. Results indicate that LDH release was significantly increased with ROS (H2O2) treatment and lowered in the SAC and H2O2 co-treatment groups versus ‘vehicle with ROS’.
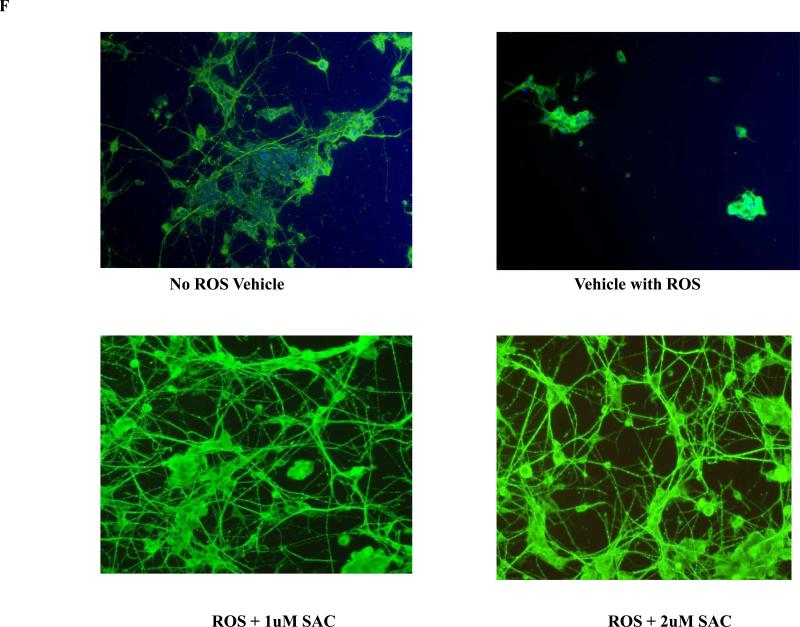
Fig. 1F: Differentiated PC12 cell co-treated with ROS and SAC: Cellular morphology Visualization of the morphology of the cells after SAC and ROS co-treatment by immunocytochemistry (ICC) technique using α-tubulin (green) as a cellular marker. Data revealed a significant loss of cells and altered cellular morphology in ‘vehicle with ROS’ group. SAC co-treatment with H2O2 increased the cell number and also maintained the intercellular connections between the cells.
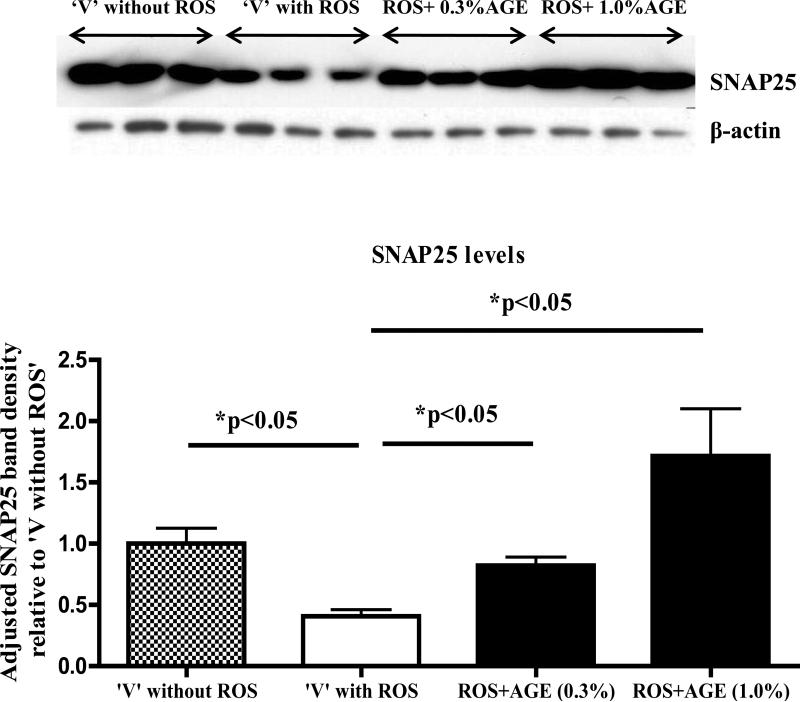
AGE treatment restores pre-synaptic proteins in the neuronal cells against ROS mediated insults
Western immunoblotting analysis with cell lysate (CL) showed approximately 60% decrease in the levels of the pre-synaptic protein SNAP25 in ‘vehicle with ROS’ group versus ‘no ROS vehicle’. Notably, levels of SNAP25 were significantly increased in CL samples of both 0.3% and 1.0% AGE co-treated with ROS treatment groups versus ‘vehicle with ROS’ (Fig.2).
Fig. 2. Differentiated PC12 cell co-treated with ROS and AGE: analysis of pre-synaptic protein SNAP25.
Western immunoblot analysis of the pre-synaptic protein SNAP25 from the cell lysate of the experiment mentioned in Fig.1 showed significance increase in levels of SNAP25 in AGE (0.3% and 1.0%) and 200μM H2O2 co-treated differentiated PC12 cells versus ‘vehicle with ROS’. SNAP25 band densities were adjusted with the β-actin band signals and plotted relative to ‘vehicle without ROS’ with error bars, which represent mean and standard deviation of the experiment performed in triplicate.
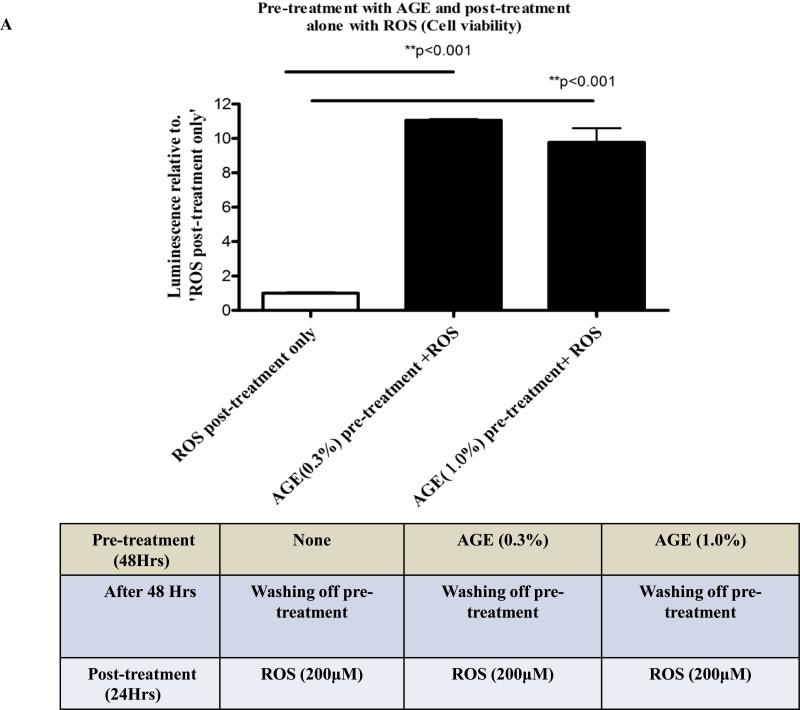
AGE pre-treatment rescues neuronal cells from ROS mediated insults
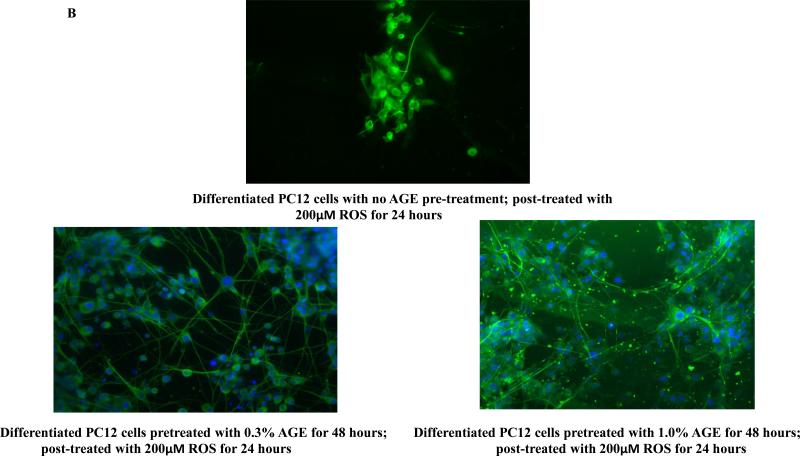
To address the ‘neurorescue’ property of AGE, we have treated neuronally differentiated PC12 cells with 0.3% or 1.0% AGE alone for 48 hours. The dietary supplements were removed and the cells were washed and post-treated with 200μM of H2O2 alone for 24 hr. At the end of 24 hr of H2O2 treatment, we measured cell viability by CTG and monitored cellular morphology by immunocytochemistry. We have observed ~80% increase in cell viability in 0.3% and 1.0% ‘AGE pre-treatment plus ROS only post-treatment’ group versus ‘no AGE pre-treatment plus ROS only post-treatment’ group (Fig.3A). Cellular morphology revealed extensive loss and altered morphology with destruction of neurites in the cells of ‘no AGE pre-treatment plus ROS only post-treatment’ group. Notably, cells in ‘AGE pre-treatment plus ROS only post-treatment’ group had intact cellular morphology (Fig. 3B), suggesting neurorescue ability of AGE, which overcomes the ROS-mediated insults.
Fig. 3A. Differentiated PC12 cell pre-treated with AGE and post-treated with ROS: Cell viability assay.
A novel neuro-rescue property of AGE was shown in this CTG data, where differentiated PC12 cells were pre-treated with 0.3% and 1.0% AGE without the presence of H2O2 for 48 hours and then treated with 200μM of H2O2 alone for 24 hours. Cell viability was almost completely lost by ROS (H2O2) without any prior treatment of AGE. Notably, there was a significant increase in cell viability in the AGE pre-treated and H2O2 post-treated cells versus cells which were only post-treated with H2O2.
Fig. 3B: Differentiated PC12 cell pre-treated with AGE and post-treated with ROS: Cellular morphology Cellular morphology of the previous experiment was studied by immunocytochemistry using α-tubulin (green) as a cytoskeleton marker and DAPI (blue) as nuclear marker. A gross reduction in cell number with complete loss of neuritic outgrowth was observed in the cells which were not pre-treated with AGE but post-treated with 200μM of H2O2 for 24 hours. AGE pre-treatment for 48 hours not only rescues neuronal cells from dying, but also preserves neurite connections even in the presence of ROS (H2O2).
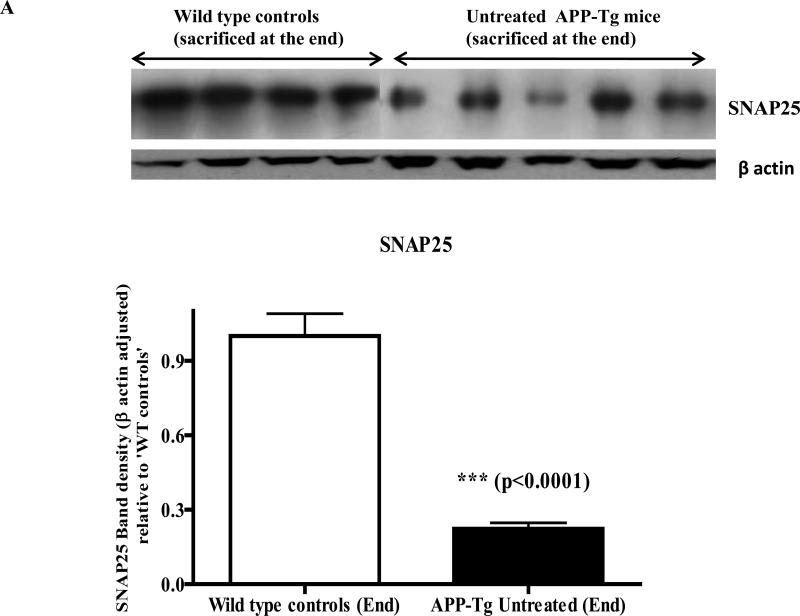
Pre-synaptic proteins (SNAP25 and synaptophysin) are decreased in the brain lysate of APP Tg. versus wild type mice
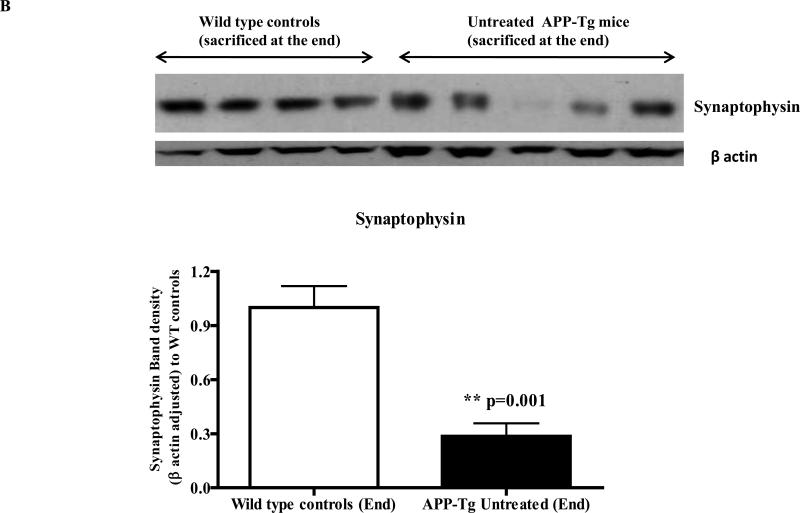
Western immunoblotting analyses of the brain lysate to evaluate the loss of pre-synaptic proteins due to excess Aβ deposition in the untreated APP Tg mice versus wild type mice showed a significant difference in levels of pre-synaptic proteins between these two groups of animals. Both levels of SNAP25 and synaptophysin were significantly reduced (~75%) in the brain lysate of Tg mice versus those of age matched wild type controls (p<0.0001 and p<0.001 respectively) (Fig. 4A and 4B).
Fig.4A. Levels of SNAP25 in wild type versus APP-Tg mice brain lysate.
Western immunoblotting with wild type and APP Tg mice brain lysates revealed a significant decrease in brain levels of the pre-synaptic protein SNAP25 in the untreated APP Tg mice versus wild type controls of the same age. SNAP25 band densities were adjusted with the corresponding β-actin band densities and plotted relative to ‘wild type controls’ (n=4 for wild type mice and n=5 for Tg mice).
Fig. 4B: Levels of synaptophysin in wild type versus APP-Tg mice brain lysate: A similar significant decrease in the brain levels of another pre-synaptic protein synaptophysin was observed in the untreated Tg mice versus wild type controls of the same age. Synaptophysin band densities were adjusted with the corresponding β-actin band densities and plotted relative to ‘wild type controls’.
AGE treatment preserves the levels of pre-synaptic proteins (SNAP25 and synaptophysin) in the brain of Tg mice
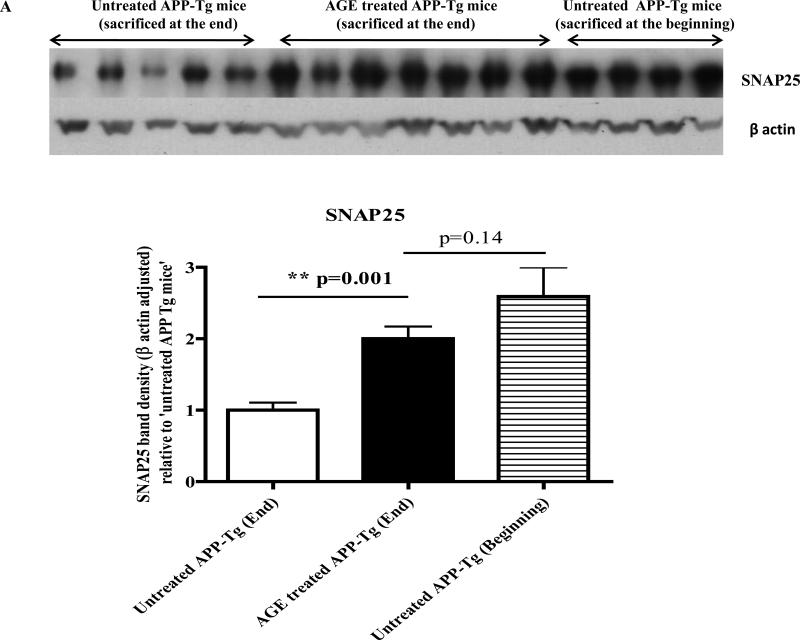
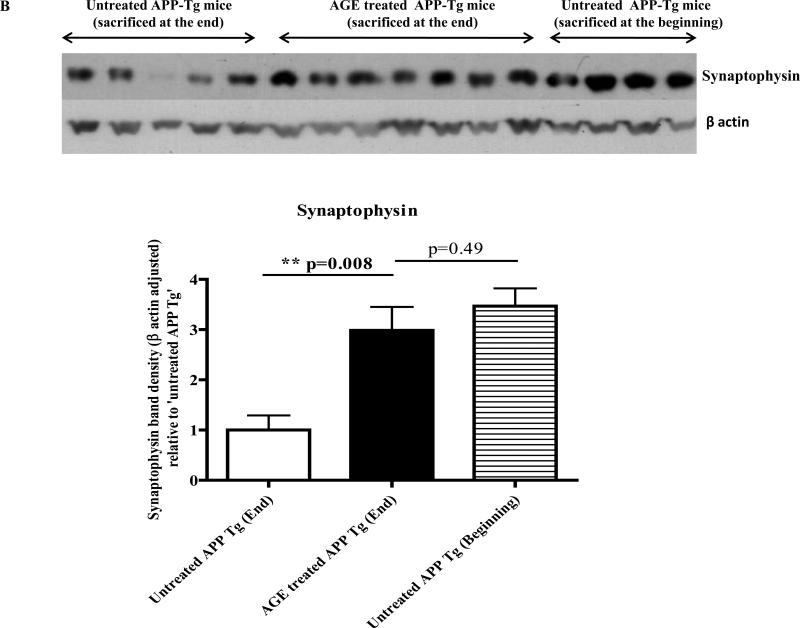
Western immunoblotting of brain lysate of APP-Tg mice revealed that 4 weeks treatment with a special diet containing 2% AGE significantly increased levels of both SNAP25 and synaptophysin versus 4 weeks isocaloric control diet fed Tg mice (p=0.001 and p=0.008 respectively) (Fig. 5A and 5B). Levels of both SNAP25 and synaptophysin were decreased in a time dependent manner in the brain of untreated Tg mice. For example, SNAP25 and synaptophysin levels were decreased approximately by half in the brain lysate of untreated Tg mice at the end of 4-week experiment versus untreated Tg mice at the beginning of the experiment (zero week). Interestingly, the treatment of Tg mice with AGE restored or preserved the levels of both SNAP25 and synaptophysin to the level at the beginning of the experiment.
Fig. 5A. Levels of SNAP25 in the brain lysate of AGE treated APP-Tg mice versus untreated APP-Tg mice.
Western immunoblotting of the mice brain lysate showed a gradual time dependent decrease in brain levels of SNAP25 in untreated APP Tg mice, which were sacrificed after the 4-week experiment versus Tg mice sacrificed at the beginning of the 4-week experiment. Treatment of AGE significantly increases brain levels of SNAP25 versus untreated mice. There is no significant difference in brain levels of SNAP25 in AGE treated Tg mice sacrificed at the end of 4-week experiment versus untreated Tg mice sacrificed at the beginning of the experiment. Beta actin adjusted SNAP25 band densities were plotted relative to ‘untreated APP Tg mice’ sacrificed at the end of the experiment. (n=5 for untreated Tg mice; n=7 for AGE treated Tg mice and n=4 for untreated Tg mice sacrificed at the beginning of the 4-week experiment).
Fig. 5B: Levels of synaptophysin in the brain lysate of AGE treated APP-Tg mice versus untreated APP-Tg mice: Similar results of SNAP25 were obtained in brain levels of synaptophysin as detected by Western immunoblotting. There is a significant increase in the levels of synaptophysin in AGE treated APP Tg mice versus untreated. A time dependent decrease in the levels of synaptophysin was also observed in Tg mice brain and no significant change was detected in the brain levels of synaptophysin between untreated Tg mice sacrificed at the beginning of the 4-week experiment versus AGE treated Tg mice sacrificed at the end of the experiment. Beta actin adjusted synaptophysin band densities were plotted relative to ‘untreated APP Tg mice’ sacrificed at the end of the experiment.
Thus AGE treatment could prevent time-dependent decline of SNAP25 and synaptophysin in APP Tg mice brain.
SAC mimics the results of AGE in cell culture and in vivo
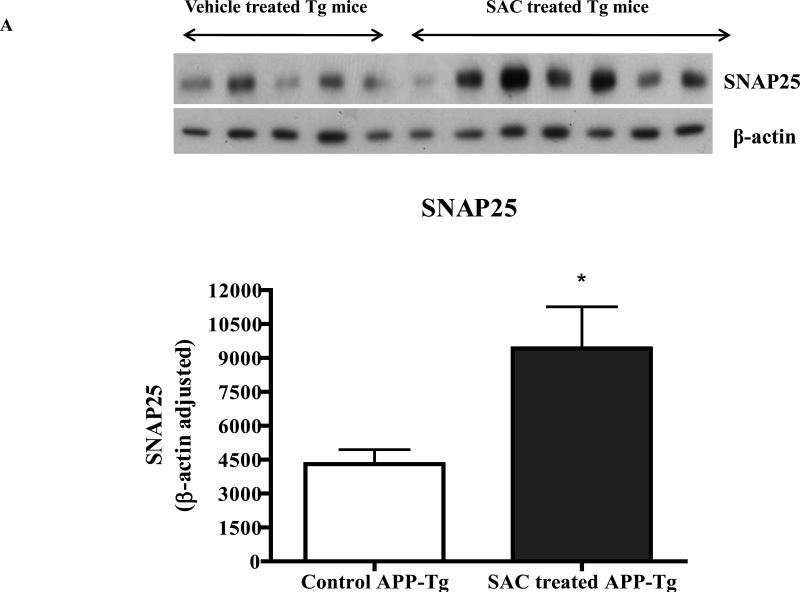
Since AGE is a mixture of several active and inactive ingredients, we hypothesized that AGE's effects observed in this study are mostly mediated by the active and most dominant constituent SAC. We observed potent neuroprotective property of SAC, when neuronal cells were co-treated with SAC and ROS, which was revealed independently by CTG, LDH and morphology analyses (Fig. 1D, 1E and 1F). Further, 4 months treatment of APP Tg mice with 20mg/ kg of SAC also caused robust increase in levels of SNAP25 and synaptophysin versus non-treated APP Tg mice (Fig. 6A and 6B), which is in accordance with the results obtained by AGE treatment to the Tg mice.
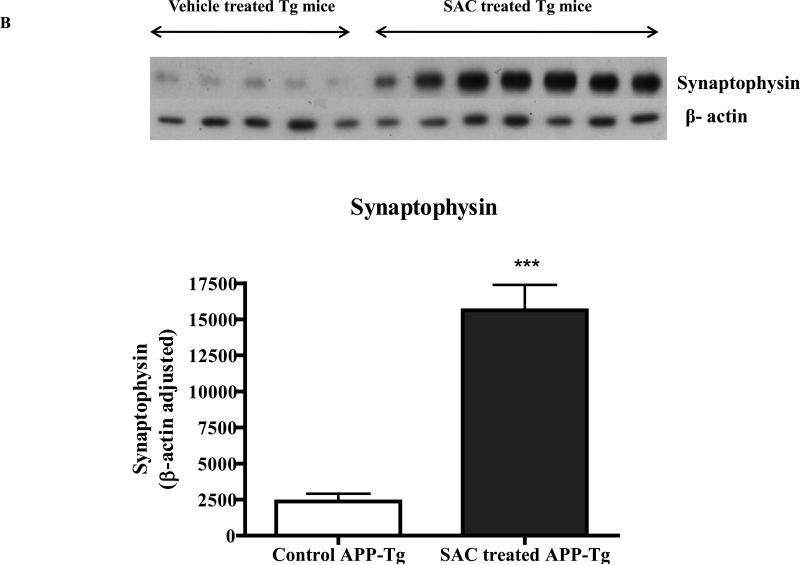
Fig. 6A and Fig. 6B. Levels of SNAP25 and synaptophysin in the brain lysate of SAC treated APP-Tg mice versus untreated APP-Tg mice.
Western immunoblot analyses of four months SAC (20mg/kg/day) treated APP-Tg mice brain lysate resulted in significant increase in the levels of pre-synaptic proteins SNAP25 and synaptophysin.
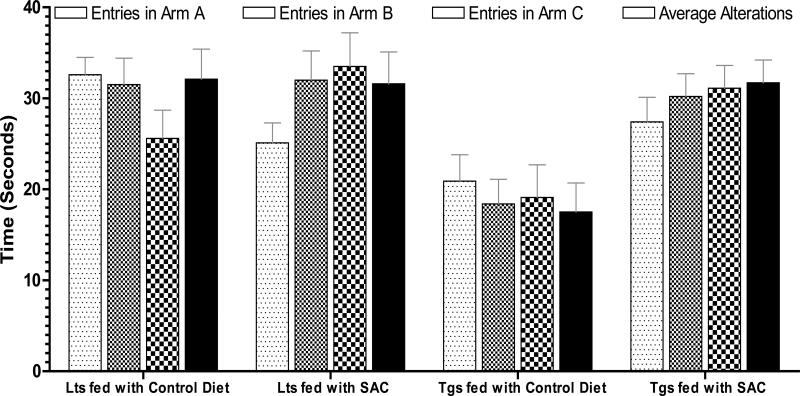
Treatment of 2% AGE was found to improve the hippocampal based memory tasks in the APP-Tg mice versus untreated APP-Tg mice (Chauhan & Sandoval 2007). SAC treatment data (Fig.7) show that spontaneous exploratory behavior remained unchanged in the littermate controls, as indicated by close-range entries in all arms (24-25 entries) and equal number of average alterations in all arms (31-32 alterations). Compared to littermate controls, the spontaneous exploratory behavior was declined by 1.25-fold reduced number of entries in all arms in the Tgs fed with isocaloric control diet, and by 1.8-fold reduced average alterations in all arms at (p<0.0003, unpaired 2-tailed t-test). Feeding of SAC seemed to restore this deficit almost to a normal level as evidenced by 1.3-fold restored number of entries in all arms, and by 1.8-fold restored average alterations in all arms (p<0.0001, unpaired 2-tailed t-test).
Fig. 7. Effect of SAC treatment in Y maze spontaneous exploration.
Hippocampal-based spontaneous alterations and exploration behavior in Y maze after SAC treatment in Tg2576 mice. Note that, impaired exploratory behavior in Tgs fed with isocaloric control diet, and restored spontaneous exploration in Tgs fed with SAC diet.
Like SAC, we also tested another compound present in garlic, diallyl disulfide (DAD) in cell culture based studies. However, DAD failed to exert any neuro-protection/neurorescue effects against ROS mediated insults (data not shown).
Discussion
Restricting the progression and/or delaying the onset of the disease are the current strategies in AD treatment. Statistical analyses revealed that, if the onset of the disease can be delayed by 2 years, the prevalence of AD would approximately decrease by 2 million after 50 years (Brookmeyer et al. 1998). Identifying risk factors and minimizing their effect can potentially be an effective strategy to delay the disease progression associated with AD. For example, it has been proposed that controlling hyperglycemia, obesity and hypertension can delay the onset of AD (Yaffe 2007). In addition, studies have found that moderate physical exercise, engagement in intellectual activity such as reading, increased social interaction and consumption of diet containing fruit and vegetables have beneficial effects in the onset of AD (Angevaren et al. 2008, Stern 2006, Gillette Guyonnet et al. 2007, Lahiri 2006). Likewise, early diagnosis is another major area of AD research. In addition to molecular areas, recent advancements in brain imaging techniques may lead to the identification of markers well ahead of its onset (Mosconi et al. 2010, Nordberg et al . 2010). However, treatment options for AD are still limited.
To date, cholinesterase inhibitors (ChEI) such as donepezil, rivastigmine, galantamine and a partial NMDA receptor antagonist such as memantine are the only FDA approved drugs to treat AD (Ray et al. 2009) . The main purpose of ChEI is to inhibit enzymatic degradation of the neurotransmitter acetylcholine, resulting in increased amount of acetylcholine in the synaptic terminals(Pepeu & Giovannini 2010). Memantine, which is a partial NMDA receptor antagonist, protects neurons from glutamate induced excitatory damages (Alley et al. 2009). However, none of the FDA approved ChEI or memantine can completely restrict or reverse the disease progression associated with AD. As already mentioned, ROS-mediated damage to the neurons presumably precedes neuropathology associated with AD, suggesting that the intake of anti-oxidants might be beneficial in preventing or treating AD. Previous studies demonstrate that the intake of anti-oxidant rich food has a significant effect in the prevention of cognitive decline (Gillette Guyonnet et al. 2007). For example, it has been observed that higher intake of fruit and vegetables may reduce the risk of dementia (Hughes et al. 2009). However, Women Health Study (WHS) found that the oral administration of 600IU vitamin E to 6377 older women did not produce any significant cognitive benefit (Kang et al. 2006). Melatonin, which also possesses potential anti-oxidant and free radical scavenging properties, was shown to reduce the production of ROS from Aβ-metal ion complex (Zatta et al. 2003), and pre-clinical study did reveal that melatonin treatment reduces Aβ peptide in mice (Lahiri et al. 2004). However, convincing evidences for any potential benefit of melatonin in ameliorating symptoms of AD has not yet been generated. Similarly, many other anti-oxidants were tested in pre-clinical and clinical settings to evaluate their effects as potential therapeutic agents in the treatment of AD, but none of them convincingly demonstrated any effect in restricting or reversing the symptoms associated with AD (Cummings & Cole 2002).
Since AD is thought to have a multi-factorial etiology (Lahiri et al. 2003), we hypothesize that an agent that modulates multiple and diverse mechanisms and sites of action would be an effective candidate as a potential drug to restrict the development of AD. A large “Indo-US”epidemiological study revealed approximately 3.7 fold less incidence of AD in Indian elderly when compared to a reference US population (Chandra et al. 2001). Environmental factors including dietary habits like consumption of plant derived substances can potentially be responsible for this decreased incidence. In this context, curcumin was shown to have ‘pleiotropic’ mechanisms and several pre-clinical studies revealed its potential roles in ameliorating neuroinflammation associated with AD (Ray & Lahiri 2009, Frautschy & Cole 2010). However, poor aqueous solubility of curcumin may represent one of the reasons for not achieving its effects in clinical settings (Bisht et al. 2007).
Earlier it was shown that AGE prevents H2O2 mediated insults to the synaptosomes obtained from young rats (Brunetti et al. 2009). In our study, we have observed a direct neuro-protective effect of AGE and SAC, when neuronal cells were co-treated with both AGE or SAC and H2O2. Previously, it was documented that AGE and SAC treatment to the same APP-Tg mice studied herein, resulted in a decrease in the brain levels of both Aβ (1-40) and Aβ (1-42) (Chauhan & Sandoval 2007). Spatial learning and spatial memory retention were also improved in those mice that were treated with AGE and SAC. As ROS can be generated by the Aβ-glia or Aβ-metal ions interaction, independent action of AGE or SAC in decreasing Aβ and scavenging ROS represent a potential synergistic effect as a neuro-protective agent for the treatment of AD. In this study, AGE was also found to exert neuro-preserving action in vitro, when the neuronal cells were pre-treated with AGE for 48 hours and then challenged with toxic H2O2. These findings suggest that AGE's effect of preserving neuronal cells under oxidative stress is just not due to neutralizing ROS but modification of cell's defensive mechanisms against ROS. Our findings also suggest that the neuro-protection property of AGE against oxidative insults can be mediated by the action of SAC present in AGE. Antioxidant property of SAC was previously observed in different experimental models (Ide & Lau 1999, Imai et al. 1994, Maldonado et al. 2003). It was also reported that deposition of Aβ peptide in brain parenchyma facilitates cellular apoptosis by inducing caspases (Varma et al. 2009). AGE was found to have caspase 3 inhibitory effect, which might slow down the extent of neuronal loss caused by deposition of Aβ peptide (Jackson et al. 2002). Furthermore, treatment of AGE to Alzheimer's Tg2576 mice revealed decreased levels of hyperphosphorylation of tau protein in the central nervous system (Chauhan 2006).
It has been proposed that diffuse synaptic loss occurs in AD due to synergistic actions of generated ROS and Aβ oligomers (Selkoe 2002). Postmortem brain analysis of AD patients revealed a reduction in levels of pre-synaptic proteins synaptophysin and growth associated protein 32 (GAP 32) (Masliah et al. 2001). Decreased levels of pre-synaptic proteins that correspond with severity of the disease have also been observed. While the exact mechanism of synaptic damage by Aβ oligomers is still not fully understood, the alteration in NGF signaling pathway and abnormal calcium influxes following activation of NMDA receptors by globular Aβ oligomers play important roles (Yamamoto et al. 2007, Shankar et al. 2007). Since generalized neuronal and synaptic losses are major consequences as a result of Aβ deposition in the brain interstitium, the protection of neuron as well as synapses remains one of the major therapeutic strategies in AD. We have previously reported that rivastigmine increases the levels of two pre-synaptic proteins SNAP25 and synaptophysin in fetal primary neurons (Bailey & Lahiri), and reasoned that rivastigmine treatment imparts preservation of neurons and synapses. A similar neuro-preservative effect of memantine has also been observed in both primary neurons and differentiated PC12 cells (Bailey, Ray & Lahiri, unpublished data). However, whether these FDA-approved drugs can protect neurons in the presence of oxidative insult is not fully elucidated.
In this study, we have observed a time dependent loss of pre-synaptic proteins, SNAP25 and synaptophysin in the APP-Tg mice brain, consistent with the pathological consequence of gradual deposition of Aβ and subsequent neuro-inflammation. Interestingly, independent treatment of AGE and SAC significantly increased brain levels of SNAP25 and synaptophysin and in fact, restored their levels. We have also observed no significant change in brain levels of SNAP25 and synaptophysin in AGE treated mice sacrificed at the end of 4-week treatment period versus untreated Tg mice sacrificed at the beginning of the experiment. SAC (20mg/kg) also significantly increased the hippocampus based memory in APP-Tg mice. These findings demonstrate the potential for AGE and SAC to preserve pre-synaptic proteins and the pathology of the disease. Therefore, preservation of pre-synaptic proteins by AGE and SAC could be preserving neurons from ROS or Aβ mediated damage. Generalized loss of cholinergic neurons in AD results in severe cholinergic deficits. In this context, we have measured ChAT activity in differentiated human SK-N-SH cells as described earlier (Ray et al. 2009) and observed preservation of choline acetyltransferase (ChAT) activity by AGE and SAC from ROS mediated damage in differentiated human SK-N-SH cells (data not shown). Consistent with this hypothesis, we measured the levels one of the post synaptic markers PSD-95 in the brain lysate of AGE and SAC treated Tg mice, and observed an increasing trend of PSD-95 in AGE treated Tg mice, albeit not significantly (Ray & Lahiri, unpublished data). However, recent findings revealed that the change in the mRNA levels of PSD-95 in AD brain is region specific. For example, no change in PSD-95 mRNA levels was observed in hippocampus or occipital cortex of AD postmortem brain tissue, while the levels of PSD-95 were significantly decreased in inferior temporal cortex region (Proctor et al. 2010). One explanation of this could be a compensatory increase in the levels of post-synaptic proteins in response to severe synaptic loss seen in frontal cortex and hippocampus (Leuba et al. 2008). Earlier it was shown that SAC can destabilize Aβ fibrils in vitro (Gupta & Rao 2007). Furthermore, Aβ induced neuro-inflammation can also be ameliorated by SAC because of its ability to inhibit the activation of nuclear factor kappa beta (NFκB) (Ide & Lau 2001). Mechanistically, the property of Aβ lowering and consequent preservation of pre-synaptic proteins by AGE could be due to effects of SAC. Thus, this novel property of the garlic compounds may have significant implications in the context of drug development in AD.
In summary, our findings indicate that AGE treatment protects neuronal cells from ROS-mediated oxidative insults, and preserves the levels of pre-synaptic proteins that define pre-synaptic terminal structure. In addition, AGE treatment reverses ROS mediated decline in cholinergic function of the neuronal cells by increasing in levels of neuronal ChAT activity. Furthermore, loss of pre-synaptic proteins because of excessive deposition of Aβ in APP-Tg mice brain can also be prevented by oral AGE treatment. Previous studies demonstrate that AGE supplement can be beneficial in minimizing cardiovascular and diabetic risk factors contributing to AD. Because of the pleiotropic effects of AGE (discussed above), AGE and SAC can be developed as a potential therapeutic agent in the treatment of AD. Ultimately, these findings warrant evaluation of AGE's clinical potency in larger clinical settings.
Supplementary Material
Acknowledgement
We thank Bryan Maloney, Jason Bailey, Justin Long, Cindy Morgan and John Spence. This work was supported by grants from Alzheimer's Associations (Zenith Award); and the National Institutes of Health (AG18379 and AG18884) to DKL
Abbreviations used
- AD
Alzheimer disease
- Aβ
amyloid beta protein
- AGE
aged garlic extract
- APP
amyloid precursor protein
- ChAT
choline acetyltransferase
- ChEI
cholinesterase inhibitors
- LDH
lactate dehydrogenase
- LSM
low serum medium
- NGF
nerve growth factor
- NMDA
N-methyl-D-aspartate
- PPARγ
peroxisome proliferator-activated receptor γ
- PC12
rat pheochromocytoma
- RA
retinoic acid
- ROS
reactive oxygen species
- SAC
S-allyl-L-cysteine
- SNAP25
synaptosomal associated protein of 25 kDa
- Tg
transgenic
Footnotes
Conflict of interest: Dr. Lahiri is a member of the scientific advisory board of QR Pharma, Inc, Randon, PA. He and other co-authors of this study are declaring no financial relationship with “Wakunaga of America Ltd.”
References
- A.A 2009 Alzheimer's disease: Facts and figures. Alzheimer's Association. 2009 doi: 10.1016/j.jalz.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Alley GM, Bailey JA, Chen D, Ray B, Puli LK, Tanila H, Banerjee PK, Lahiri DK. Memantine lowers amyloid-beta peptide levels in neuronal cultures and in APP/PS1 transgenic mice. J Neurosci Res. 2009;88:143–154. doi: 10.1002/jnr.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008:CD005381. doi: 10.1002/14651858.CD005381.pub2. [DOI] [PubMed] [Google Scholar]
- Bailey JA, Lahiri DK. A novel effect of rivastigmine on pre-synaptic proteins and neuronal viability in a neurodegeneration model of fetal rat primary cortical cultures and its implication in Alzheimer's disease. J Neurochem. 112:843–853. doi: 10.1111/j.1471-4159.2009.06490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl C, Sagara Y. Mechanism of amyloid beta protein induced neuronal cell death: current concepts and future perspectives. J Neural Transm Suppl. 1997;49:125–134. doi: 10.1007/978-3-7091-6844-8_14. [DOI] [PubMed] [Google Scholar]
- Berginc K, Zakelj S, Ursic D, Kristl A. Aged garlic extract stimulates p-glycoprotein and multidrug resistance associated protein 2 mediated effluxes. Biol Pharm Bull. 2009;32:694–699. doi: 10.1248/bpb.32.694. [DOI] [PubMed] [Google Scholar]
- Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnology. 2007;5:3. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek C. Garlic reduces dementia and heart-disease risk. J Nutr. 2006;136:810S–812S. doi: 10.1093/jn/136.3.810S. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti L, Menghini L, Orlando G, et al. Antioxidant effects of garlic in young and aged rat brain in vitro. J Med Food. 2009;12:1166–1169. doi: 10.1089/jmf.2008.0176. [DOI] [PubMed] [Google Scholar]
- Chandra V, Pandav R, Dodge HH, Johnston JM, Belle SH, DeKosky ST, Ganguli M. Incidence of Alzheimer's disease in a rural community in India: the Indo-US study. Neurology. 2001;57:985–989. doi: 10.1212/wnl.57.6.985. [DOI] [PubMed] [Google Scholar]
- Chauhan NB. Anti-amyloidogenic effect of Allium sativum in Alzheimer's transgenic model Tg2576. J Herb Pharmacother. 2003;3:95–107. [PubMed] [Google Scholar]
- Chauhan NB. Effect of aged garlic extract on APP processing and tau phosphorylation in Alzheimer's transgenic model Tg2576. J Ethnopharmacol. 2006;108:385–394. doi: 10.1016/j.jep.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Chauhan NB, Sandoval J. Amelioration of early cognitive deficits by aged garlic extract in Alzheimer's transgenic mice. Phytother Res. 2007;21:629–640. doi: 10.1002/ptr.2122. [DOI] [PubMed] [Google Scholar]
- Chauhan NB, Siegel GJ. Effect of PPF and ALCAR on the induction of NGF- and p75-mRNA and on APP processing in Tg2576 brain. Neurochem Int. 2003a;43:225–233. doi: 10.1016/s0197-0186(03)00006-8. [DOI] [PubMed] [Google Scholar]
- Chauhan NB, Siegel GJ. Intracerebroventricular passive immunization with anti-Abeta antibody in Tg2576. J Neurosci Res. 2003b;74:142–147. doi: 10.1002/jnr.10721. [DOI] [PubMed] [Google Scholar]
- Coraci IS, Husemann J, Berman JW, Hulette C, Dufour JH, Campanella GK, Luster AD, Silverstein SC, El-Khoury JB. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer's disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am J Pathol. 2002;160:101–112. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Cole G. Alzheimer disease. JAMA. 2002;287:2335–2338. doi: 10.1001/jama.287.18.2335. [DOI] [PubMed] [Google Scholar]
- Durak I, Aytac B, Atmaca Y, Devrim E, Avci A, Erol C, Oral D. Effects of garlic extract consumption on plasma and erythrocyte antioxidant parameters in atherosclerotic patients. Life Sci. 2004a;75:1959–1966. doi: 10.1016/j.lfs.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Durak I, Kavutcu M, Aytac B, Avci A, Devrim E, Ozbek H, Ozturk HS. Effects of garlic extract consumption on blood lipid and oxidant/antioxidant parameters in humans with high blood cholesterol. J Nutr Biochem. 2004b;15:373–377. doi: 10.1016/j.jnutbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Cole GM. Why pleiotropic interventions are Needed for Alzheimer's disease. Mol Neurobiol. 2010;41:392–409. doi: 10.1007/s12035-010-8137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh C, Lahiri DK. Increased vulnerability of neuronal cell lines to sodium nitroprusside-mediated toxicity is caused by the decreased level of nitric oxide metabolites. J Mol Neurosci. 1999;13:77–92. doi: 10.1385/JMN:13:1-2:77. [DOI] [PubMed] [Google Scholar]
- Gillette Guyonnet S, Abellan Van Kan G, Andrieu S, et al. IANA task force on nutrition and cognitive decline with aging. J Nutr Health Aging. 2007;11:132–152. [PubMed] [Google Scholar]
- Greber S, Lubec G, Cairns N, Fountoulakis M. Decreased levels of synaptosomal associated protein 25 in the brain of patients with Down syndrome and Alzheimer's disease. Electrophoresis. 1999;20:928–934. doi: 10.1002/(SICI)1522-2683(19990101)20:4/5<928::AID-ELPS928>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Gupta VB, Rao KS. Anti-amyloidogenic activity of S-allyl-L-cysteine and its activity to destabilize Alzheimer's beta-amyloid fibrils in vitro. Neurosci Lett. 2007;429:75–80. doi: 10.1016/j.neulet.2007.09.042. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Hughes TF, Andel R, Small BJ, et al. Midlife Fruit and Vegetable Consumption and Risk of Dementia in Later Life in Swedish Twins. Am J Geriatr Psychiatry. 2009;18:413–420. doi: 10.1097/JGP.0b013e3181c65250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa M, Ryu K, Yoshida J, Ide N, Yoshida S, Sasaoka T, Sumi S. Antioxidant effects of tetrahydro-beta-carboline derivatives identified in aged garlic extract. Biofactors. 2002;16:57–72. doi: 10.1002/biof.5520160302. [DOI] [PubMed] [Google Scholar]
- Ide N, Lau BH. S-allylcysteine attenuates oxidative stress in endothelial cells. Drug Dev Ind Pharm. 1999;25:619–624. doi: 10.1081/ddc-100102217. [DOI] [PubMed] [Google Scholar]
- Ide N, Lau BH. Garlic compounds minimize intracellular oxidative stress and inhibit nuclear factor-kappa b activation. J Nutr. 2001;131:1020S–1026S. doi: 10.1093/jn/131.3.1020S. [DOI] [PubMed] [Google Scholar]
- Imai J, Ide N, Nagae S, Moriguchi T, Matsuura H, Itakura Y. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Med. 1994;60:417–420. doi: 10.1055/s-2006-959522. [DOI] [PubMed] [Google Scholar]
- Jackson R, McNeil B, Taylor C, Holl G, Ruff D, Gwebu ET. Effect of aged garlic extract on caspase-3 activity, in vitro. Nutr Neurosci. 2002;5:287–290. doi: 10.1080/10284150290032012. [DOI] [PubMed] [Google Scholar]
- Jacoby RO, Fox JG. Federal support for training in laboratory animal medicine should be reinstated. Lab Anim Sci. 1999;49:348. [PubMed] [Google Scholar]
- Kang JH, Cook N, Manson J, Buring JE, Grodstein F. A randomized trial of vitamin E supplementation and cognitive function in women. Arch Intern Med. 2006;166:2462–2468. doi: 10.1001/archinte.166.22.2462. [DOI] [PubMed] [Google Scholar]
- Kosuge Y, Koen Y, Ishige K, Minami K, Urasawa H, Saito H, Ito Y. S-allyl-L-cysteine selectively protects cultured rat hippocampal neurons from amyloid beta-protein- and tunicamycin-induced neuronal death. Neuroscience. 2003;122:885–895. doi: 10.1016/j.neuroscience.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Lahiri DK. Where the actions of environment (nutrition), gene and protein meet: beneficial role of fruit and vegetable juices in potentially delaying the onset of Alzheimer's disease. J Alzheimers Dis. 2006;10:359–361. doi: 10.3233/jad-2006-10402. discussion 363-354. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Chen D, Ge YW, Bondy SC, Sharman EH. Dietary supplementation with melatonin reduces levels of amyloid beta-peptides in the murine cerebral cortex. J Pineal Res. 2004;36:224–231. doi: 10.1111/j.1600-079X.2004.00121.x. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Farlow MR, Sambamurti K, Greig NH, Giacobini E, Schneider LS. A critical analysis of new molecular targets and strategies for drug developments in Alzheimer's disease. Curr Drug Targets. 2003;4:97–112. doi: 10.2174/1389450033346957. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Maloney B, Zawia NH. The LEARn model: an epigenetic explanation for idiopathic neurobiological diseases. Mol Psychiatry. 2009;14:992–1003. doi: 10.1038/mp.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK, Maloney B. The “LEARn” (Latent Early-life Associated Regulation) model integrates environmental risk factors and the developmental basis of Alzheimer's disease, and proposes remedial steps. Exp Gerontol. 2010;45:291–296. doi: 10.1016/j.exger.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H, Weiler R, Fischer P, Bancher C, Jellinger K, Floor E, Danielczyk W, Seitelberger F, Winkler H. Synaptic pathology in Alzheimer's disease: immunological data for markers of synaptic and large dense-core vesicles. Neuroscience. 1992;46:1–8. doi: 10.1016/0306-4522(92)90003-k. [DOI] [PubMed] [Google Scholar]
- Leuba G, Walzer C, Vernay A, Carnal B, Kraftsik R, Piotton F, Marin P, Bouras C, Savioz A. Postsynaptic density protein PSD-95 expression in Alzheimer's disease and okadaic acid induced neuritic retraction. Neurobiol Dis. 2008;30:408–419. doi: 10.1016/j.nbd.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Li T, Ito K, Sumi S, Fuwa T, Horie T. Protective effect of aged garlic extract (AGE) on the apoptosis of intestinal epithelial cells caused by methotrexate. Cancer Chemother Pharmacol. 2009;63:873–880. doi: 10.1007/s00280-008-0809-4. [DOI] [PubMed] [Google Scholar]
- Maldonado PD, Barrera D, Rivero I, Mata R, Medina-Campos ON, Hernandez-Pando R, Pedraza-Chaverri J. Antioxidant S-allylcysteine prevents gentamicin-induced oxidative stress and renal damage. Free Radic Biol Med. 2003;35:317–324. doi: 10.1016/s0891-5849(03)00312-5. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Jr., Morris JC. Altered expression of synaptic proteins occurs early during progression of Alzheimer's disease. Neurology. 2001;56:127–129. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- Morihara N, Ushijima M, Kashimoto N, Sumioka I, Nishihama T, Hayama M, Takeda H. Aged garlic extract ameliorates physical fatigue. Biol Pharm Bull. 2006;29:962–966. doi: 10.1248/bpb.29.962. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Berti V, Glodzik L, Pupi A, De Santi S, de Leon MJ. Pre-Clinical Detection of Alzheimer's Disease Using FDG-PET, with or without Amyloid Imaging. J Alzheimers Dis. 2010;20:843–854. doi: 10.3233/JAD-2010-091504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Masamoto K, Sumiyoshi H, Kunihiro K, Fuwa T. [Effect of raw and extracted-aged garlic juice on growth of young rats and their organs after peroral administration (author's transl)]. J Toxicol Sci. 1980;5:91–112. doi: 10.2131/jts.5.91. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Rinne JO, Kadir A, Langstrom B. The use of PET in Alzheimer disease. Nat Rev Neurol. 2010;6:78–87. doi: 10.1038/nrneurol.2009.217. [DOI] [PubMed] [Google Scholar]
- Opazo C, Huang X, Cherny RA, et al. Metalloenzyme-like activity of Alzheimer's disease beta-amyloid. Cu-dependent catalytic conversion of dopamine, cholesterol, and biological reducing agents to neurotoxic H(2)O(2). J Biol Chem. 2002;277:40302–40308. doi: 10.1074/jbc.M206428200. [DOI] [PubMed] [Google Scholar]
- Park JH, Park YK, Park E. Antioxidative and antigenotoxic effects of garlic (Allium sativum L.) prepared by different processing methods. Plant Foods Hum Nutr. 2009;64:244–249. doi: 10.1007/s11130-009-0132-1. [DOI] [PubMed] [Google Scholar]
- Pepeu G, Giovannini MG. Cholinesterase inhibitors and memory. Chem Biol Interact. 2010;187:403–408. doi: 10.1016/j.cbi.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Proctor DT, Coulson EJ, Dodd PR. Reduction in Post-Synaptic Scaffolding PSD-95 and SAP-102 Protein Levels in the Alzheimer Inferior Temporal Cortex is Correlated with Disease Pathology. J Alzheimers Dis. 2010;21:795–811. doi: 10.3233/JAD-2010-100090. [DOI] [PubMed] [Google Scholar]
- Ray B, Banerjee PK, Greig NH, Lahiri DK. Memantine treatment decreases levels of secreted Alzheimer's amyloid precursor protein (APP) and amyloid beta (Abeta) peptide in the human neuroblastoma cells. Neurosci Lett. 2009;470:1–5. doi: 10.1016/j.neulet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B, Lahiri DK. Neuroinflammation in Alzheimer's disease: different molecular targets and potential therapeutic agents including curcumin. Curr Opin Pharmacol. 2009;9:434–444. doi: 10.1016/j.coph.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Ray B, Simon JR, Lahiri DK. Determination of high-affinity choline uptake (HACU) and choline acetyltransferase (ChAT) activity in the same population of cultured cells. Brain Res. 2009;1297:160–168. doi: 10.1016/j.brainres.2009.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski M, Pankiewicz J, Scholtzova H, et al. Amyloid-beta deposition is associated with decreased hippocampal glucose metabolism and spatial memory impairment in APP/PS1 mice. J Neuropathol Exp Neurol. 2004;63:418–428. doi: 10.1093/jnen/63.5.418. [DOI] [PubMed] [Google Scholar]
- Schmidt ML, Zhukareva V, Newell KL, Lee VM, Trojanowski JQ. Tau isoform profile and phosphorylation state in dementia pugilistica recapitulate Alzheimer's disease. Acta Neuropathol. 2001;101:518–524. doi: 10.1007/s004010000330. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Clearing the brain's amyloid cobwebs. Neuron. 2001;32:177–180. doi: 10.1016/s0896-6273(01)00475-5. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashikanth KN, Basappa SC, Sreenivasa Murthy V. A comparative study of raw garlic extract and tetracycline on caecal microflora and serum proteins of albino rats. Folia Microbiol (Praha) 1984;29:348–352. doi: 10.1007/BF02875970. [DOI] [PubMed] [Google Scholar]
- Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:S69–74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- Varma R, Chai Y, Troncoso J, Gu J, Xing H, Stojilkovic SS, Mattson MP, Haughey NJ. Amyloid-beta induces a caspase-mediated cleavage of P2X4 to promote purinotoxicity. Neuromolecular Med. 2009;11:63–75. doi: 10.1007/s12017-009-8073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Kovacs DM, Yan R, Wong PC. The beta-secretase enzyme BACE in health and Alzheimer's disease: regulation, cell biology, function, and therapeutic potential. J Neurosci. 2009;29:12787–12794. doi: 10.1523/JNEUROSCI.3657-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Yaffe K. Metabolic syndrome and cognitive disorders: is the sum greater than its parts? Alzheimer Dis Assoc Disord. 2007;21:167–171. doi: 10.1097/WAD.0b013e318065bfd6. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Matsubara E, Maeda S, Minagawa H, Takashima A, Maruyama W, Michikawa M, Yanagisawa K. A ganglioside-induced toxic soluble Abeta assembly. Its enhanced formation from Abeta bearing the Arctic mutation. J Biol Chem. 2007;282:2646–2655. doi: 10.1074/jbc.M606202200. [DOI] [PubMed] [Google Scholar]
- Zatta P, Tognon G, Carampin P. Melatonin prevents free radical formation due to the interaction between beta-amyloid peptides and metal ions [Al(III), Zn(II), Cu(II), Mn(II), Fe(II)]. J Pineal Res. 2003;35:98–103. doi: 10.1034/j.1600-079x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.