Abstract
Both environmental stress and anxiety may represent important risk factors for Alzheimer's disease (AD) pathogenesis. Previous studies demonstrate that restraint stress is associated with increased amyloid beta (Aβ) and decreased brain-derived neurotrophic factor (BDNF) levels in the brain. Aβ deposition, synaptic loss, and neurodegeneration define major hallmarks of AD, and BDNF is responsible for the maintenance of neurons. In contrast to restraint stress, repeated injections of sub-anxiogenic doses of the corticotrophin releasing factor receptor agonist urocortin1 (Ucn1) administered in the basolateral amygdala (BLA) of rats elicits persistent anxiety-like responses. We hypothesized that both restraint stress and Ucn1-induced anxiety would contribute to a neurobiological abnormality that would change the levels of Aβ precursor protein (APP) and Aβ as well as BDNF and pre-synaptic markers. In the first experiment, adult male Wister rats (N=5) were subjected to three-hour restraint, as compared to unstressed controls. In the second experiment, adult male Wistar rats (N=6) were subjected to sub-anxiogenic doses of Ucn1 (6 fmol/100 nl) administered in the BLA, as compared to controls. Following each respective treatment, the social interaction (SI) test was performed to measure anxiety-like behavior. Protein studies were then conducted to quantify levels of APP, Aβ, BDNF and presynaptic proteins in the prefrontal cortex (PFC). In both experiments, we detected differences in either corticosterone levels or the SI test associated with a stress response. Our findings indicate that both restraint stress and Ucn1 administration in the BLA lead to increased APP and Aβ deposition. However, restraint-induced stress leads to reductions in the levels of BDNF and presynaptic markers, while Ucn1-induced anxiety is associated with increases in the levels of each respective protein. This demonstrates a convergent role for stress response and Ucn1-induced anxiety in the regulation of APP and Aβ, but opposing roles for each respective treatment in the regulation of BDNF and presynaptic markers.
Keywords: Aging, Alzheimer, Anxiety, APP, BDNF, Brain, CNS, Dementia, Mental illness, Restraint stress, Social interaction, Synaptic proteins, Ucn1
INTRODUCTION
Alzheimer's disease (AD) is a complex neurodegenerative disorder that is influenced by multiple factors including genetics, the environment, and gene × environment interactions (Lahiri et al., 2009). To date, a growing body of evidence has implicated psychological stress and anxiety as potential contributing factors to the development of AD (Wilson et al., 2005, Csernansky et al., 2006, Palmer et al., 2007). A major hallmark feature of AD is the deposition of the amyloid-β (Aβ) peptide. In patients with AD, Aβ peptide is deposited as plaques in the central nervous system (CNS), and Aβ deposition is associated with neurodegeneration in AD (Selkoe, 2008). Previous studies in rodents demonstrate that an acute stressor leads to increases in the formation of Aβ peptide, and these increases can be detected in the levels of both amyloid-β precursor protein (APP) messenger RNA (mRNA) and Aβ peptides (Rosa et al., 2005, Kang et al., 2007). Due to its effects on the levels of APP and Aβ in the CNS, these findings provide evidence that stress may be a potential contributing factor for the development of AD. Likewise, the downstream effects of stress on neurotrophic factors and presynaptic proteins also represent important molecular targets associated with AD pathophysiology (Tapia-Arancibia et al., 2008).
APP is a transmembrane protein that is cleaved by β and γ secretase to generate Aβ, and Aβ deposition forms plaques observed in AD patients (Sambamurti et al., 2002). APP can be cleaved in neuronal and non-neuronal cells by two different proteolytic pathways. For instance, the α-secretase protein cleaves APP within its Aβ domain to produce sAPPα. This ‘non-amyloidogenic pathway’ precludes the production of the Aβ peptide (Ray et al., 2009b). On the contrary, β-secretase cleaves the N-terminus of the Aβ peptide sequence of APP, and then γ-secretase further cleaves the protein to produce Aβ peptide, a mechanism defined as the ‘amyloidogenic’ pathway (Sambamurti et al., 2002). This mechanism leads to the production of Aβ with 42 amino acids residue (Aβ 1-42) and Aβ with 40 amino acids residue (Aβ 1-40). The larger form of Aβ (i.e. Aβ 1-42) leads to more aggregates than the shorter form (i.e. Aβ 1-40) in AD patients. Deposited Aβ peptide, especially Aβ (1-42), can lead to severe neuro-inflammation and neurodegeneration due to the production of reactive oxygen species (ROS). In AD patients, significant decreases in the levels of brain derived neurotrophic factor (BDNF) have been documented in hippocampal and cortical regions (Hock et al., 2000). In addition, previous studies demonstrate that a single or repeated restraint-induced stress in rats leads to decreases in BDNF mRNA levels in the hippocampus (Smith et al., 1995). BDNF and other neurotrophins regulate multiple cellular functions by supporting the development, the differentiation and the maintenance of neurons (Cohen-Cory et al., 1996). Therefore, neurotrophins are essential for normal brain function throughout life.
Corticotrophin releasing factor (CRF) plays a critical role in activating the behavioral and physiological responses to stress. Its biological function is carried out through activation of two receptor subtypes, corticotropin-releasing factor receptor 1 (CRFR1) and CRF receptor 2 (CRFR2). CRFR1 and CRFR2 receptors are 70% homologous at the protein level and contain a putative signal peptide, an extra cellular N-terminal domain (ECD1) and seven transmembrane domains. CRFR1 receptors are distributed throughout the brain, whereas the location of the CRFR2 receptors is more restricted to specific brain regions (Chalmers et al., 1995, Van Pett et al., 2000). The mammalian family of ligands for the CRFR1 and CRFR2 receptors includes CRF, urocortin I (UCN1), UCN II, and UCN III. These ligands differ in their tissue distribution and receptor pharmacology. For example, CRF binds to CRFR1 with high affinity, whereas UCN1 binds with high affinity to both CRFR1 and CRFR2 (Lovenberg et al., 1995). UCN II and UCN III appear to be selective for CRFR2 (Lewis et al., 2001, Inoue et al., 2003).
CRF is the principal neuroregulator of the hypothalamic-pituitary-adrenal (HPA) axis, and is the major mediator of the stress response (Bale and Vale, 2004). Following a stressor, CRF is released from the paraventricular nucleus (PVN) of the hypothalamus activating the HPA axis. CRF then binds to CRFR1 in the anterior pituitary resulting in the secretion of adrenal corticotrophic hormone (ACTH). ACTH then stimulates the release of glucocorticoids (i.e. corticosterone) from the adrenal cortex that act via a negative-feedback system to inhibit further CRF release from the hypothalamus. Corticosterone binds primarily to two receptor types including mineralocorticoid receptors (MR) and glucocorticoid receptors (GR). In response to stress, CRFR2 may function as an inhibitory or modulatory receptor to dampen HPA activation (Bale and Vale, 2004).
In humans, chronic stress is associated with the development of psychiatric disorders in susceptible individuals including anxiety and depression (Arborelius et al., 1999, Rosenkranz et al., 2010). Additionally, chronic stress leads to changes in the amygdala in rodents, a brain region implicated in both anxiety and fear-based learning (Bale and Vale, 2004). For instance, both electrical and pharmacological stimulation of the amygdala induces an enhanced cardiovascular response and behavioral arousal consistent with a fight-or-flight response (Kapp et al., 1982, al Maskati and Zbrozyna, 1989). By selectively targeting the basolateral amygdala (BLA) using pharmacological manipulation, previous studies demonstrate that the amygdala also regulates social aspects of anxiety and fear-based learning (Sanders and Shekhar, 1995, Sajdyk and Shekhar, 2000, Sajdyk et al., 2008). For example, mimicking repeated episodes of the stress response, repeated sub-anxiogenic doses of the CRF receptor agonist urocortin1 (Ucn1) microinjected into the basolateral amygdala (BLA) of rats once a day for 5 consecutive days (termed ‘priming’) leads to the development of pathological anxiety in that long-lasting behavioral changes are observed in social interaction (SI) and elevated plus maze (EPM) tests of anxiety (Rainnie et al., 2004). Likewise, rats primed with Ucn1 in the BLA demonstrated both increased anxiety-like behaviors as well as physiological sensitivity to intravenous sodium lactate infusions (Sajdyk and Shekhar, 2000). This physiological response to lactate infusion has been documented in subjects with panic or posttraumatic stress disorders, but not social or generalized anxiety disorders.
Given the previously documented involvement of stress and anxiety in the regulation of AD biomarkers, we hypothesized that restraint stress and repeated stimulation of CRF receptors within the BLA would lead to dysregulation in biomarkers associated with AD. We observed significant increases in total intracellular APP and Aβ peptide (x-40) with each respective condition, but only detected an increase in the level of Aβ (x-42) following three-hour (hr) restraint-induced stress. Interestingly, three-hr restraint stress negatively regulates BDNF and pre-synaptic proteins, while Ucn1 administration into the BLA positively regulates these proteins. Together, these findings reveal an important role for stress in the regulation of APP and Aβ in rats, and define BDNF as a potential marker of interest associated with synaptic integrity and the pathophysiology of AD.
MATERIALS AND METHODS
Animals
Male Wistar rats (275–300 g); Harlan Laboratories, Indianapolis, IN, USA) were used in these experiments. Upon arrival, the animals were housed individually in a temperature-controlled room (22°C) and had access to food and water ad libitum. The room was maintained at 12–12 hr light/dark cycle with light on at 0700 hr. Animal care procedures were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and the Guidelines of the Indiana University–Purdue University Indianapolis Institutional Animal Care and Use Committee.
Experimental Protocol
In experiment 1, the rats assigned to the ‘restraint stress’ group (N=5) were placed in a decapicone for three hrs (Brain TreeScientific, Braintree, MA, USA). The unstressed control rats (N=5) were kept in their home cage during this time period. Social interaction was measured immediately after the restraint stress. In experiment 2, the rats received a daily bilateral i.c. microinjection into the BLA with either vehicle [Veh,1% bovine serum albumin/100 nl/side] (N=6) or a sub-anxiogenic dose of Urocortin 1 [ (Ucn1), 6 fmoles/100 nl/side] (n=6) for five consecutive days. Urocortin1 (Sigma-Aldrich, St Louis, MO, USA) was dissolved in a vehicle of 1% bovine albumin in distilled water. The rats were tested for differences in social interaction, as compared to controls 30 minutes following the priming microinjection on D5. The brains were harvested for protein studies immediately after completion of the SI test of each experiment.
Surgical Procedures
For experiment 2, BLA cannulation surgeries were conducted on the rats at least 1 week following arrival from the supplier. Rats were anesthetized with isoflurane (2.5%) and placed in a stereotaxic apparatus. Bilateral injection cannulas (26 gauge; Plastics One, Roanoke, VA, USA) were implanted into the BLA [anteroposterior (AP): –2.1; mediolateral (ML): +5.0; dorsoventral (DV): –8.0; incisor bar: –3.3 mm] according to a standard stereotaxic atlas of the rat brain (Paxinos and Watson, 1986). The cannulas were secured to the skull with three stainless steel screws (2.8 mm; Plastics One) and locktite adhesive (Applied Industrial Technologies, Indianapolis, IN, USA). After completion of surgery, all animals received buprenex (Sigma, St. Louis, MO; 1 mg/kg, s.c.) and were placed on a warming pad until they had fully recovered from the anesthetic. The rats were allowed to recover in their home cages for at least 5 days prior to any behavioral testing.
Social interaction test
Following three-hr restraint or Ucn1- priming injections into the BLA, the social interaction (SI) test was utilized to characterize the anxiety-like response to each respective condition. A day before the SI test, the rats were exposed to the test room for at least 30 minutes and then placed into the SI apparatus alone for a 5 minute habituation session. A previously standardized version of the SI test was used to measure social interaction, in which the experimental rats were placed in an open field (0.9 m long × 0.9 m wide with walls 0.3 m high) with a novel male Wistar rat. During the five minute test, the total amount of time the treated rat initiated interaction with the partner rat was recorded (sniffing, grooming, etc.), as described previously (Shekhar and Keim, 1997). All tests were videotaped and independently scored at a later time by two individuals who were unaware of the animals’ treatment using cumulative stopwatches. Between each session, the apparatus were cleaned with 70% ethanol.
Dissection and preparation of brain lysate
After the SI test all animals were anaesthetized and promptly decapitated. The brains were carefully removed, and the frontal cortices were dissected and stored in -80°C freezer for further analysis. Brain dissections were carried out as described earlier (Sajdyk et al., 2008). To prepare protein lysates, the frozen tissues were homogenized in Tris-HCl buffered saline (TBS) [pH 7.6] containing 140 mM NaCl, 3 mM KCl, 25 mM Tris-HCl (pH 7.6), 5 mM EDTA, 2mM Phenanthroline, 0.5% SDS, EDTA-free protease inhibitor cocktail that was supplemented with protease inhibitor (Roche, Indianapolis, IN, USA). The resulting samples were then homogenized using ‘polytron’ homogenizer, and were centrifuged at 4°C (12,000g) for 10 minutes to obtain TBS soluble fraction of the tissue. This fraction was subjected to protein estimation using the Bradford assay, as previously described (Ray et al., 2009a). The resulting volume of supernatant containing a fixed amount of protein was analyzed for Western immunoblotting.
Corticosterone assay in the plasma in rats following restraint stress verses controls
After the SI test, trunk blood was collected from the rats following restraint stress and in controls. Whole blood was centrifuged at 10,000×g to collect plasma. Plasma samples were aliquoted and frozen at -80°C freezer. To measure corticosterone, a competitive EIA assay was used (Enzo Life Sciences, Plymouth Meeting, PA, USA). Briefly, 97.5 μl of plasma was added with 2.5 μl of corticosterone displacement reagent to displace bound corticosterone present in plasma. The plasma samples were diluted 1:20, and the assay was carried out as per manufacturer's protocol.
Western immunoblotting
Equal amounts of protein from the denatured lysates from each respective experimental and control groups were loaded in 10% Bis-Tris ‘Criterion’ polyacrylamide gels (BioRad, Hercules, CA, USA) and separated at 180 V for 2 hrs. Proteins were then electrophoretically transferred onto a PVDF membrane (BioRad) using the chilled transfer buffer (25 mM Tris base, 200mM Glycine and 20% Methanol) at 30V for 5 hrs at 4°C. After the protein transfer, the membrane was blocked in 5% nonfat milk in a Tris buffered saline, (pH 7.4) containing 0.05% Tween-20 (TBST) at room temperature (RT) for 1 hr. Different parts of the membrane were then probed with 22C11 (Amyloid Precursor protein, Millipore, Billerica, MA, USA) and β-actin (Sigma-Aldrich, USA) antibody. Brain homogenate (15μg of protein) was treated with 1(N) HCL to bring the pH down to 2.5 and incubated at room temperature for 15 minutes. The acid treated lysates were subsequently treated with 1(N) NaOH to bring back to normal neutral pH. Acid treatment increases detectable amount of neurotrophic factors in the sample (Okragly and Haak-Frendscho, 1997). Acid treated samples were mixed with Laemmli buffer and ran in a 10% SDS-PAGE, as previously described. The membrane was then probed with BDNF (1:1000 dil) (Abcam, Cambridge, MA, USA) and β-actin antibody (1: 100,000 dil). Levels of pre-synaptic proteins (SNAP25; 1:10,000dil and syntaxin6; 1: 3000 dil) were also measured in a similar fashion using specific antibodies (Millipore and BD Bioscience, Rockville, MD, USA, respectively). Detection of protein band signals was achieved by adding chemoluminescent buffer (GE, Buckinghamshire, UK) to the blot, which was immediately photographed using GE chemoluminescent detection film. A specific strip of the membrane was also probed with monoclonal anti-β-actin antibody (Sigma) for normalization purposes.
ELISA
ELISA was utilized to detect BDNF in an acid treated brain homogenate according to the manufacturer's protocol (Promega, Madison, WI, USA). The resulting BDNF levels (in pg/ml) were normalized by protein content of the lysates (μg/ml) and the unit was converted to pg/μg of lysate. Additionally, sensitive chemiluminescence Aβ assays were utilized to measure Aβ (x-40) and Aβ (x-42) in the rat cortical homogenate (Covance, Princeton, NJ, USA). The individual kit measures Aβ (x-40) and Aβ (x-42), respectively. (x-40) ELISA kit detects full length sequence of Aβ (1-40) and also peptides generated by N-terminal cleavages, such as Aβ (3-40), Aβ (11-40) etc. Similarly (x-42) ELISA kit detects full length sequence of Aβ (1-42) and also peptides generated by N-terminal cleavages, such as Aβ (3-42), Aβ (11-42) etc. Chemiluminescence signals obtained in Aβ ELISAs were normalized by the protein content of the lysates and plotted as ‘% control’.
Statistical analyses
Statistical analyses were performed by SPSS using a student's t-test, and the results were plotted using GraphPad Prism 4.0 software (GraphPad Software, La Jolla, CA, USA). All data were presented as mean ± SEM, and p-value < 0.05 were considered significant for all analyses. For SI, a one-tailed t-test was performed, which was consistent with our a priori hypothesis.
RESULTS
Both three-hr restraint and repeated Ucn1 injections into the BLA lead to decreases in social interaction in rats
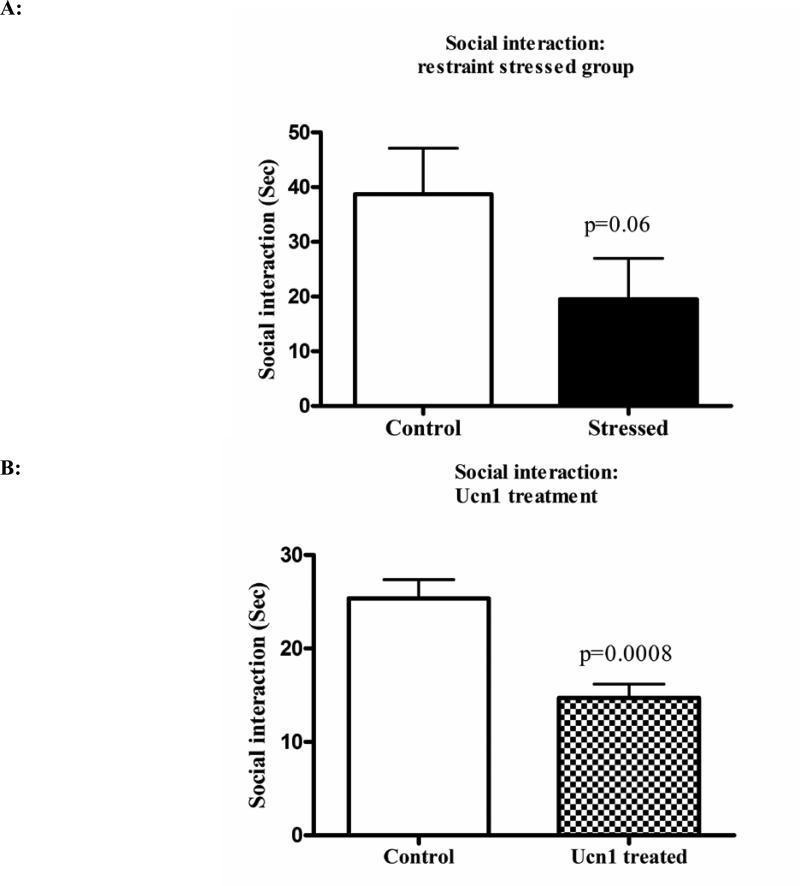
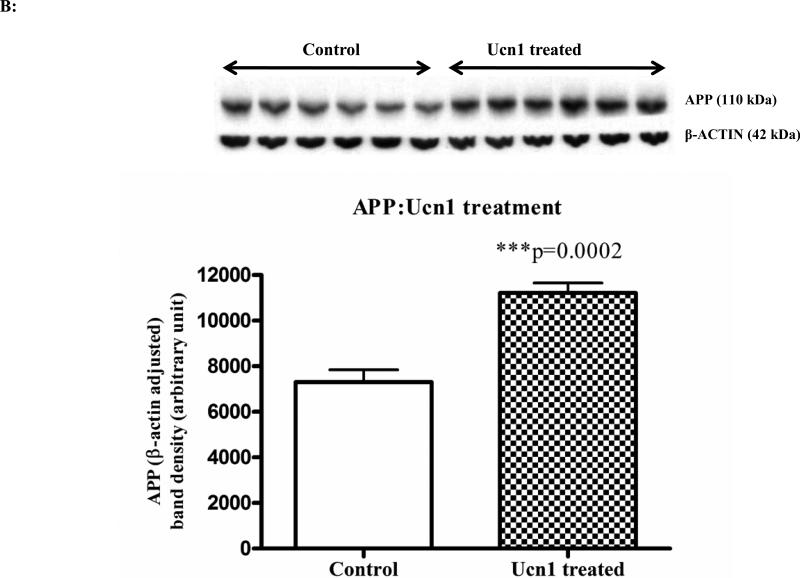
The social interaction test was performed to measure the effects of three-hr restraint and repeated Ucn1 administration on anxiety-like behavior in rats. Both three-hr restraint stress, and Ucn1 administration resulted in decreases in social interaction in rats (p=0.06 and 0.0008 respectively) (Figure 1A and 1B). To confirm that three-hr restraint stress leads to the activation of the HPA-axis, plasma corticosterone levels were determined following restraint stress. Plasma corticosterone was significantly increased in the plasma of rats following restraint stress versus controls (Figure 1C).
Figure 1A. Social interaction of control and stressed animals after three hrs of restraint stress.
The social interaction test was conducted to measure anxiety-like behavior in rats. Following three hrs of restraint stress, we have observed a trend in decreased social interaction in stressed rats (P=0.06 by one tailed t-test).
Figure 1B: Social interaction of vehicle and Ucn1-treated animals: Following repeated Ucn1 injections into the BLA, the rats showed significant decreases in social interactions consistent with increased anxiety-like behavior.
Figure 1C: Plasma corticosterone levels in control and restraint stressed animals: Plasma corticosterone was measured after three hrs restraint stress from both stressed and control animals. A competitive EIA assay was utilized to measure corticosterone and the levels were significantly higher in the stressed animals versus controls.
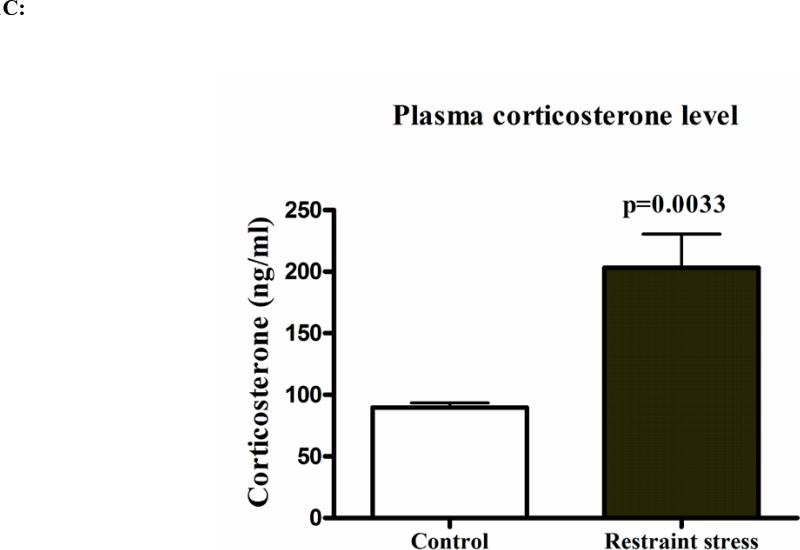
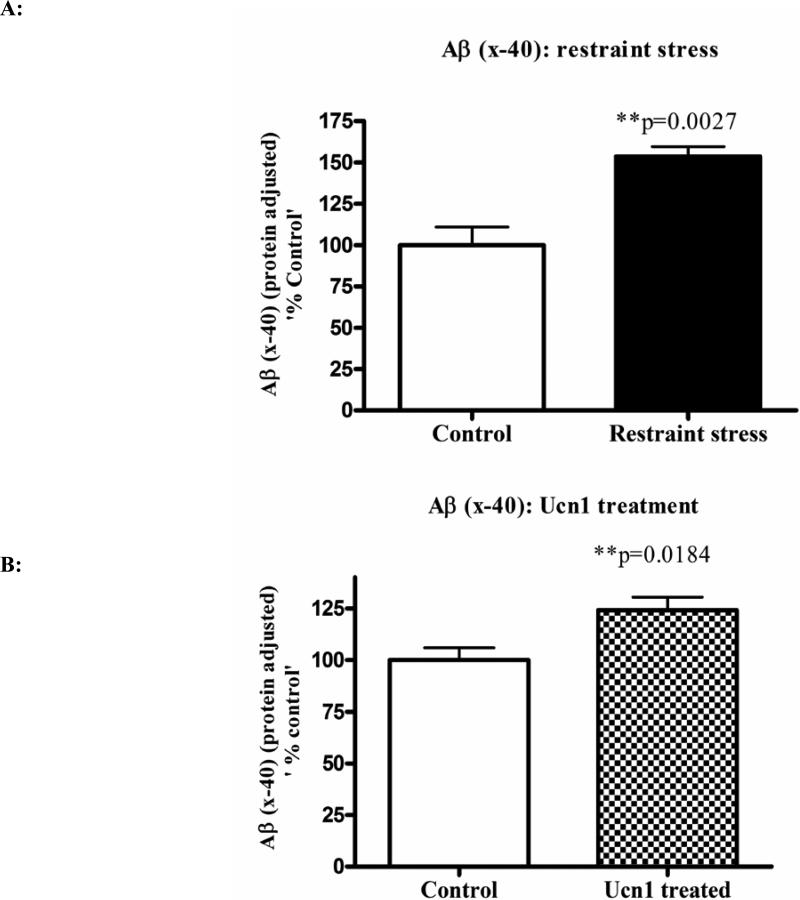
Both three-hr restraint and repeated Ucn1 injections into the BLA increases intracellular levels of APP and amyloid-β (x-40)
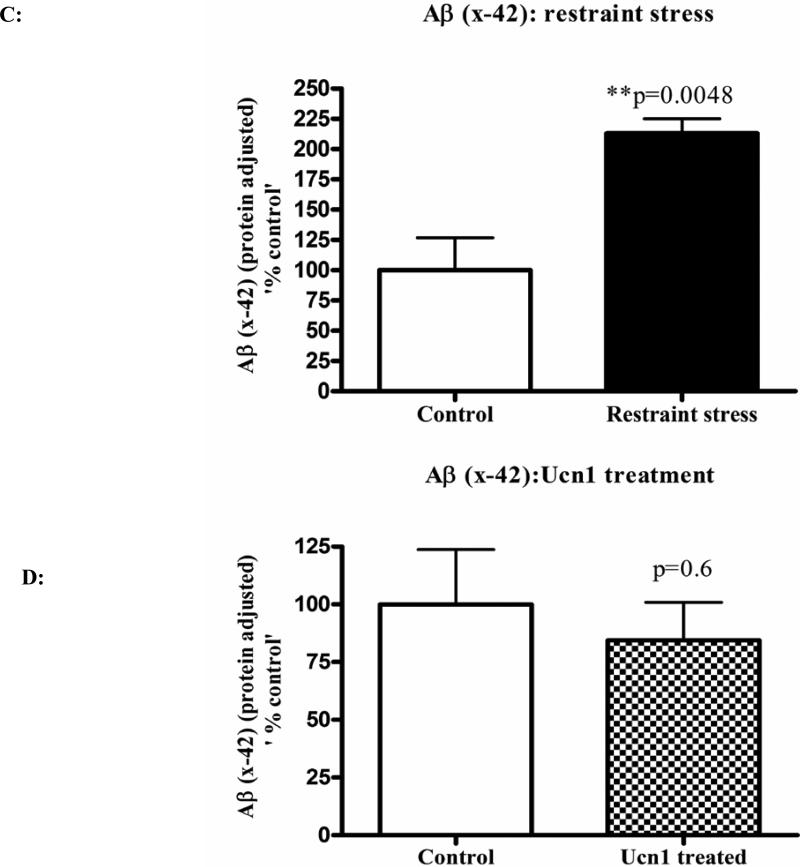
Following the social interaction test, the rats were decapitated, and brain lysates were isolated from cortical tissue. Western immunoblotting revealed a significant increase (p=0.008 and p=0.0002 respectively)) in the levels of total intracellular APP following both three-hr restraint and repeated Ucn1 injections into the CNS (Figure 2A and 2B). The total APP bands were normalized with β-actin bands. The levels of Aβ (x-40) were significantly increased (p=0.0027) in the cortex following both three-hr restraint stress and repeated Ucn1 injections verses controls (Figure 3A and 3B). While we observed a significant increase (p=0.0048) in the level of Aβ (x-42) in the frontal cortex following restraint stress (Figure 3C), repeated Ucn1 injections into the CNS did not affect cortical levels of Aβ (x-42) (p=0.6) (Figure 3D).
Figure 2A. Levels of total APP in the frontal cortex of control and restraint stressed animals.
Frontal cortex of control and stressed animals were homogenized in Tris- HCl buffer supplemented with protease inhibitors cocktail. Equal amount of protein samples from both control and stressed groups were loaded in 10% polyacrylamide mini gel, and Western immunoblot was performed to measure the levels of total APP. APP band density was normalized with β-actin bands. Normalized APP bands showed a significant increase in the frontal cortex of restrained stressed animals versus controls.
Figure 2B: Levels of total APP in the frontal cortex of control and Ucn1-treated animals: Western immunoblot analysis of frontal cortex brain lysate from vehicle and Ucn1-treated animals was performed as described previously in ‘Figure 3A’. Normalized APP band density showed a significant increase in frontal cortex of Ucn1-treated animals versus controls.
Figure 3A & 3B. Levels of Aβ (x-40) in the cortical lysates.
To determine the levels of Aβ (x-40) in the cortical lysates, a sensitive chemiluminescent ELISA was used. This sandwich ELISA measures Aβ (x-40) in rodent and human brain samples and has negligible cross reactivity with Aβ (x-42). The ELISA values were normalized by the total protein content of the lysates and plotted as ‘% control’. Normalized results revealed a significant increase in the levels of Aβ (x-40) in the cortical lysates of both restraint stressed and Ucn1-treated animals versus control/vehicle treated animals.
Figure 3C and 3D: Levels of Aβ (x-42) in the cortical lysates: Aβ (x-42) levels in the cortical lysates were measured by a sensitive and specific chemiluminescent ELISA as per manufacturer's protocol. Aβ (x-42) signals were normalized and plotted in the same way as described in ‘Figure 3A and 3B’. Normalized Aβ (1-42) showed a significant increase in the cortex of restraint stressed animals versus controls. However, Aβ (x-42) levels did not differ between the cortical lysates of vehicle and Ucn1-treated animals.
Three-hr restraint decreases and repeated Ucn1 injections the BLA increases intracellular levels of BDNF, respectively
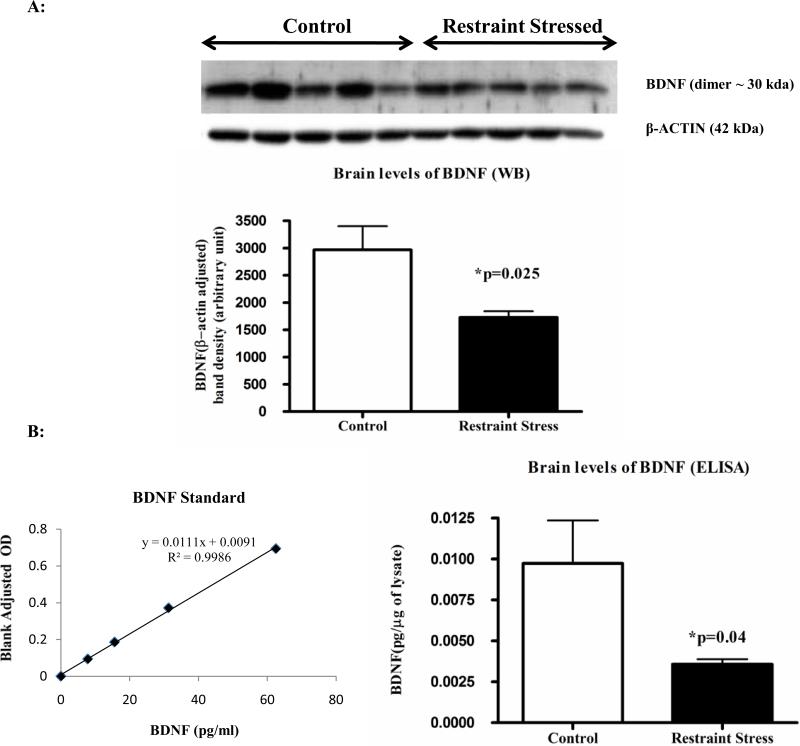
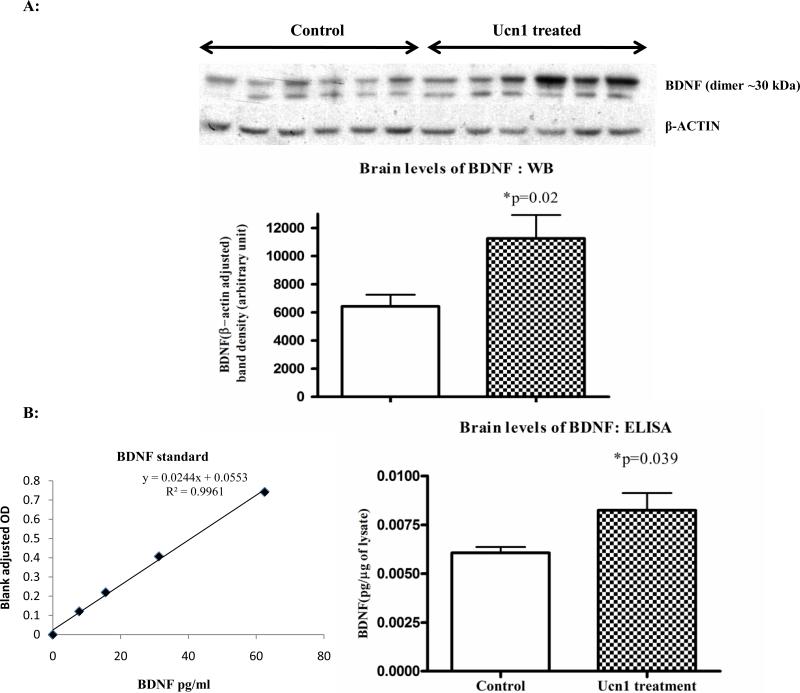
Following three-hr restraint stress, we observed a significant decrease (p=0.025) in the brain levels of BDNF by Western immunoblotting in the stressed rats versus controls (Figure 4A). On the contrary, the repeated injection of Ucn1 resulted in increased levels of BDNF (p=0.02) in the frontal cortex of Ucn1 primed animals verses controls (Figure 5A). These results were further confirmed utilizing an ELISA that is sensitive to BDNF detection (Figure 4B and 5B). For ELISA, individual BDNF values (pg/ml) were converted to pg/μg by normalizing protein content of the corresponding brain lysate as measured by the Bradford assay.
Figure 4A. Levels of BDNF in the frontal cortex of control and restraint stressed animals: Western immunoblot analysis.
Acid treated frontal cortex lysates were analyzed by western immunoblotting to detect the levels of BDNF (for details see ‘Materials and Methods’). BDNF band densities (primarily BDNF dimer) were normalized by β-actin signals. Normalized BDNF showed a significant decrease in the cortex of animals underwent 3 hrs restraint stress versus controls.
Figure 4A: Levels of BDNF in the frontal cortex of control and restraint stressed animals: ELISA analysis. To confirm the BDNF Western immunoblot results, BDNF levels were measured in acid treated cortical lysates by a sensitive colorimetric ELISA. BDNF values (pg/ml) were converted to pg/μg, normalized by protein contents of the lysates and plotted. BDNF ELISA also revealed a significant decrease in the cortex of restraint stressed animals versus controls.
Figure 5A. Levels of BDNF in the frontal cortex of vehicle and Ucn1-treated animals: Western immunoblot analysis.
BDNF Westernblotting was performed with the cortical lysates of vehicle and Ucn1-treated animals in a similar way described in ‘Figure 4A’. β-actin adjusted BDNF band densities (BDNF dimer) showed a significant increase in the cortical lysates of Ucn1-treated animals versus vehicle. The lower band observed in this figure is due to secondary ‘artifact’ and excluded from the analyses.
Figure 5B: Levels of BDNF in the frontal cortex of vehicle and Ucn1-treated animals: ELISA analysis. To confirm the findings of ‘Figure 3A’, a sensitive ELISA was used to determine the levels of BDNF in the acid treated cortical lysates of vehicle and Ucn1-treated animals. BDNF values (pg/ml) were converted to pg/μg, normalized by protein contents of the lysates and plotted. Results showed a significant increase in the cortical lysates of Ucn1-treated animals versus vehicle.
Three-hr restraint decreases whereas repeated Ucn1 injections into the BLA increases intracellular levels of pre-synaptic proteins, respectively
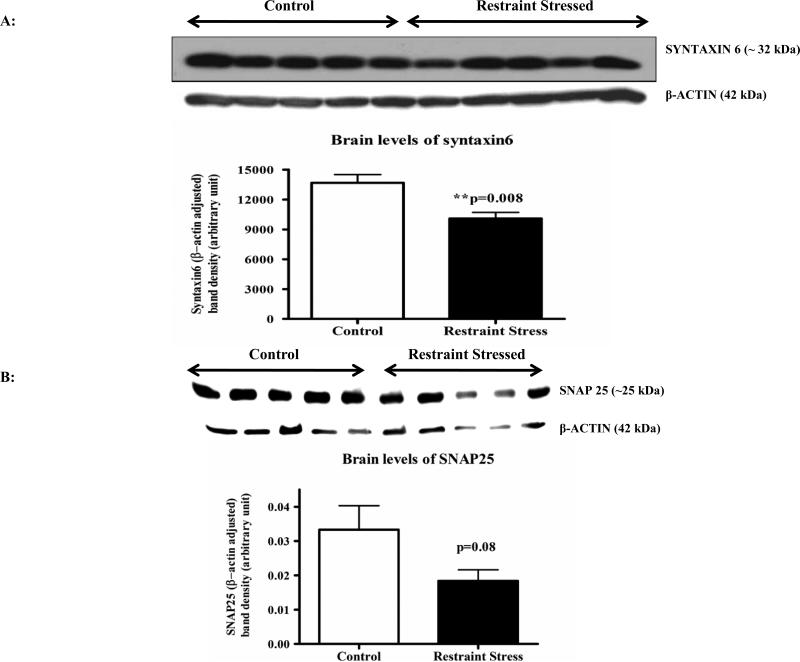
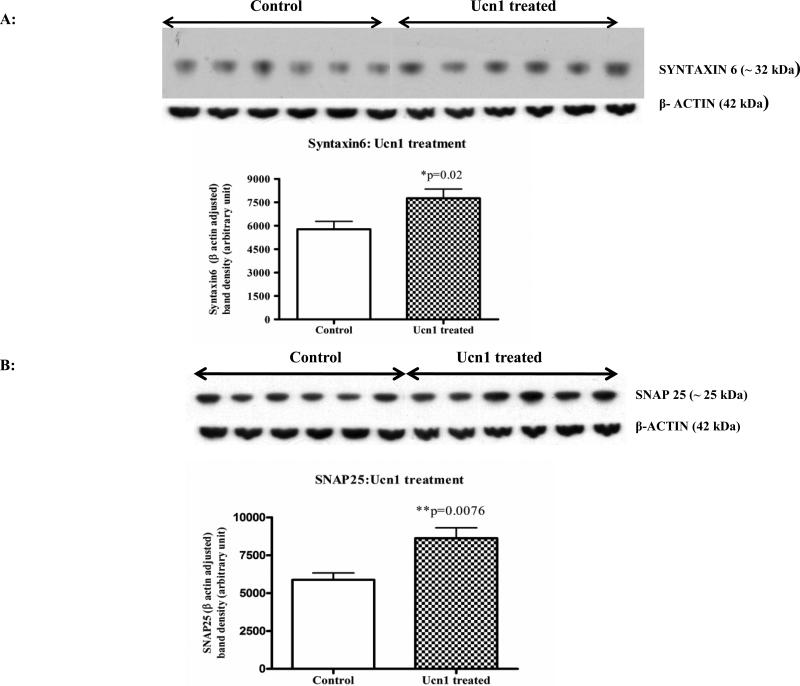
Pre-synaptic markers provide an important biological measure of neuronal integrity as well as synaptic plasticity. Western immunoblot analyses of pre-synaptic proteins syntaxin6 revealed a significant decrease (p=0.008) in the cortex following three hr restraint versus controls (Figure 6A), and a decreasing trend for SNAP25 levels (p=0.08; Figure 6B). Following repeated Ucn1 injections, significant increases in the levels of syntaxin6 (p=0.02) and SNAP25 (p=0.0076) were detected in the cortical lysate versus controls (Figure 7A and 7B).
Figure 6A. Levels of syntaxin6 in the cortical lysates of control and restraint stressed animals.
Western immunoblot analyses of cortical lysates of control and restrained stressed animals were carried out with specific monoclonal syntaxin6 antibody, which recognizes one of the important pre-synaptic proteins syntaxin6. The bands were normalized by β-actin signals. Normalized syntaxin6 bands showed a significant decrease in the lysates of restraint stressed animals versus controls.
Figure 6B: Levels of SNAP25 in the cortical lysates of control and restraint stressed animals: Western immunoblot analyses of the same samples mentioned in ‘Figure 6A’ for another pre-synaptic protein SNAP25 showed a decreasing trend (p=0.08) in the cortical lysates of restraint stressed animals versus controls.
Figure 7A. Levels of SNAP25 in the cortical lysates of vehicle and Ucn1-treated animals.
Western immunoblot analyses of cortical lysates of vehicle and Ucn1-treated animals were carried out to detect the levels of cortical SNAP25. SNAP25 bands were normalized by β-actin signals and plotted. Normalized SNAP25 showed a significant increase in the cortex of Ucn1-treated animals versus vehicle treated animals.
Figure 7B: Levels of syntaxin6 in the cortical lysates of vehicle and Ucn1-treated animals: Western immunoblot analyses revealed a significant increase in the levels of syntaxin6 in the cortical lysates of Ucn1-treated animals versus vehicle treated animals.
Discussion
In the present study, we observed reductions in social interaction associated with both three-hr restraint stress and Ucn1 repeated injections into the BLA. Additionally, we found significant increases in total intracellular APP and Aβ peptide (x-40) associated with each respective condition. However, a significant increase was only observed in the level of Aβ (x-42) following three-hr restraint-induced stress. While we observed significant decreases in the level of BDNF in the cortical lysate from rats after three-hr restraint stress, Ucn1 administration resulted in significant increases in the level of BDNF in the cortex. Western immunoblotting and ELISA studies revealed decreases in the levels of pre-synaptic proteins (syntaxin6 and SNAP25) in the cortical lysate following three-hr restraint stress; however, the levels of SNAP25, a pre-synaptic protein of SNARE complex, was significantly increased in the cortical lysate following Ucn1-induced anxiety.
Late onset AD is the most common cause of dementia in the elderly population, and often displays a sporadic mode of transmission (Lahiri et al., 2003). As opposed to a purely genetic approach to evaluate the risk factors, more emphasis in recent years has focused on the contribution of environmental factors associated with AD (Migliore and Coppede, 2009, Zawia et al., 2009). For example, an epidemiological study in Nigeria revealed no association between the well known risk gene APOE ε 4 and AD in a local population, which further emphasizes the role of environmental factors in the pathogenesis of AD (Gureje et al., 2006). Consistent with other environmental factors that underlie AD, recent studies indicate that stress may also represent an important risk factor (Smith et al., 2005, Wilson et al., 2005).
A previous study demonstrated that acute restraint stress leads to increases in the levels of Aβ in brain interstitial fluid (ISF), and the effect was mediated by a corticotropin-releasing factor (CRF)-dependent mechanism (Kang et al., 2007). In this study, it was postulated that the increase in the levels of ISF Aβ is likely due to the increase in neuronal activity associated with CRF, which was increased in response to stress. In a cell culture based study, APP processing leads to a shift towards an intracellular route following stimulation by heat shock protein in human astrocyte (Shepherd et al., 2000). Additionally, a recent study using gene expression profiling demonstrates a 1.64-fold increase in APP (App) expression in DBA/2J mice following the force swim test, a behavioral test that activates a stress response (Tsolakidou et al., 2010). However, the effect of acute restraint stress on APP has not yet been addressed. In the present study, we observed a significant increase (~55%) in the levels of APP in frontal cortex following restraint stressed versus unstressed rats. Because APP was significantly increased in the frontal cortical lysate following restraint stress, we further assayed the levels of Aβ peptide (both x-40 and x-42). We detected significant increases in Aβ peptide (both x-40 and x-42). This increase in Aβ can either result from increased intracellular processing of APP, the up-regulation of BACE-1 or a direct effect that can be attributed to the action of CRF on neurons (Tamagno et al., 2005, Kang et al., 2007). While the exact mechanism that underlies this increase in Aβ peptides following restraint stress is not fully understood, a shift in APP processing towards the intracellular compartment lead to an “amyloidogenic state” in the neuron (LaFerla et al., 2007). In the present study, we observed a significant increase in corticosterone levels following restraint stress. Therefore, increases in corticosterone and other hormones in response to the activation of the HPA axis following a stressful condition may independently up-regulate the levels APP and BACE-1, which may ultimately contribute to the formation of Aβ peptides (Green et al., 2006). A previous study has also shown that CRF itself can independently stimulate neurons that can lead to the release of more Aβ peptides (Kang et al., 2007).
In addition to the effects on APP and Aβ, we also detected significant decreases in the levels of BDNF in the frontal cortex following restraint stress versus unstressed rats. In a previous study, a significant decrease in BDNF positive cells was detected following chronic isolation in frontal cortex and hippocampus that is consistent with our findings (Chen et al., 2008). Additionally, a previous study found that 6 hrs of restraint stress in rats is also associated with significant decreases in BDNF mRNA levels in the hippocampus (Murakami et al., 2005). Because BDNF plays a critical role in the regulation of synaptic plasticity, we hypothesized that acute restraint stress would lead to decreases in the levels of pre-synaptic proteins. We found significant decreases in the levels of the pre-synaptic protein synataxin-6 in the prefrontal cortical lysate following restraint stressed versus unstressed rats. In addition, the levels of the pre-synaptic protein SNAP25 were also lower in the stressed group; however, the difference did not reach significance. Consistent with these findings, previous findings indicate that BDNF treatment in organotypic hippocampal slice culture results in an increase in both the number of synapses as well as docked synaptic vesicles (Tyler and Pozzo-Miller, 2001). Interestingly, the lack of BDNF can also play important roles in trafficking of APP. For example, the exogenous administration of BDNF in primary neuron culture leads to decreases in Aβ production mediated through up-regulation of SORLA (Rohe et al., 2009). Hence, decreased levels of BDNF following restraint stress may also contribute to the increases in Aβ production following restraint stress.
Stress resulting from physical restraint leads to a complex physiological response, and involves multiple structures in the CNS including the amygdala and the hypothalamus. To specifically target the effects of the amygdaloid nuclei on APP and Aβ, site directed injections of Ucn1 were conducted into the BLA, a brain region that is known to mediate the effects of CRF on anxiety. Interestingly, different molecular sequelae were observed following repeated activation of CRF receptors with Ucn1 treatment, as compared to restraint stress. Ucn1 is a peptide that shows sequence homology with both urotensin 1 and CRF (Vaughan et al., 1995), and produces anxiety-like behavior in rodents (Moreau et al., 1997). In fact, the site directed injection of Ucn1 into the BLA of rats acts as a potent anxiogenic peptide, and leads to a more robust effect on anxiety-like behavior than that of CRF (Sajdyk et al., 1999). Therefore, we hypothesized that the levels of APP and Aβ peptides would also be increased in Ucn1-treated rat in the frontal cortex. In the present study, we observed a robust increase in the levels of APP in Ucn1 injected rats consistent with our findings in rats following restraint stress. Additionally, we observed a significant increase in the level of Aβ (x-40) in the frontal cortical lysate of Ucn1-treated rats versus untreated controls. However, the levels of Aβ (x-42) were left unchanged. These findings suggest that the increases APP may underlie the increases seen in Aβ peptides that were observed following both Ucn1 treatment and restraint stress.
In contrary to the decreases observed following restraint stress, we observed significant increases in the levels of BDNF in the frontal cortex of Ucn1 injected rats. Although chronic stressors increase APP and other markers of AD in adult rodents (Dong et al., 2008), a similar increase in cortical BDNF levels are seen in very early adolescent rats following short-term social isolation stress where significant synaptic reorganization is thought to occur (Meng et al., 2011). Additionally, a previous study demonstrates that CRFR1 receptor signaling in cerebellar granular cells leads to increases in BDNF mRNA levels (Bayatti et al., 2005). Likewise, CRFR1 receptor signaling in locus coeruleus also increases BDNF signaling via ERK-MAPK cascade (Bayatti et al., 2005). Since Ucn1 also has primary stimulatory effects on CRFR2 receptor, the increase in the levels of BDNF may potentially be due to CRFR2 mediated effects on neurons projecting from the amygdala to the prefrontal cortex.
Consistent with the increase of BDNF in the frontal cortex following Ucn1 injections into the BLA, we also observed significant increases in the levels of pre-synaptic proteins SNAP25 and syntaxin6 in Ucn1 injected rats versus controls. Hence, repeated Ucn1 injections into the BLA nucleus results in a complex cascade of signal transduction events. Our findings suggest that the increases in APP and Aβ peptide and BDNF may result from the effects on CRFR1 receptors. Likewise, the increases in BDNF may underlie the increases in the levels of pre-synaptic proteins SNAP25 and syntaxin6. A previous study has revealed that decreases in BDNF levels are mediated by Aβ (1-42) (Hjorth et al., 2010). Interestingly, the BDNF level is associated with phagocytosis of Aβ (1-42) by macrophases (Asami et al., 2006). In a cell culture model, BDNF was found to protect neurons from Aβ (1-42)-mediated damage. Therefore, increases in the levels of BDNF may be responsible for the lack of increase in Aβ (x-42) levels in the frontal cortex in Ucn1 injected rats. Ultimately, the increases seen in BDNF and pre-synaptic proteins could be due to compensatory mechanism in response to chronic Ucn1 injections into the BLA associated with increases in APP and Aβ generation.
Mechanistically, whether the aforementioned restraint-induced stress or Ucn1-induced anxiety triggers cellular oxidative stress remains unclear. It is known that aging and neurodegenerative disorders are associated with increased cellular oxidative stress; however, we have not directly assayed oxidative stress markers in the present work because of the experimental design. Nevertheless, we do not rule out the possibility that the induced stress tested in this work may cause oxidative stress resulting from different reactive oxygen species such as superoxide, hydrogen peroxide and hydroxyl radicals. The effect of oxidative stress and growth factors in the regulation of neuronal gene expression, such as APP and BACE, has been studied (Lahiri and Nall, 1995, Ge et al., 2004, Lahiri et al., 2006). Further, transcriptional activation of APP gene by stress was previously reported (Dewji et al., 1995). The interaction of various transcription factors with the BACE1 promoter can modulate synaptic plasticity, neuronal apoptosis and oxidative stress, which are relevant to the pathogenesis of AD (Lahiri et al., 2006). It would be interesting to speculate the underlying mechanism. Nuclear factor erythroid 2-related factor 2 (NrF2), which protects against Aβ (Kanninen et al., 2008), is known to play a role in antioxidant defense in the cell and has been shown to be neuroprotective in an animal model making an interesting target for studies into preventing or reversing neurodegeneration in dementias (de Vries et al., 2008, Ohtsuji et al., 2008). Analysis of the transcription factor binding sites on both APP and BACE, using the TESS program (Transcriptional Element Search System) reveals sites for heat shock element and the transcription factor Nrf2 in the APP and BACE1 promoter (Lahiri and Robakis, 1991, Sambamurti et al., 2004). Another study suggests that p-hydroxybenzyl alcohol (HBA) protects against brain damage by modulating cytoprotective genes, such as NrF2, and neurotrophic factors, including BDNF (Kam et al., 2011). The above results taken with our present data suggest that restraint-induced stress may cause cellular oxidative stress, which results in down regulation of cytoprotective genes such as BDNF and in upregulation of APP gene expression leading to the amyloidogenic pathway. Thus, restraint-induced stress triggers APP and Aβ peptide expression at the cost of cytoprotective BDNF and synaptic proteins. Our present study would have great translational implication in understanding the neurobiology and therapeutic targets for the stress-related psychiatric disorders, such as AD, anxiety, depression and schizophrenia.
In conclusion, our findings identified significant increases in APP and Aβ levels following both restraint stress and sub-anxiogenic doses of Ucn1 administered into the BLA. Because the regulation of APP and Aβ deposition represent biological markers that are associated with AD pathogenesis, environmental stressors and persistent anxiety may represent predisposing factors that may contribute to AD pathogenesis. Furthermore, these findings demonstrate a negative role for restraint stress and a positive role for Ucn1-induced anxiety in the regulation of BDNF and presynaptic markers. These results indicate that the levels of APP and Aβ are likely regulated by distinct mechanisms from BNDF and pre-synaptic markers following restraint stress and repeated Ucn1 injections into the BLA. Ultimately, the effects of stress and persistent anxiety likely define important factors that can contribute to AD. Overall, the present molecular-driven study in vivo may contribute a great translational impact for AD and anxiety, and pave the way for identifying biomarkers and novel therapeutic strategies for these devastating disorders.
RESEARCH HIGHLIGHTS.
➢ Environmental stress and anxiety may be risk factors for Alzheimer's disease (AD).
➢ We observed that both restraint stress and Ucn1 inj. in the BLA lead to changes in APP and Aβ.
➢ Restraint-induced stress leads to reductions in the levels of BDNF and presynaptic markers.
➢ Ucn1-induced anxiety is associated with increases in the levels of each respective protein.
➢ Therefore, this study identifies putative biomarkers for AD and anxiety disorders.
Acknowledgements
We thank Justin Long and Jason bailey for technical assistance. We are also thankful to Philip Johnson for fruitful discussion. This work was supported by grants from the National Institute of Health (AG 18379 and AG 18884) to DKL and (RO1 MH065702) to AS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFRERENCE
- al Maskati HA, Zbrozyna AW. Stimulation in prefrontal cortex area inhibits cardiovascular and motor components of the defence reaction in rats. J Auton Nerv Syst. 1989;28:117–125. doi: 10.1016/0165-1838(89)90084-2. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Asami T, Ito T, Fukumitsu H, Nomoto H, Furukawa Y, Furukawa S. Autocrine activation of cultured macrophages by brain-derived neurotrophic factor. Biochem Biophys Res Commun. 2006;344:941–947. doi: 10.1016/j.bbrc.2006.03.228. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bayatti N, Hermann H, Lutz B, Behl C. Corticotropin-releasing hormone-mediated induction of intracellular signaling pathways and brain-derived neurotrophic factor expression is inhibited by the activation of the endocannabinoid system. Endocrinology. 2005;146:1205–1213. doi: 10.1210/en.2004-1154. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JX, Li W, Zhao X, Yang JX. Effects of the Chinese traditional prescription Xiaoyaosan decoction on chronic immobilization stress-induced changes in behavior and brain BDNF, TrkB, and NT-3 in rats. Cell Mol Neurobiol. 2008;28:745–755. doi: 10.1007/s10571-007-9169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S, Escandon E, Fraser SE. The cellular patterns of BDNF and trkB expression suggest multiple roles for BDNF during Xenopus visual system development. Dev Biol. 1996;179:102–115. doi: 10.1006/dbio.1996.0244. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, Morris JC. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiatry. 2006;163:2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries HE, Witte M, Hondius D, Rozemuller AJ, Drukarch B, Hoozemans J, van Horssen J. Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic Biol Med. 2008;45:1375–1383. doi: 10.1016/j.freeradbiomed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Dewji NN, Do C, Bayney RM. Transcriptional activation of Alzheimer's beta-amyloid precursor protein gene by stress. Brain Res Mol Brain Res. 1995;33:245–253. doi: 10.1016/0169-328x(95)00131-b. [DOI] [PubMed] [Google Scholar]
- Dong H, Yuede CM, Yoo HS, Martin MV, Deal C, Mace AG, Csernansky JG. Corticosterone and related receptor expression are associated with increased beta-amyloid plaques in isolated Tg2576 mice. Neuroscience. 2008;155:154–163. doi: 10.1016/j.neuroscience.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge YW, Maloney B, Sambamurti K, Lahiri DK. Functional characterization of the 5' flanking region of the BACE gene: identification of a 91 bp fragment involved in basal level of BACE promoter expression. FASEB J. 2004;18:1037–1039. doi: 10.1096/fj.03-1379fje. [DOI] [PubMed] [Google Scholar]
- Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer's disease. J Neurosci. 2006;26:9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureje O, Ogunniyi A, Baiyewu O, Price B, Unverzagt FW, Evans RM, Smith-Gamble V, Lane KA, Gao S, Hall KS, Hendrie HC, Murrell JR. APOE epsilon4 is not associated with Alzheimer's disease in elderly Nigerians. Ann Neurol. 2006;59:182–185. doi: 10.1002/ana.20694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth E, Frenkel D, Weiner H, Schultzberg M. Effects of immunomodulatory substances on phagocytosis of abeta(1-42) by human microglia. Int J Alzheimers Dis. 2010 doi: 10.4061/2010/798424. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock C, Heese K, Hulette C, Rosenberg C, Otten U. Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch Neurol. 2000;57:846–851. doi: 10.1001/archneur.57.6.846. [DOI] [PubMed] [Google Scholar]
- Inoue K, Valdez GR, Reyes TM, Reinhardt LE, Tabarin A, Rivier J, Vale WW, Sawchenko PE, Koob GF, Zorrilla EP. Human urocortin II, a selective agonist for the type 2 corticotropin-releasing factor receptor, decreases feeding and drinking in the rat. J Pharmacol Exp Ther. 2003;305:385–393. doi: 10.1124/jpet.102.047712. [DOI] [PubMed] [Google Scholar]
- Kam KY, Yu SJ, Jeong N, Hong JH, Jalin AM, Lee S, Choi YW, Lee CK, Kang SG. p-hydroxybenzyl alcohol prevents brain injury and behavioral impairment by activating Nrf2, PDI, and neurotrophic factor genes in a rat model of brain ischemia. Mol Cells. 2011 doi: 10.1007/s10059-011-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JE, Cirrito JR, Dong H, Csernansky JG, Holtzman DM. Acute stress increases interstitial fluid amyloid-beta via corticotropin-releasing factor and neuronal activity. Proc Natl Acad Sci U S A. 2007;104:10673–10678. doi: 10.1073/pnas.0700148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanninen K, Malm TM, Jyrkkanen HK, Goldsteins G, Keksa-Goldsteine V, Tanila H, Yamamoto M, Yla-Herttuala S, Levonen AL, Koistinaho J. Nuclear factor erythroid 2-related factor 2 protects against beta amyloid. Mol Cell Neurosci. 2008;39:302–313. doi: 10.1016/j.mcn.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Gallagher M, Underwood MD, McNall CL, Whitehorn D. Cardiovascular responses elicited by electrical stimulation of the amygdala central nucleus in the rabbit. Brain Res. 1982;234:251–262. doi: 10.1016/0006-8993(82)90866-6. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Farlow MR, Sambamurti K, Greig NH, Giacobini E, Schneider LS. A critical analysis of new molecular targets and strategies for drug developments in Alzheimer's disease. Curr Drug Targets. 2003;4:97–112. doi: 10.2174/1389450033346957. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Ge YW, Rogers JT, Sambamurti K, Greig NH, Maloney B. Taking down the unindicted co-conspirators of amyloid beta-peptide-mediated neuronal death: shared gene regulation of BACE1 and APP genes interacting with CREB, Fe65 and YY1 transcription factors. Curr Alzheimer Res. 2006;3:475–483. doi: 10.2174/156720506779025224. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Maloney B, Zawia NH. The LEARn model: an epigenetic explanation for idiopathic neurobiological diseases. Mol Psychiatry. 2009;14:992–1003. doi: 10.1038/mp.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK, Nall C. Promoter activity of the gene encoding the beta-amyloid precursor protein is up-regulated by growth factors, phorbol ester, retinoic acid and interleukin-1. Brain Res Mol Brain Res. 1995;32:233–240. doi: 10.1016/0169-328x(95)00078-7. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Robakis NK. The promoter activity of the gene encoding Alzheimer beta-amyloid precursor protein (APP) is regulated by two blocks of upstream sequences. Brain Res Mol Brain Res. 1991;9:253–257. doi: 10.1016/0169-328x(91)90009-m. [DOI] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q, Li N, Han X, Shao F, Wang W. Effects of adolescent social isolation on the expression of brain-derived neurotrophic factors in the forebrain. Eur J Pharmacol. 2011;650:229–232. doi: 10.1016/j.ejphar.2010.09.061. [DOI] [PubMed] [Google Scholar]
- Migliore L, Coppede F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat Res. 2009;674:73–84. doi: 10.1016/j.mrgentox.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Moreau JL, Kilpatrick G, Jenck F. Urocortin, a novel neuropeptide with anxiogenic-like properties. Neuroreport. 1997;8:1697–1701. doi: 10.1097/00001756-199705060-00027. [DOI] [PubMed] [Google Scholar]
- Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res. 2005;53:129–139. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Ohtsuji M, Katsuoka F, Kobayashi A, Aburatani H, Hayes JD, Yamamoto M. Nrf1 and Nrf2 play distinct roles in activation of antioxidant response element-dependent genes. J Biol Chem. 2008;283:33554–33562. doi: 10.1074/jbc.M804597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okragly AJ, Haak-Frendscho M. An acid-treatment method for the enhanced detection of GDNF in biological samples. Exp Neurol. 1997;145:592–596. doi: 10.1006/exnr.1997.6500. [DOI] [PubMed] [Google Scholar]
- Palmer K, Berger AK, Monastero R, Winblad B, Backman L, Fratiglioni L. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology. 2007;68:1596–1602. doi: 10.1212/01.wnl.0000260968.92345.3f. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 2. Academic; New York: [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B, Bailey JA, Sarkar S, Lahiri DK. Molecular and immunocytochemical characterization of primary neuronal cultures from adult rat brain: Differential expression of neuronal and glial protein markers. J Neurosci Methods. 2009a;184:294–302. doi: 10.1016/j.jneumeth.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B, Banerjee PK, Greig NH, Lahiri DK. Memantine treatment decreases levels of secreted Alzheimer's amyloid precursor protein (APP) and amyloid beta (Abeta) peptide in the human neuroblastoma cells. Neurosci Lett. 2009b doi: 10.1016/j.neulet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohe M, Synowitz M, Glass R, Paul SM, Nykjaer A, Willnow TE. brain-derived neurotrophic factor reduces amyloidogenic processing through control of SORLA gene expression. J Neurosci. 2009;29:15472–15478. doi: 10.1523/JNEUROSCI.3960-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa ML, Guimaraes FS, de Oliveira RM, Padovan CM, Pearson RC, Del Bel EA. Restraint stress induces beta-amyloid precursor protein mRNA expression in the rat basolateral amygdala. Brain Res Bull. 2005;65:69–75. doi: 10.1016/j.brainresbull.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry. 2010;67:1128–1136. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, Morin M, Gehlert DR, Urban JH, Shekhar A. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J Neurosci. 2008;28:893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A. Sodium lactate elicits anxiety in rats after repeated GABA receptor blockade in the basolateral amygdala. Eur J Pharmacol. 2000;394:265–273. doi: 10.1016/s0014-2999(00)00128-x. [DOI] [PubMed] [Google Scholar]
- Sambamurti K, Greig NH, Lahiri DK. Advances in the cellular and molecular biology of the beta-amyloid protein in Alzheimer's disease. Neuromolecular Med. 2002;1:1–31. doi: 10.1385/NMM:1:1:1. [DOI] [PubMed] [Google Scholar]
- Sambamurti K, Kinsey R, Maloney B, Ge YW, Lahiri DK. Gene structure and organization of the human beta-secretase (BACE) promoter. FASEB J. 2004;18:1034–1036. doi: 10.1096/fj.03-1378fje. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52:701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, Keim SR. The circumventricular organs form a potential neural pathway for lactate sensitivity: implications for panic disorder. J Neurosci. 1997;17:9726–9735. doi: 10.1523/JNEUROSCI.17-24-09726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd CE, Bowes S, Parkinson D, Cambray-Deakin M, Pearson RC. Expression of amyloid precursor protein in human astrocytes in vitro: isoform-specific increases following heat shock. Neuroscience. 2000;99:317–325. doi: 10.1016/s0306-4522(00)00197-4. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Rosen KM, Pola R, Magrane J. Stress proteins in Alzheimer's disease. Int J Hyperthermia. 2005;21:421–431. doi: 10.1080/02656730500133165. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Parola M, Bardini P, Piccini A, Borghi R, Guglielmotto M, Santoro G, Davit A, Danni O, Smith MA, Perry G, Tabaton M. Beta-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J Neurochem. 2005;92:628–636. doi: 10.1111/j.1471-4159.2004.02895.x. [DOI] [PubMed] [Google Scholar]
- Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev. 2008;59:201–220. doi: 10.1016/j.brainresrev.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Tsolakidou A, Czibere L, Putz B, Trumbach D, Panhuysen M, Deussing JM, Wurst W, Sillaber I, Landgraf R, Holsboer F, Rein T. Gene expression profiling in the stress control brain region hypothalamic paraventricular nucleus reveals a novel gene network including amyloid beta precursor protein. BMC Genomics. 2010;11:546. doi: 10.1186/1471-2164-11-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21:4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Bennett DA, Li Y, Bienias JL, Mendes de Leon CF, Evans DA. Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Neurology. 2005;64:380–382. doi: 10.1212/01.WNL.0000149525.53525.E7. [DOI] [PubMed] [Google Scholar]
- Zawia NH, Lahiri DK, Cardozo-Pelaez F. Epigenetics, oxidative stress, and Alzheimer disease. Free Radic Biol Med. 2009;46:1241–1249. doi: 10.1016/j.freeradbiomed.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]