Abstract
The integration of extrinsic and intrinsic signals is required to preserve the self-renewal and tissue regenerative capacity of adult stem cells, while protecting them from malignant conversion or loss of proliferative potential by death, differentiation or senescence. Here we review emerging signaling circuitries regulating stem cell fate, with emphasis on epithelial stem cells. Wnt, mTOR, GPCRs, Notch, Rho GTPases, YAP and DNA and histone methylases are some of the mechanisms that allow stem cells to balance their regenerative potential and the initiation of terminal differentiation programs, guaranteeing appropriate tissue homeostasis. Understanding the signaling circuitries regulating stem cell fate decisions might provide important insights into cancer initiation and numerous human pathologies that involve the progressive loss of tissue-specific adult stem cells.
Introduction: Stem cell fate
Tissue homeostasis and repair upon injury depend on the regenerative capacity of distinct tissue-specific adult stem cell populations. These stem cells are characterized by their ability to self renew during the entire lifespan of the organism, and by their ability to differentiate into the distinct cell types that constitute each specific organ. Adult stem cells are maintained in particular microenvironments within each tissue, referred to as niches [1]. Numerous intrinsic signals as well as microenvironmental cues from their niche allow stem cells to maintain epigenetic marks enabling their self-renewal. On the other hand, a constant communication with their niche enables adult stem cells to perceive and respond to environmental changes, balancing their growth and regenerative potential or initiating terminal differentiation programs. The latter may provide a fail-safe mechanism to avoid dysplastic cell growth or amplification of the stem cell pool, while maintaining appropriate tissue homeostasis. Tissue renewal mediated by adult stem cells is crucial in organs that are continuously exposed to environmental assaults, such as the hematopoietic system, in which bone-marrow derived hematopoietic cells need to be produced continuously, and the epithelial cells covering the digestive track and skin, in which exfoliated cells have to be constantly renewed. The reduction or depletion of the stem cell population has drastic consequences in the physiology of these rapidly self regenerating tissues.
Among the rapidly self-renewing tissues in the adult body, the multilayer epidermis of the skin and mucosae is an excellent system to study adult stem cell biology. Location and markers of skin stem cells have been already reviewed extensively elsewhere [2,3]. Essentially, in the epidermal layer of the skin there are at least two main stem cell populations that contribute to tissue homeostasis and regeneration: the hair follicle stem cells and the interfollicular basal stem/progenitor cells. The epidermal stem cells harboring self-renewal capacity and their immediate descendants, known as transient amplifying cells, can proliferate only when attached to extracellular matrix (ECM) components of the basal membrane, and undergo terminal differentiation once they leave the basal layer. Stem cells must retain their ability to self renew in order to maintain tissue homeostasis, while they need to exit this self-renewal cycle and instead proliferate and differentiate when instructed by microenvironmental cues, including in response to tissue injury. This balance between self-renewal and proliferation and terminal differentiation is strictly regulated and its deregulation can have severe consequences, including cancer. Paradoxically, it has become evident in recent years that signaling pathways that induce stem cell proliferation may also be responsible for stem cell exhaustion and depletion. Indeed, when exposed to persistent proliferative signals, the stem cell compartment undergoes a transient amplification of the progenitor cell population, followed by a depletion of the tissue regenerative cells. Hence, we can postulate that stem cells are endowed with a protective mechanism that results in cell differentiation, death or senescence -- which we refer to as the DDS response (Fig 1) -- upon the aberrant stimulation of proliferative pathways, thereby preventing tumor progression. On the other hand, stem cell depletion by the activation of the DDS response can contribute to reduced tissue regenerative capacity and accelerated aging. Hence, understanding the signaling circuitries regulating self-renewal capacity and DDS entry and escape within the stem/progenitor compartment might provide important insights into cancer initiation and a host of pathologies that involve the progressive loss of tissue-specific regenerating adult stem cells
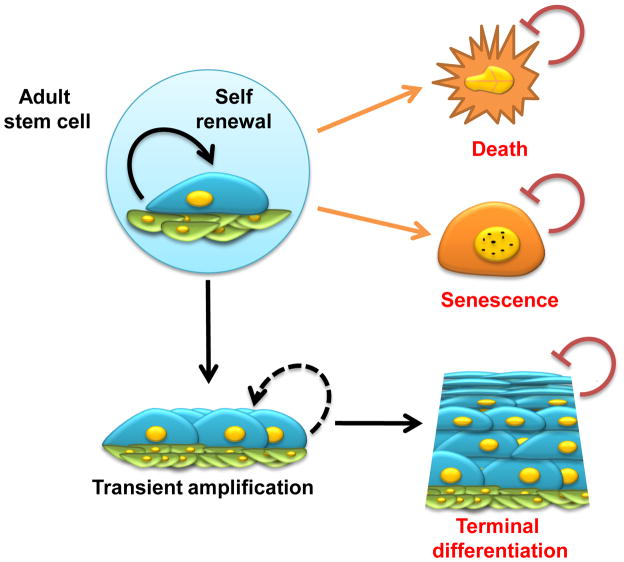
Figure 1. Stem cell fate.
An exquisite balance between microenvironmental cues from the niche and cell autonomous signals is required to preserve the self-renewal and tissue regenerative capacity of stem cells. Under physiological situations, epithelial stem cells undergo asymmetric cell division, thus self-renewing within their niche and generating daughter cells that proliferate rapidly, in a process known as transient amplification, followed by terminal differentiation. Environmental and intrinsic processes, including oncogenic stress, can cause stem cells to undergo cell death or senescence. The activation of cell differentiation, death or senescence programs -- DDS response -- results in the loss of proliferative potential of adult stem cells. The demise of stem cells by DDS activation may represent a natural protective barrier, preventing the incorporation of genetic and epigenetic alterations into self-renewing multipotent cells, and thus its devastating consequences, including tumor formation. On the other hand, stem cell depletion resulting from the activation of the DDS response may contribute to reduced tissue regenerative capacity and accelerated aging.
Wnt and mTOR: Stem cell maintenance vs stem cell exhaustion
The Wnt pathway has received the most attention in stem cell biology, as Wnt-signaling is involved in adult stem cell self-renewal and proliferation in many organs and cellular systems [4–6]. There are 19 human Wnt family members, which are secreted cysteine-rich glycoproteins that initiate signaling by interacting with the N-terminal extracellular cysteine-rich region of the Frizzled family of seven-span transmembrane receptors, and with either LRP5 or LRP6, two members of the low density-lipoprotein receptor-related (LDL-R) protein family. Wnt stimulates a variety of intracellular signaling routes, including the best understood canonical Wnt/β-catenin pathway wherein β-catenin is stabilized and translocates to the nucleus, and the noncanonical pathway involving the kinase (JNK), the small GTPase Rho, and calcium signaling (reviewed in [6,7]). The canonical Wnt/β-catenin pathway represents a key regulator of stem cell self-renewal (Fig 2A) [5,8]. For example, activation of β-catenin by Wnt contributes to the inhibition of keratinocytes differentiation [9], induction of hair follicle formation [10], and maintenance of proliferation of neuronal progenitors [11]. However, expression of a dominant active form of β-catenin exerts a negative effect on hematopoietic stem cells, leading to multilineage differentiation impairment, and the consequent loss of stem cell activity [12,13]. In this regard, we found that long-term Wnt1-induced signaling in the epithelial compartment of the skin causes initially the rapid growth of the hair follicle. But, surprisingly, this was followed by the disappearance of the epidermal stem cell compartment and progressive, premature hair loss [14]. While Wnt1 expression induces the activation of both β-catenin and mammalian Target of Rapamcyin (mTOR), stem cell exhaustion was uniquely associated with mTOR activation [14]. Hence, while Wnt may be required for the maintenance of the stem cell pool, prolonged activation of Wnt may cause cell senescence and the exhaustion or demise of the stem cell compartment by the persistent activation of mTOR (Fig 2A).
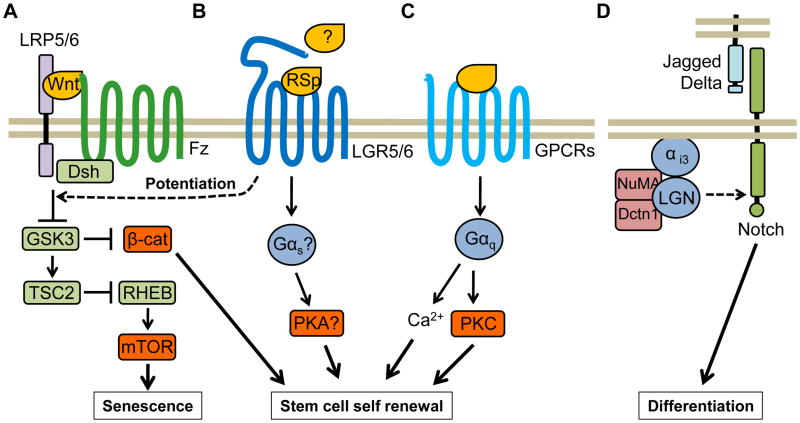
Figure 2. Signaling circuitries controlling stem cell fate decisions.
A) Wnt family members initiate signaling by interacting with Frizzled (FZ) receptors and LRP5 or LRP6. Wnt stimulates a variety of intracellular signaling routes, including the canonical β-catenin pathway wherein β-catenin is stabilized due to GSK3 inhibition and translocates to the nucleus, where it promotes stem cell self-renewal. However, GSK3 inhibition may also result in the activation of mTOR due to inactivation of the TSC2, which inhibits RHEB, thereby initiating mTOR-driven stem cell senescence programs. B) The GPCR-like LGR5 and 6 are expressed in stem cells, where they potentiate Wnt signaling when bound to R-spondins (RSp). LGR5/6 may be able to activate G proteins in response to R-spondins or other ligands. C) The release of the neurotransmitter acetylcholine promotes the growth of stem cells in the salivary gland by acting on Gαq-coupled muscarinic 1 (M1) receptors though Ca+2 release and activation of PKC. D) Notch signaling is dispensable for stem cell self-renewal but necessary to induce differentiation of epithelial stem cells. LGN, NuMA and dynactin (Dctn1) control asymmetric cell division and differentiation by activating Notch signaling. The distribution of asymmetric cell division components is polarized in mitotic basal keratinocytes, where they form an apical crescent of LGN and an interacting partner, NuMA. NuMA in turn binds microtubules and interacts with cytoplasmic Dctn1. LGN is thought to be recruited to the cell membrane through Gαi3.
While emerging evidence supports the central role of the mTOR pathway in cell growth and cancer progression, the emerging picture is that increased mTOR activity perturbs the ability of the whole organism to cope with stress, causing premature senescence and aging. Indeed, decreasing mTOR activity results in increased lifespan in multiple organisms, ranging from yeast, flies and worms, to mice [15]. The molecular mechanisms underlying this paradoxical contribution of mTOR to cancerous growth while promoting stem cell senescence and reducing organismal life span are poorly understood. In this context, we hypothesize that adult stem cells are prone to senescence in response to mTOR activation, thereby providing a mechanism protecting from malignant transformation of adult stem cells.
GPCRs
G protein coupled receptors represents the largest family of cell surface molecules involved in signal transmission [16]. These receptors transduce signals specified by a large variety of stimuli, which converge to increase the activity of one or more of the four families of G protein α subunits, Gαs, Gαi, Gαq, and Gα12, and their associated Gβγ heterodimers [17]. GPCRs play multiple roles in regulating stem cell maintenance (Fig 2A). A direct example of how GPCRs can control stem cells has been recently nicely documented during salivary gland development [18]. By using as a model cytokeratin 5 expressing (K5+) basal epithelial – derived stem cells from the salivary gland, it was recently shown that parasympathetic innervation maintains these K5+ progenitor cells during epithelial organogenesis, and that the release of the neurotransmitter acetylcholine promotes the growth of these K5+ progenitor cells in the salivary gland by acting on a Gαq-coupled muscarinic 1 (M1) receptors [18]. This provides an example of how innervation of an organ can be linked to the development of functional secretory units upon expansion and differentiation of its epithelial progenitor stem cells.
Members of the leucine-rich G protein-coupled receptor family, LGR5 and LGR6, have recently emerged as potential G protein-linked receptors playing a central role in the maintenance and function of a variety of adult epithelial stem cell populations. In the skin, LGR5 marks the hair follicle stem cells and contributes to all hair cell lineages, but not to the interfollicular epidermis or sebaceous glands [19]. LGR5 also contributes to the maintenance of the crypt stem cells of the intestine [20]. LGR5 expression is dependent on Wnt [21] and LGR5 associates with the Frizzled/Lrp Wnt receptor complex [22]. It was recently shown that the secreted Wnt pathway agonists R-spondins can bind to LGR4, LGR5 and LGR6 and potentiate Wnt signaling [22,23], allowing to augment short-range Wnt signals. LGR6 positive cells in the skin give rise to the sebaceous gland and the interfollicular epithelia, and this stem cell population is likely to represent the prenatal multipotent precursor of all skin cell lineages [24]. Interestingly, unlike LGR5, LGR6 expression is independent of Wnt signaling [24]. A picture thus emerges in which the LGR6 expressing stem cell pool can renew sebaceous and interfollicular cells throughout life independently of Wnt function, while regeneration of hair follicles depends on a LGR5+ stem cell pool under the control of Wnt signaling. At the biochemical level, LGR5 and LGR6 are closely related to LH, FSH, and TSH family of G protein-linked hormone glycoprotein receptors [25]. However, no direct activation of particular G proteins in response to R-spondins has been detected, suggesting that either this family of stem cell markers act independently of G proteins, or raising the possibility that additional yet to be identified ligands promoting G protein signaling might exist, which would warrant further investigation.
Notch
Distinct from many other agonist/receptor systems, Notch signaling relies on non-diffusible ligands (Delta, Delta like and Jagged) which are integral membrane proteins that engage and activate surface receptors of immediately adjacent cells. The role of Notch signaling in cell proliferation and differentiation is highly cell specific. In keratinocytes, Notch signaling is essential to promote differentiation and its absence can enhance tumor formation [26]. Even more, inactivating mutations in Notch have been reported to be an early event in squamous cell carcinoma [27], highlighting again the importance of the deployment of differentiation pathways to prevent stem cell transformation. Similarly, in the epithelium lining the airways, Notch signaling has been shown to be dispensable for stem cell self-renewal but necessary to induce differentiation of the basal stem cells during tissue regeneration [28]. Asymmetric cell division and Notch signaling act in a common pathway promoting the basal to suprabasal switch and differentiation in the mouse epidermis [29]. Recent studies also suggest a cross-talk between Notch and G protein function. The heterotrimeric G protein α subunit Gαi3 and its GPCR-independent G protein regulator LGN show strong apical localization in skin progenitors, and LGN deficiency inhibit asymmetrical cell divisions and skin stratification, mainly due to a defective activation of Notch [29]. LGN (also named Gpsm2), NuMA and dynactin (Dctn1) control asymmetric cell division in the mouse epidermis by reorienting mitotic spindles to achieve perpendicular divisions, which in turn promotes stratification and differentiation by compartmentalizing Notch signaling suprabasally (Fig 2B) [29].
Other signaling pathways controlling stem cell fate
An exquisite balance between physical microenvironmental cues and cell autonomous signals is required to preserve adult stem cell self-renewal. For example, signaling events that mediate the interaction between the basement membrane and the basal epidermal stem cells are required to maintain their stem-like state. Basal keratinocytes, including epithelial stem cells, display an enhanced tendency to differentiate when cell attachment is restricted, partially due to the stimulation of the Serum Response Factor (SRF) through the activation of one of its cofactors, MAL [30]. SRF activation enhances expression of c-Fos and JunB [30], two key components of the AP-1 transcription factor which controls the expression of proteins involved in multiple differentiated epithelial cell functions [31]. Therefore, biomechanical sensing mechanisms link changes in the epithelial stem cell niche with the expression of differentiation gene programs, thereby controlling epidermal stem cell fate decisions [32]. Cell–matrix interactions may be particularly important in the case of wound healing, which involves stem cell migration. Epithelial stem cells lacking the small GTPase Rac1 fail to undergo shape changes and migrate on the provisional ECM at the wound edge [33]. Rac1-depleted cells do not migrate, but instead differentiate and thus exit prematurely from the stem cell pool, resulting in defective wound closure [33]. In addition, it is known that blockade of the downstream target of Rho, ROCK, with pharmacological inhibitors can enhance stem cell maintenance [34,35]. These studies highlight the importance of stem cell differentiation gene programs activated by cell surface receptors impinging on Rho proteins and their regulated signaling circuitries (Fig 3A).
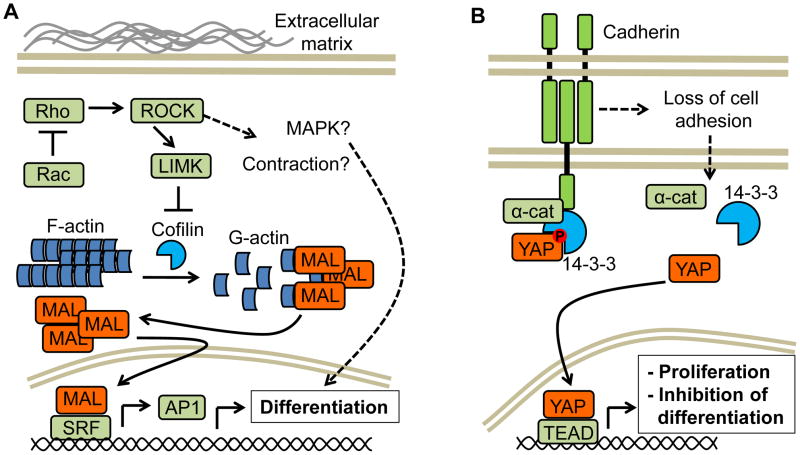
Figure 3. Other signaling pathways controlling stem cell fate.
A) MAL is normally retained in the cytosol in an inactive state while bound to monomeric actin (G-actin). Signaling pathways that activate Rho (also due to inactivation of Rac) lead to ROCK and LIMK activation, resulting in the phosphorylation of the actin severing protein cofilin and accumulation of fibrillar actin (F-actin). This change relieves MAL from the inhibitory activity of G-actin, and free MAL can then bind SRF in the nucleus, enhancing the expression of multiple SRF regulated genes, including key components of the AP-1 transcription factor which controls the expression of proteins involved in multiple differentiated epithelial cell function. Rho GTPase stimulation may result also in activation of cell contraction or mitogen-activated protein kinase (MAPK) cascades, such as the JNK and p38 pathways, that can lead to the activation of differentiation programs. B) Yes-associated protein (YAP) activity appears to be regulated by α-catenin (α-cat). In differentiating keratinocytes, phosphorylated (inactive) Yap binds 14-3-3 proteins and is sequestered by α-catenin, preventing its dephosphorylation and activation. The dissolution of cell-cell contacts causes dissociation and depletion of α-catenin and, concomitantly, dissociation of YAP from 14-3-3 proteins. YAP can be then dephosphorylated and translocates to the nucleus, where it binds the TEA domain (TEAD) transcription factor and induces stem cell proliferation while repressing differentiation gene programs.
Another emerging player controlling epithelial progenitor maintenance and growth is the Yes-associated protein (YAP) (Fig 3B). Yap acts as a transcriptional co-activator that is repressed downstream of the Hippo tumor suppressor signaling pathway [36]. YAP regulates embryonic and pluripotent stem cell self-renewal and differentiation [37]. YAP is expressed in epidermal skin progenitor cells, were it maintains self renewal and the undifferentiated state [38]. In vivo, elevation of nuclear YAP in cells of the basal layer of the skin results in the expansion of proliferative basal epidermal progenitors, while YAP knockdown results in failure of skin expansion due to lack of proliferation and differentiation of epithelial stem cells [38,39]. The effects of YAP seem to be mediated by the TEA domain (TEAD) transcription factor. Interestingly, the upstream regulation of Yap1 activity seems to be mediated by α-catenin. In differentiating keratinocytes, phosphorylated (inactive) Yap binds 14-3-3 proteins and is sequestered by α-catenin, preventing its dephosphorylation and activation [39]. YAP dephosphorylation may require the protein phosphatase 2A (PP2AC) [39] but the nature of the kinase that phosphorylates YAP is still unknown. The importance of this pathway is highlighted by the fact that the tumor suppressor activity of α-catenin is dependent on its role in inactivating YAP [40]. Finally, YAP activity has been also shown to be controlled by extracellular matrix stiffness and Rho GTPases [41], adding to the emerging complexity of YAP regulation.
Epigenetic regulation of stem cell fate
The seemingly irreversible transition between stemness and differentiation is also marked by epigenetic changes that dictate the availability of promoters accessible for transcription factors, adding one extra layer of complexity to the transcriptional regulation of stem cell function. These transitions are marked essentially by modifications in the methylation state of DNA and histones (Fig 4). In epithelial cells, DNA and histone methylation repressor complexes seem to play an important role in controlling the proliferative potential of basal progenitors by preventing the recruitment of transcriptional activators to genes involved in differentiation and senescence. Members of the polycomb repressive complex (PRC) 2 (Ezh1/2 and Eed) and PRC1 (Bmi1, Cbx2, and Pcgf2) are expressed in basal epithelial progenitor cells and downregulated suprabasally [31,42]. Deletion of Ezh1 and Ezh2 in the skin leads to a complete loss of H3K27 trimethylation and to the concomitant activation of Ink4a/p16 and Ink4b/Arf gene expression [31,42], which encode the tumor suppressive proteins p16 and Arf, respectively. While p16 binds CDKs and prevents the inhibitory phosphorylation of the retinoblastoma tumor suppressor protein (Rb), Arf inhibits Mdm2 function leading to the activation of the tumor suppressor p53 [43], thus together exerting a potent antiproliferative response. Activation of Ink4a/p16 and Ink4b/Arf expression in hair follicle stem cells leads to their rapid depletion and consequent block of hair growth [42]. The PRC1 protein BMI1 also regulates epithelial stem cell self-renewal trough the control of the Ink4a/Ink4b locus [44–46], likely through interactions with PRC2 members [47]. DNA silencing by methylation is also important to maintain the progenitor function and self renewal of stem cells. DNA methyltransferase 1 (DNMT1) has been found to be enriched in undifferentiated epidermal progenitors and its depletion leads to loss of progenitor function and premature differentiation due to the expression of Ink4a/Ink4b [48]. The opposite function is performed by demethylases, which hence regulate the switch between stem cell self renewal and differentiation. For example, the histone demethylase JMJD3 is known to induce epidermal progenitor differentiation [49].
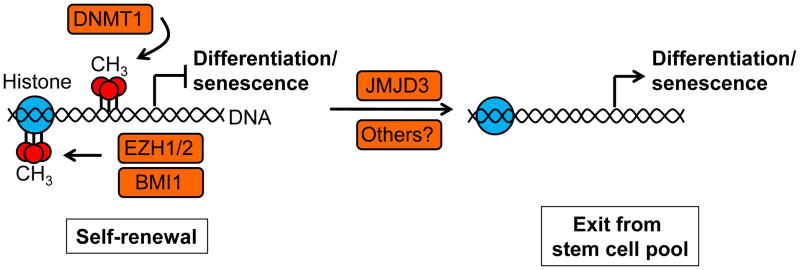
Figure 4. Epigenetic regulation of stem cell fate.
Members of the polycomb repressive complex (PRC) 2 (Ezh1/2) and PRC1 (Bmi1) and DNA methyltransferase 1 (DNMT1) are expressed in basal epithelial progenitor cells and promote the methylation of histones and DNA, preventing the recruitment of transcriptional activators to genes involved in differentiation and senescence (such as the Ink4a/p16 and Ink4b/Arf locus), promoting stem cell self-renewal. The opposite function is performed by demethylases, including the histone demethylase JMJD3, which hence regulates the switch between stem cell self renewal and differentiation/senescence resulting in the loss of proliferative potential of adult stem cells.
At the moment is not clear what links signaling pathways promoting stem cell renewal or differentiation with the regulation of DNA and histone methylation. A crosstalk exists between histone methylation patterns and de novo DNA methylation through the targeting of DNMTs to specific genomic regions [50]. On the other hand, posttranslational modifications can regulate the activity of methyltransferases. For example, modifications mediated by AKT1 and SET7 have been shown to regulate the activity of DNMT1 [51]. Finally, co-factor availability is emerging as another potential regulator of demethylases [52], thus potentially linking cellular metabolism with the regulation of the epigenetic state.
Conclusions
The maintenance of tissue specific stem cell involves an exquisite balance between microenvironmental cues and cell autonomous signals. Indeed, the integration of extrinsic and intrinsic signals is required to preserve self-renewal and tissue regenerative capacity of adult stem cells. Due to their self-renewal capacity and extended lifespan, this stem cell population may be prone to malignant transformation. However, various lines of evidence suggest that the demise of stem cells by death, senescence, or differentiation - which we refer to as the DDS response - triggered by extrinsic or intrinsic cellular stress pathways might represent a key mechanism protecting stem cells from aberrant cell growth and malignant reprogramming. On the other hand, this natural cancer-preventive strategy deployed by stem cells comes at a cost, as stem cell depletion by the activation of cell senescence programs can contribute to reduced tissue regenerative capacity and accelerated aging. Ultimately, understanding the signaling circuitries regulating stem cell fate decisions might provide important insights into cancer initiation and a host of pathologies that involve the progressive loss of tissue-specific regenerating adult stem cells.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Dental and Craniofacial Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 2.Barker N, Bartfeld S, Clevers H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell. 2010;7:656–670. doi: 10.1016/j.stem.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 5.Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- 6.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 7.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 8.Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003;17:1189–1200. doi: 10.1101/gad.1086903. [DOI] [PubMed] [Google Scholar]
- 9.Zhu AJ, Watt FM. beta-catenin signalling modulates proliferative potential of human epidermal keratinocytes independently of intercellular adhesion. Development. 1999;126:2285–2298. doi: 10.1242/dev.126.10.2285. [DOI] [PubMed] [Google Scholar]
- 10.Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 11.Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB, 3rd, Birchmeier W, Birchmeier C. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 12.Kirstetter P, Anderson K, Porse BT, Jacobsen SEW, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006;7:1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- 13.Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, Leutz A. Hematopoietic stem cell and multilineage defects generated by constitutive [beta]-catenin activation. Nat Immunol. 2006;7:1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- 14••.Castilho RM, Squarize CH, Chodosh LA, Williams BO, Gutkind JS. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 2009;5:279–289. doi: 10.1016/j.stem.2009.06.017. This paper shows that persistent expression of Wnt in mouse epidermis leads to hyperproliferation of epithelial stem cells, ultimately causing them to undergo senescence and exhausting the stem cell niche. Importantly, stem cell senescence depends on Wnt-mediated activation of the mTOR pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 17.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7 :79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 18••.Knox SM, Lombaert IMA, Reed X, Vitale-Cross L, Gutkind JS, Hoffman MP. Parasympathetic Innervation Maintains Epithelial Progenitor Cells During Salivary Organogenesis. Science. 2010;329:1645–1647. doi: 10.1126/science.1192046. This study provides an example of how innervation of an organ can be linked to the development of functional secretory units upon expansion and differentiation of its epithelial progenitor stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 20.Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, et al. Lgr5+ve Stem Cells Drive Self-Renewal in the Stomach and Build Long-Lived Gastric Units In Vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Barker N, Clevers H. Tracking down the stem cells of the intestine: strategies to identify adult stem cells. Gastroenterology. 2007;133:1755–1760. doi: 10.1053/j.gastro.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 22••.de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011 doi: 10.1038/nature10337. This study identifies a ligand for the stem cell orphan receptors LGR4/5/6 and shows that it can potentiate Wnt signaling (similarly to reference [23]) [DOI] [PubMed] [Google Scholar]
- 23••.Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/β-catenin signaling. Proceedings of the National Academy of Sciences. 2011 doi: 10.1073/pnas.1106083108. This study identifies a ligand for the orphan receptor LGR4/5 and shows that it can potentiate Wnt signaling (similarly to reference [22]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24••.Snippert HJ, Haegebarth A, Kasper M, Jaks V, Van Es JH, Barker N, Van De Wetering M, Van Den Born M, Begthel H, Vries RG, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. Shows that LGR6+ epithelial stem cells represent the prenatal multipotent precursors of all adult skin cell lineages. Also shows that the LGR6 stem cell pool might be independent of Wnt signaling. [DOI] [PubMed] [Google Scholar]
- 25.Hsu SY, Kudo M, Chen T, Nakabayashi K, Bhalla A, van der Spek PJ, van Duin M, Hsueh AJ. The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol Endocrinol. 2000;14:1257–1271. doi: 10.1210/mend.14.8.0510. [DOI] [PubMed] [Google Scholar]
- 26.Dotto GP. Crosstalk of Notch with p53 and p63 in cancer growth control. Nat Rev Cancer. 2009;9:587–595. doi: 10.1038/nrc2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, Fakhry C, Xie T-X, Zhang J, Wang J, et al. Exome Sequencing of Head and Neck Squamous Cell Carcinoma Reveals Inactivating Mutations in NOTCH1. Science. 2011 doi: 10.1126/science.1206923. Highlights the importance of the deployment of differentiation pathways to prevent epithelial stem cell transformation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rock Jason R, Gao X, Xue Y, Randell Scott H, Kong Y-Y, Hogan Brigid LM. Notch-Dependent Differentiation of Adult Airway Basal Stem Cells. Cell Stem Cell. 2011;8 :639–648. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Williams SE, Beronja S, Pasolli HA, Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–358. doi: 10.1038/nature09793. Provides evidence the importance of asymmetric cell division in promoting tissue growth and architecture in the mammalian skin, and investigated the underlying mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Connelly JT, Gautrot JE, Trappmann B, Tan DWM, Donati G, Huck WTS, Watt FM. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nature Cell Biology. 2010;12:711–718. doi: 10.1038/ncb2074. Identifies biomechanical sensing mechanisms that link the physical shape of the stem cell microenvironment to epithelial stem cell fate decisions. [DOI] [PubMed] [Google Scholar]
- 31.Ezhkova E, Pasolli HA, Parker JS, Stokes N, Su Ih, Hannon G, Tarakhovsky A, Fuchs E. Ezh2 Orchestrates Gene Expression for the Stepwise Differentiation of Tissue-Specific Stem Cells. Cell. 2009;136:1122–1135. doi: 10.1016/j.cell.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iglesias-Bartolome R, Gutkind JS. Keeping the epidermal stem cell niche in shape. Cell Stem Cell. 2010;7:143–145. doi: 10.1016/j.stem.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 33•.Castilho RM, Squarize CH, Leelahavanichkul K, Zheng Y, Bugge T, Gutkind JS. Rac1 is required for epithelial stem cell function during dermal and oral mucosal wound healing but not for tissue homeostasis in mice. PLoS One. 2010;5:e10503. doi: 10.1371/journal.pone.0010503. Highlights the importance of stem cell differentiation/senescence gene programs activated by Rho proteins and their regulated signaling circuitries. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen G, Hou Z, Gulbranson DR, Thomson JA. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell. 2010;7:240–248. doi: 10.1016/j.stem.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Y, Zhu X, Hahm HS, Wei W, Hao E, Hayek A, Ding S. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc Natl Acad Sci U S A. 2010;107:8129–8134. doi: 10.1073/pnas.1002024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan D. The Hippo Signaling Pathway in Development and Cancer. Developmental Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, Chinnaiyan A, Israel MA, Goldstein LS, Abujarour R, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–1118. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Pasolli HA, Fuchs E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2270–2275. doi: 10.1073/pnas.1019603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. Shows the in vivo relevance of Yap1 in epidermal progenitor proliferation and its regulation by alpha-catenin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silvis MR, Kreger BT, Lien W-H, Klezovitch O, Rudakova GM, Camargo FD, Lantz DM, Seykora JT, Vasioukhin V. {alpha}-Catenin Is a Tumor Suppressor That Controls Cell Accumulation by Regulating the Localization and Activity of the Transcriptional Coactivator Yap1. Sci Signal. 2011;4:ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 42••.Ezhkova E, Lien WH, Stokes N, Pasolli HA, Silva JM, Fuchs E. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes and Development. 2011;25:485–498. doi: 10.1101/gad.2019811. Shows the relevance of histone methylation pathways in the maintenance of the progenitor function and self-renewal capacity of epithelial stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collado M, Blasco MA, Serrano M. Cellular Senescence in Cancer and Aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Caramel J, Lacroix M, Le Cam L, Sardet C. E4F1 connects the Bmi1-ARF-p53 pathway to epidermal stem cell-dependent skin homeostasis. Cell Cycle. 2011;10:866–867. doi: 10.4161/cc.10.6.14974. [DOI] [PubMed] [Google Scholar]
- 45.Cordisco S, Maurelli R, Bondanza S, Stefanini M, Zambruno G, Guerra L, Dellambra E. Bmi-1 Reduction Plays a Key Role in Physiological and Premature Aging of Primary Human Keratinocytes. J Invest Dermatol. 2010;130:1048–1062. doi: 10.1038/jid.2009.355. [DOI] [PubMed] [Google Scholar]
- 46.Lacroix M, Caramel J, Goguet-Rubio P, Linares LK, Estrach S, Hatchi E, Rodier G, Lledo G, de Bettignies C, Thépot A, et al. Transcription factor E4F1 is essential for epidermal stem cell maintenance and skin homeostasis. Proceedings of the National Academy of Sciences. 2010;107:21076–21081. doi: 10.1073/pnas.1010167107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang M-H, Hsu DS-S, Wang H-W, Wang H-J, Lan H-Y, Yang W-H, Huang C-H, Kao S-Y, Tzeng C-H, Tai S-K, et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12:982–992. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- 48••.Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA. DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature. 2010;463:563–567. doi: 10.1038/nature08683. Shows the relevance of histone methylation pathways to maintain the progenitor function and self-renewal capacity of epithelial stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sen GL, Webster DE, Barragan DI, Chang HY, Khavari PA. Control of differentiation in a self-renewing mammalian tissue by the histone demethylase JMJD3. Genes & Development. 2008;22:1865–1870. doi: 10.1101/gad.1673508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Denis H, Ndlovu N, Fuks F. Regulation of mammalian DNA methyltransferases: a route to new mechanisms. EMBO Rep. 2011;12:647–656. doi: 10.1038/embor.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Esteve PO, Chang Y, Samaranayake M, Upadhyay AK, Horton JR, Feehery GR, Cheng X, Pradhan S. A methylation and phosphorylation switch between an adjacent lysine and serine determines human DNMT1 stability. Nat Struct Mol Biol. 2011;18:42–48. doi: 10.1038/nsmb.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, Ito S, Yang C, Xiao MT, Liu LX, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. Links co-factor availability as an emerging potential regulator of demethylases, thus potentially linking cellular metabolism with the regulation of the epigenetic state. [DOI] [PMC free article] [PubMed] [Google Scholar]