Abstract
Objective
Children with arthritis experience frequent pain, but the predictors of daily pain variations are largely unidentified. The goal of this study was to examine sleep quality as a predictor of pain in children with arthritis and to determine whether mood moderates this relationship.
Methods
In this prospective, longitudinal study children with polyarticular arthritis (n = 51, ages 8–16 years) tracked daily symptoms including sleep quality over 2 months. Self-reported daily pain intensity, as indicated on a visual analog scale, was used as the primary outcome measure in multilevel models.
Results
Poorer sleep quality was associated with higher next-day pain ratings (p < .01). Mood moderated this relationship such that as positive mood increased, the relationship between poor sleep quality and high pain weakened (p < .01). Daily pain did not predict nightly sleep quality (p > .05).
Conclusions
Sleep quality is an important predictor of pain in children with arthritis. These findings add to the growing body of literature on the utility of daily diaries for analyzing patterns of pain, sleep, and mood in children with chronic painful conditions.
Keywords: juvenile arthritis, sleep quality, disease-related pain, positive mood, daily pain diaries
Children with juvenile arthritis face the health consequences of ongoing, recurrent pain as well as functional and psychosocial burdens. Daily arthritis-related pain is common, and most importantly, daily pain has been shown to predict activity cutbacks (Schanberg, Anthony, Gil, & Maurin, 2003; Schanberg, Gil, Anthony, Yow, & Rochon, 2005). Thus, it is critical to identify predictors of pain in children with juvenile arthritis in order to guide the development of interventions to promote symptom reduction and improve quality of life. Previous research has detected weak associations between disease-related variables and pain (Malleson et al., 2004), suggesting that other factors are involved.
Recently, sleep has been identified as an important aspect of the disease experience in children with chronic pain (Chambers, Corkum, & Rusak, 2008; Valrie et al., 2007a), but little research to date has examined sleep as a predictor of pain in children with arthritis. Sleep has been measured via both objective (e.g., polysomnography) and subjective (e.g., standardized questionnaires) methods and the few published cross-sectional studies suggest that children with arthritis experience significant sleep disturbances (Amos, Curry, Drutz, Frost & Warren, 1997; Zamir, Press, Asher, & Tarasiuk, 1998; Passarelli et al., 2006). For example, Bloom, et al. (2003) found that parents of children with arthritis rated their children as having significantly more sleep anxiety, night awakenings, parasomnia, sleep anxiety, and sleep disordered breathing. Moreover, children’s overall sleep reports were significantly related to pain ratings in this study. In a study using objective sleep measures, Passarelli and colleagues (2006) found significantly higher sleep disturbances, characterized by lower sleep efficiency, higher arousal index, and frequent leg movements in children and adolescents with arthritis than in matched controls. Despite preliminary evidence of sleep problems in children with arthritis, no studies to date have had children track variations in sleep and pain under naturalistic circumstances over several weeks in order to examine sleep as a predictor of juvenile arthritis pain. Prospective research designs are well-suited for studying the associations among symptoms and behaviors that are expected to fluctuate over time, such as pain (Stinson et al., 2008) and sleep (Hanson & Chen, 2010).
Although sleep difficulties have been documented in children with arthritis, the etiology of sleep problems in juvenile arthritis remains unclear (Labyak, Bourguignon, & Docherty, 2003). Several potential mechanisms have been hypothesized as causing sleep problems in children. Physiological mechanisms have been proposed related to neural circuitry and neurotransmitters underlying sleep and circadian rhythms (Schwartz & Roth, 2008). Other potential mechanisms relate to emotional (e.g., anxiety) or behavioral problems (e.g., bedtime refusal) interfering with sleep (Chorney, Detweiler, Morriss, & Kuhn, 2008; Meltzer & Mindell, 2006). Previous studies of sleep in children with arthritis suggested pain as the cause of sleep problems (Bloom et al., 2002, Passarelli et al., 2006) and no other disease-specific variables have been examined as predictors of sleep problems. However, conceptual models of sleep and pain suggest a complex, bidirectional relationship (Lewin & Dahl, 1999) and recent research in pediatric pain populations has provided more support for sleep as a predictor of pain than the alternate direction of influence. For example, in Ward et al.’s (2008) polysomnography study of children with arthritis, evening pain did not significantly predict sleep parameters measured during the laboratory sleep assessment. A recent longitudinal study by Lewandowski, et al. (2010) also provides support for studying sleep as a predictor of daily pain. Using a sample of adolescents with chronic pain unrelated to a chronic medical illness and a healthy control group, Lewandowski, et al. (2010) found that sleep variables significantly predicted next-day pain reports, but contrary to study hypotheses, daytime pain did not predict sleep at night. Based on these studies and the research priority of identifying predictors of pain in juvenile arthritis, the current study examined sleep as a predictor of pain. A global rating of sleep quality at night was used as the primary sleep measure in order to determine if the subjective experience of sleep influences reported pain.
Another unique feature of the current study was to examine the contribution of psychological factors, namely mood, to the pain-sleep relationship. Previous research has suggested that children with arthritis are at an increased risk for internalizing problems (LeBovidge, Lavigne, Donenberg, & Miller, 2003), and the associations between mood, sleep, and pain have long been recognized (Argoff, 2007; Buysse, Germain, Nofzinger, & Kupfer, 2006) in the general psychiatric and behavioral medicine literature, but remain to be established in children with painful medical illnesses. Few studies have examined how these factors are related in pediatric pain populations and only one study of children with chronic or recurrent pain has examined the role of mood in the daily relationship between pain and sleep (Valrie et al., 2008).
In order to identify predictors of daily pain variability, the current study examined daily relationships between sleep, mood, and pain in children and adolescents with polyarticular juvenile arthritis over a two-month period. We hypothesized that poorer sleep quality would predict higher pain ratings the following day. Similarly, we expected children with poorer sleep quality overall to have higher pain, on average. We also hypothesized that the relationship between nightly sleep and next-day pain would be weakened at increasing levels of positive mood, such that positive mood would buffer the effects of poor sleep quality on the previous night.
Method
Child and parent dyads were recruited as part of a larger, longitudinal study of 51 children ages 8–16 years with polyarticular arthritis (Schanberg et al., 2003, 2005). Potential participants in the larger study were excluded if they were currently using psychotropic medications or systemic steroids. To date, two publications regarding the correlates of pain in children with arthritis have resulted from this line of inquiry (Schanberg et al., 2003, 2005). The current study uniquely focused on variations in sleep, pain, and mood by analyzing data obtained from the child-completed daily diary.
Participants
Patients were recruited from the Pediatric Rheumatology Clinic at a major medical center in the Southeast. All participants experienced arthritis onset prior to the age of 16 years, had arthritis in 5 or more joints (i.e., polyarticular arthritis) during the first 6 months of disease, and had experienced arthritis for at least 6 weeks. The study sample consisted of 51 patients (33 girls, 18 boys) between the ages of 8–16 years (m = 12.4 years, SD = 2.8), and one of their parents. One additional child completed study procedures, but withdrew consent during the diary completion period.
Measures
Daily diary
The daily diary measure used for this study was based on a previous version of the diary (Gil et al., 2000, 2003; Schanberg et al., 2000) developed for children with chronic illnesses such as sickle cell disease or chronic arthritis. Specific variables from the diary analyzed for the present study include pain, sleep quality, and mood.
Arthritis-related pain intensity
Children were asked to rate their average level of pain for the day on a 100 mm visual analogue scale (VAS) taken from the Pediatric Pain Questionnaire (Varni, Thompson, & Hanson, 1987), anchored by “No hurting, No discomfort, No pain” and “Hurting a whole lot, Very uncomfortable, Severe pain.” The reliability and validity of visual analogue scales has been established for measuring pediatric musculoskeletal pain (Varni et al., 1987, McGrath et al., 1996).
Sleep Quality
Each day, children reported the previous night’s sleep quality. Children were provided a 100 mm VAS anchored by “Did not sleep well” and “Slept very well” and were prompted to “Put a mark on the line to show how well you slept last night.” No other sleep items were captured on the diary, thus ratings on the 100 mm VAS were used as the sleep measure used in statistical models. The use of daily sleep measures has been found to be reliable and valid in comparison to physiological sleep measures (Gaina, Sekine, Chen, Hamanishi, & Kagamimori, 2004; Sadeh, Raviv, & Gruber, 2000) and is considered optimal for assessing sleep patterns (Knab & Engel-Sittenfeld, 1983; Wolfson et al., 2003). Recent research in children with sickle cell disease (Valrie et al., 2007a, 2007b, 2008) further supports the reliability and validity of daily sleep diaries using a similar sleep quality item. Overall, results of these studies found adequate consistency between parent and child baseline reports and daily reports, further supporting the use of daily diaries in measuring sleep in a pediatric population. However, consistent with previous research, Valrie and colleagues (2007a) found the average daily report of sleep quality to be significantly higher than baseline reports of sleep quality, suggesting that daily reports are less influenced by recall bias than baseline measures.
Mood
The Facial Affective Scale (FAS, McGrath, de Veber, & Hearn 1985; McGrath et al., 1996) was used as a visual scale for daily mood rating. Children were instructed to select the face that best reflected their mood for the day, ranging from a very upset face (.90) to a very happy smiling face (.10). Lower FAS scores are associated with more positive mood. The FAS has been used as a measure of general mood in previous research with children (Valrie et al., 2008) and is a reliable and valid measure (McGrath et al., 1985, 1996; Sandstrom, Cillessen, & Eisenhower, 2003). The continuous measure of mood was used in statistical models as a unique predictor of daily pain.
Baseline measures
Disease severity
As part of the larger study, the pediatric rheumatologist completed a physical examination during the baseline clinic visit. Results of this examination served to inform the physician’s global assessment of disease severity rating for each child. A 100 mm VAS anchored by the points “asymptomatic” and “very severe” was used to measure disease severity. This measure has been widely used across rheumatology research and is designated as part of the core set of outcome variables in juvenile arthritis clinical research (Giannini et al., 1997).
Procedure
The study protocol was approved by the Institutional Review Board at a major medical center in the southeast. Eligible patients were contacted regarding study participation by letter and during a routine clinic visit. Upon providing informed consent, children and parents completed baseline measures, research personnel assessed each child’s ability to understand and independently complete the diary, and a research assistant trained each child to complete the daily diary. Children were provided with paper diaries and envelopes and were instructed to complete daily reports of symptoms including pain, mood, and sleep each night without the assistance of a parent for 2 months. Children completed ratings of symptom severity during that day and the previous night’s sleep quality each evening and returned the diary in a self-addressed, stamped envelope by mail the following morning. Children entered the date and time the entry was completed in a space provided at the beginning of each diary. A research assistant contacted each participant twice during the initial week and once a week thereafter throughout the recording period to reinforce the independent completion of the daily report. Parents were reimbursed $25 upon entry to the study for travel-related expenses. Children were reimbursed $0.50 for each diary completed and an extra $1.25 for each week in which all 7 daily reports were returned.
Statistical Analysis
Hypothesis testing was completed through a series of hierarchical multilevel models with serial autocorrelation residual variance structures, consistent with recommendations made by Raudenbush and Bryk (2002). An advantage of multilevel models is the ability to use all available data to estimate model parameters despite missing observations (Raudenbush et al., 2002). Variables predicting between-child differences were grand mean centered. For within-child predictors of daily pain, each observation was centered around the individual child’s mean score. Interclass correlations were calculated in order to decompose effects into within- and between-child sources of variance. Sleep quality and mood ratings as predictors of daily pain were analyzed from the same diary entry. In contrast, analysis of the alternate model predicting sleep quality was lagged so as to predict sleep quality from diary reports of pain completed the night before.
Following recommendations for moderation testing by Aiken and West (1991), levels of mood were defined statistically in order to graph the simple slopes of the relationship between sleep quality and pain by level of mood. This statistical approach allows longitudinal data to be comparable to typical ANOVA techniques for probing interactions (Aiken & West, 1991). All analyses were conducted using PROC MIXED in SAS (SAS Institute, 2008). The results of all models are presented in Table 1.
Table 1.
Hierarchical Multilevel Random Effects Analysis Predicting Pain and Sleep Quality
| Models Predicting Pain | B | t | Model Predicting Sleep Quality | B | t | ||
|---|---|---|---|---|---|---|---|
| 2 | Age | 1.05 | 1.02 | 2 | Age | −0.39 | −0.53 |
| Disease severity | 0.28 | 2.49* | Disease severity | 0.05 | 0.62 | ||
| 3 | Between-child sleep quality | −0.71 | −5.73** | Between-child pain | −0.52 | −5.07** | |
| Within-child sleep quality | −0.79 | −3.07** | Within-child pain | 0.04 | −1.05 | ||
| 4 | Between-child mood | 4.01 | 0.16 | ||||
| Within-child mood | 27.50 | 12.43** | |||||
| Within-child sleep quality × mood | −0.25 | −3.26** | |||||
Note. The initial step of each model contained no predictors per procedures as detailed by Raudenbush & Bryk (2002). Ratings of predictor variables in the models predicting pain were taken from the same diary entry, whereas ratings of predictors in the model predicting sleep were taken from the dairy entry completed the previous day.
p < .05,
p < .01
Results
Descriptive Statistics
Children in the sample completed 84% of all possible diary reports (m = 50 diaries, SD = 13.67). In total, 3.9% of diary reports were considered nonconsecutive (i.e., followed a missing report). Based on correlations and independent sample t-tests completion rate did not vary by demographic variables (i.e., age, disease severity, race, or gender) or study variables (i.e., sleep quality, pain intensity, or mood).
The mean physician global assessment of disease severity score was 31 (SD = 25), indicating that children in the sample experienced mild to moderate arthritis on average. Children reported pain on 76% (SD = 34.8%) of days during the study period with the average pain intensity rating of 29.9 (SD = 19.2) on days in which pain occurred. The mean sleep quality score across all diaries was in the moderate to high sleep quality range (M = 75.25, SD = 18.53). Despite relatively low pain and high sleep quality on average, children reported pain intensity in the upper range for the sample (>1 SD above the mean pain intensity; i.e., > 56) on 19.6% of diaries and children reported sleep quality in the lower range for the sample (>1 SD below the mean sleep quality; i.e., < 56.72) on 32% of diaries. This suggests that children in the sample experienced variability in pain and sleep quality over time. Children endorsed positive mood on 92% of days, with a mean score of 0.27 (SD = 0.17).
Model 1- Predicting the Total Variance in Pain Intensity
Initially a random effects analysis of covariance with a serial autocorrelation residual matrix was tested in order to determine the between-child variability in reported pain and the correlation of pain scores within each child. Results of the empty model indicated that approximately 65% of the variance in pain was due to differences between children, suggesting that demographic or trait variables, rather than daily variables, account for almost two thirds of the variance in reported pain.
Model 2- Age and Disease Severity as Predictors of Pain Intensity
Age and physician-rated disease severity were entered into the second model as between-child, time invariant covariates in order to determine the influence of baseline demographics. The model also included a random intercept based on the assumption that the average level of reported pain will vary across children.
Results of this model indicate that age, t(46) =1.02, p > .05, did not significantly predict pain, but disease severity did, t(46)=2.49, p < .05. Disease severity accounted for approximately 15% of the variance in pain scores between children, such that greater disease severity predicted higher reported pain across all days. The intercept for this model was significant, t(46)=8.98, p<.0001, and showed that for the average child in this sample (i.e., a 12 year, 4 month old child with a disease severity of 31.3 mm), his or her average pain score was predicted to be 25.4 mm, which is consistent with the mild range.
Model 3 – Sleep Quality as a Predictor of Pain intensity
Sleep quality within- and between-children was added to the 3rd model, in order to determine the effects of sleep on pain above and beyond the effects of age and disease severity. A random intercept was included in the model to allow for differences in initial sleep quality levels and random slopes were included to allow for a different relationship between sleep and pain for each child over time.
Children’s daily rating of the previous night’s sleep quality was significantly related to the day’s pain, t(45)= −3.07, p < .05, indicating that poorer sleep quality predicted higher reported pain. Also, children’s overall mean sleep quality significantly predicted pain, t(45) = −5.73, p < .0001. These results confirm that poorer sleep quality predicts higher reported pain from day-to-day and between children. Children’s initial sleep quality scores varied significantly between children, but the pattern of co-occurrence between sleep and pain over time was similar for all children in the sample. The interclass correlations for this model indicate that daily reported sleep quality accounts for 10% of the total within-child variance in pain. In contrast, between-child differences in sleep quality account for nearly half (49%) of the between-child variance in pain. These findings indicate that individual differences in overall sleep quality, rather than daily variations in each child’s sleep experiences, better explain the relationship between sleep quality and pain.
Model 4- Mood and the Interaction between Sleep Quality and Mood as Predictors of Pain Intensity
Three additional terms were entered into the final model (within-child mood, between-child mood, and an interaction term) and all previous model parameters remained the same. The interaction term, composed of within-child sleep quality and mood, was entered to determine if mood moderates the relationship between sleep quality and pain from day-to-day.
Within-child mood significantly predicted daily pain, t(44) = 12.43, p < .0001, but between-child mood did not predict pain, t(44)= .088, p > .05. Therefore, a child’s mean mood did not predict pain, but daily reported mood predicted the pain rating that day. The interaction was significant, t(44)= −3.26, p = .001, showing that the relationship between sleep quality on the previous night and pain that day was moderated by mood that day.
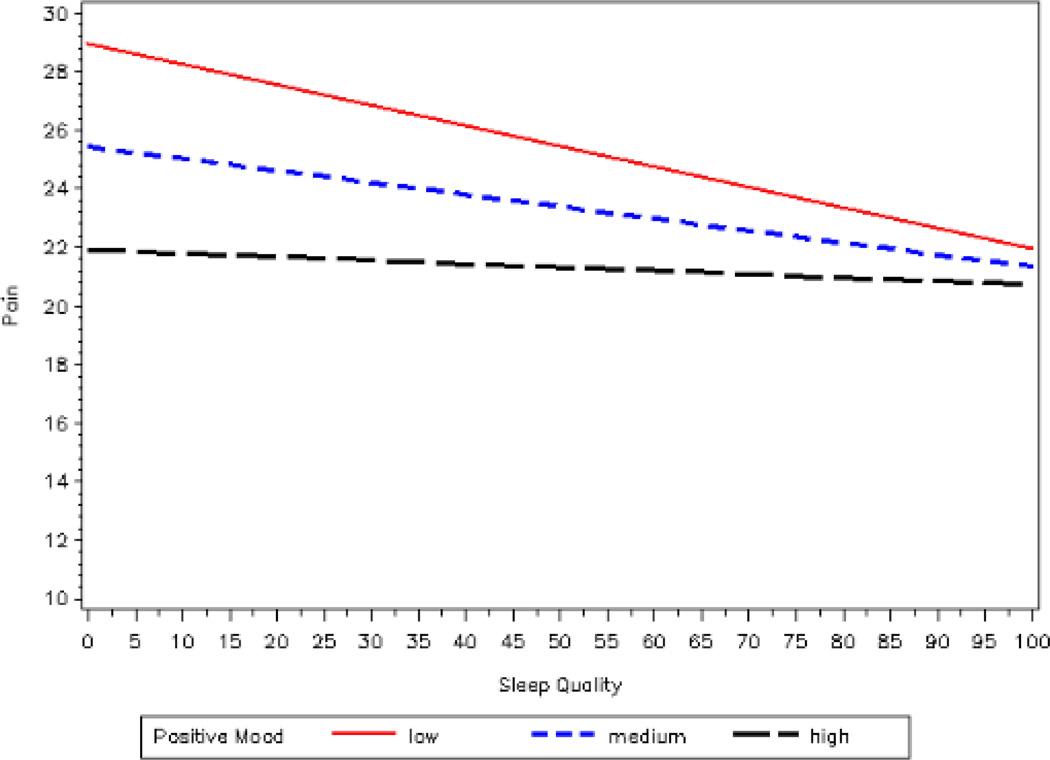
In order to further investigate how daily mood interacted with sleep quality in relation to pain, a series of simple regressions, as outlined by Aiken and West (1991), were conducted to examine the relationship at each of three levels of mood. Mood levels were defined statistically in relation to deviation from the mean: low positive mood (1 standard deviation below the mean), medium positive mood (at the mean), and high positive mood (1 standard deviation above the mean) (Aiken & West, 1991). This method allows results to be immediately comparable with standard ANOVA procedures and outcomes (Aiken & West, 1991). As seen in Figure 1, sleep quality and pain were negatively related at each level of positive mood, indicating that as sleep quality increased, pain decreased correspondingly. This relationship is modified at increasing levels of positive mood, such that as mood became more positive, the influence of poor sleep quality on pain intensity decreased.
Figure 1.
Plot of the interaction between daily sleep quality and daily mood predicting pain
Note. Lower scores on the FAS are considered indicative of more positive mood. We statistically defined the levels of mood for graphing the simple slopes based upon recommendations by Aiken and West (1991). Low positive mood was defined as 1 SD above the mean, medium positive mood was at the mean for the sample, and high positive mood was 1 SD below the mean.
Alternate model- Pain Intensity as a Predictor of Sleep Quality
Based on the model by Lewin and Dahl (1999), which proposed a bidirectional relationship between sleep and pain, a final model was analyzed in order to explore the influence of daily pain on sleep quality that night. Disease severity and age were entered into the model along with within- and between-child pain.
Results of the alternate model indicated that age, t(45)= −0.53, p>.05, disease severity, t(45) = 0.62, p > .05, and daily pain, t(45) = −1.05, p > .05, did not predict within-child sleep quality. However, between-child, mean pain significantly predicted sleep quality, t(45)=−5.07, p<.0001. These findings indicate that pain was a significant predictor of sleep quality between children, but not within children. Therefore, although average levels of pain experienced by each child predicted overall sleep quality, daily variations in reported pain did not predict variations in reported sleep quality each night.
Discussion
Consistent with study hypotheses, the results indicated that children with arthritis who endorsed poorer sleep quality on average, had higher pain intensity scores and this pattern of associations held within children, over time. This finding adds to the results of prior research in children with sickle cell disease (Valrie et al., 2007a, 2007b, 2008) suggesting that sleep may play an important role in painful childhood diseases. Surprisingly, in the present study, daily sleep quality scores accounted for only a small portion of the total within-child variance in pain, whereas between-child sleep quality accounted for nearly half of the variance in pain between children. Children’s sleep quality each night mildly influenced the pain they experienced the next day, but their usual sleep quality greatly contributed to their average self-reported pain. This finding may be due, in part, to limited variability in daily reports of sleep quality in this sample. However, these results indicate that sleep quality is a significant contributor to children’s overall pain experiences and should be routinely assessed and treated as part of clinical care and pain management. Future prospective, longitudinal research should be used to identify specific sleep behaviors and disruptions experienced by children with arthritis for the purpose of intervention development.
Similar to findings by Valrie and colleagues (2008) and consistent with the third hypothesis of the present study, mood moderated the relationship between sleep quality and pain on the following day. Although the measure of daily mood spanned positive to negative mood ratings, the average mood rating across all days was in the positive end of the spectrum. Similarly, with increasingly positive mood, the relationship between poor sleep quality and pain intensity was weaker. Consistent with a positive psychology framework, this suggests that positive affect may serve as a buffer against the harmful effects of poor sleep such that more positive affect promotes resiliency to pain in the context of poor sleep quality. Fredrickson’s (1998, 2001) broaden-and-build theory of positive emotions posits that positive affect promotes exploration and creativity, which develops social, cognitive, psychological, and physical resources. These additional resources may afford more options for coping with a stressor like poor sleep. Consistent with the stress-buffering model (Pressman & Cohen, 2005), positive affect may help facilitate the use of social resources (e.g., seeking out social interactions and support) in the context of stress. Positive affect may be related to greater use or benefit from social support, which could serve to reduce pain complaints. In a few previous studies, negative mood has been identified as a mediator between poor sleep and high pain intensity in individuals with chronic and recurrent pain (O’Brien et al., 2010; Valrie et al., 2008), suggesting that both positive and negative mood are influential in the association between sleep and pain. Future research will need to address the ways in which both positive and negative mood relate to sleep and pain in order to develop interventions aimed at mood enhancement and pain reduction in children with chronic or recurrent pain.
Another issue in the sleep experience of children with recurrent pain is the potential influence of daily pain on sleep. Consistent with previous research (Valrie et al., 2007b), an inverse relationship between sleep and pain was detected in this study and overall levels of pain predicted sleep quality between children; however, results from the current study indicate that sleep quality accounts for a larger portion of the overall variance in pain intensity. In contrast to previous longitudinal research (Valrie et al., 2007a) and theoretical models (Lewin & Dahl, 1999), daily pain did not significantly predict nightly sleep quality within each child. Of note, in contrast to the models used for hypothesis testing, analysis of the alternate hypothesis used lagged reports, such that pain intensity from one diary entry was used to predict sleep quality from the next diary entry. The difference in time between reports may account for the difference in findings. However, when using daily reports, Valrie et al. (2007a) also used lagged reports to test the influence of daytime pain (on evening 1) on sleep that night (as reported on morning 2), suggesting that the differences in results may not be due to using lagged reports. Based on these findings, it will be important to continue to examine sleep quality as a predictor of pain in juvenile arthritis.
A unique strength of this study was the use of prospective, longitudinal data collection. By implementing a prospective daily diary to track day-to-day patterns in sleep, mood, and pain, this study eliminated many potential limitations of cross-sectional studies that capture only a limited sample of behavior and symptoms. Daily diaries are preferable to retrospective summary methods for determining the frequency, characteristics, and effects of chronic and recurrent pain and other symptoms. Whereas retrospective pain assessments conducted at a single time point may introduce increased recall bias (Butz, 2004), daily diary methodology reduces recall bias, allows for naturalistic data collection, and captures day-to-day variations in symptoms such as pain (van der Brink, Bandell-Hoekstra, & Abu-Saad, 2001). Moreover, multiple observations collected over time can be analyzed using multilevel modeling which allows researchers to investigate temporal relationships amongst variables, thus capturing the unique richness of longitudinal data (Schwartz & Stone, 1998).
Although the prospective nature of daily self-reports was a significant strength of the current study, children completed the diary only once per day, in the evening. Therefore, daily experiences may have influenced recall of the previous night’s sleep quality. Although children were prompted to report the time and date each diary was completed, the procedures for completing and collecting the paper diaries provided no protection against backfilling. Thus, there is no guarantee that participants completed diary entries at the instructed or reported times. Furthermore, a number of limitations are associated with the use of paper diaries, such as the influence of motivation on entry completion (Broderick & Stone, 2006), and the influence of memory processes on any retrospective ratings (Takarangi, Garry, & Loftus, 2006). Future studies using electronic assessments of pain and sleep with more frequent monitoring and data capture in real time would address many of these concerns. Using electronic assessments, participants may receive reminder alarms to cue responses at preselected times as well as encouragement via electronic messages to promote future reporting after completing each entry. Additionally, surveys can be locked after the end of the assessment period, all data can be time stamped, and participants may complete in-the-moment reports of current symptoms and functioning using mobile electronic devices. Indeed, recent studies have begun to establish the validity of the use of electronic diaries with children with arthritis (Stinson et al., 2008; Connelly et al., 2010).
There are limitations with the assessment of sleep quality in that it depended on one global, single-item sleep quality measure. Other aspects of sleep, such as sleep duration and sleep disruption may not have been captured by this measure. Even when the individual perceives high sleep quality, specific sleep disturbances that cannot be captured via self-report (e.g., less time spent in deep sleep) may occur and adversely affect daytime functioning. As Bourguignon and colleagues (2003) point out in their review of sleep in adults with rheumatoid arthritis, individuals with chronic, specific sleep problems as captured by polysomnography (PSG), may acclimate to a certain level of sleep disturbance, influencing their perceptions of adequate sleep quality. Further research might also include physiological measures such as PSG and actigraphy to provide additional information about other sleep domains and sleep architecture. Measuring the influence of specific sleep problems as well as overall sleep quality on pain, symptoms, and function will help inform the development of effective pain and sleep management interventions.
There are also limitations related to the study sample. Children in the sample generally had mild to moderate disease, thus recruiting a sample of convenience may have led to an underrepresentation of children with severe disease. However, a significant relationship between daily sleep and pain was detected in this sample of children despite less severe disease and sleep disruptions; pain may be an even greater concern in children with more severe arthritis. Furthermore, in addition to the aspects of the disease experience incorporated in the present study, there are many other potentially important factors to include, particularly medication use. Controlling for medications is likely important as research in adults has suggested that NSAIDs have a negative effect on sleep (Murphy, Badia, Myers, Boecker, & Wright; 1994); however, research in adults with arthritis has suggested that NSAIDs may improve the subjective sleep experience (Lavie, Nahir, Lorber, & Scharf, 1991). Daily medication use was not assessed as part of this study. Therefore, the direct relationship between sleep and medications commonly used to treat pediatric arthritis remains to be determined.
In addition to other medical variables, other psychosocial predictors of sleep and pain may be important to consider in future research. Family level variables such as family environment (Hanson & Chen, 2010) and stress (Sadeh et al., 2000) may influence daily sleep. It is also possible that a stable trait variable, such as temperament or trait anxiety, may account for the significant between-child association between sleep and pain. For example, Fuller et al. (1997) found that higher trait anxiety was associated with greater sleep disruptions in adults via polysomnography. Similarly, children with anxiety disorders often experience sleep difficulties such as initiating or maintaining sleep, nightmares, and reluctance or refusal to sleep alone (Alfano, Ginsberg, & Kingery, 2007). Anxiety has also been identified as a predictor of pain in juvenile arthritis (Schanberg et al., 2003). Finally, the adult pain literature has established associations between personality traits, such as neuroticism and pain (Affleck, Tennen, Urrows, & Higgins, 1992; Goubert, Cromberz, & Van Damme, 2004) and there is some evidence that temperament is also associated with pain in children (Ranger & Campbell-Yeo, 2008). Thus, further research is needed to investigate the role of such characteristics in explaining sleep and pain in children with arthritis.
Although other factors that relate to sleep and pain in children with arthritis remain to be determined, the current findings clearly support an association between children’s sleep quality, pain, and mood. Our findings have multiple implications for clinical care. These results support the need for routine sleep assessment or sleep monitoring of children with arthritis. Healthcare providers may also consider implementing interventions targeting sleep quality when developing arthritis-pain treatment plans. In addition to sleep hygiene and pain management, interventions to improve children’s daily mood are indicated, as targeting this moderating variable is an avenue to reducing the relationship between poor sleep quality and pain.
Acknowledgement
This research was supported by an American College of Rheumatology Research and Education Foundation/Abbott Health Professional Graduate Student Research Preceptorship. Other support for this research was provided by a grant from the Office of Research on Women’s Health in conjunction with the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR-45238), as well as the Arthritis Foundation.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/hea.
Contributor Information
Maggie H. Bromberg, University of North Carolina at Chapel Hill
Karen M. Gil, University of North Carolina at Chapel Hill
Laura E. Schanberg, Duke University Medical Center
References
- Affleck G, Tennen H, Urrows S, Higgins P. Neuroticism and the pain-mood relation in rheumatoid arthritis: Insights from a prospective daily study. Journal of Consulting and Clinical Psychology. 1992;60(1):119–126. doi: 10.1037//0022-006x.60.1.119. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. London, England: Sage Publications; 1991. [Google Scholar]
- Alfano CA, Ginsburg GS, Kingery JM. Sleep-related problems among children and adolescents with anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;42:224–232. doi: 10.1097/01.chi.0000242233.06011.8e. [DOI] [PubMed] [Google Scholar]
- Amos CE, Curry MR, Drutz IE, Frost JD, Warren RW. Sleep disruption in school-aged children with JRA. Journal of Rheumatology. 1997;40:S244. [Google Scholar]
- Argoff CE. The coexistence of neuropathic pain, sleep, and psychiatric disorders: A novel treatment approach. Clinical Journal of Pain. 2007;23:15–22. doi: 10.1097/01.ajp.0000210945.27052.b3. [DOI] [PubMed] [Google Scholar]
- Bloom BJ, Owens JA, McGuinn M, Nobile C, Schaeffer L, Alario AJ. Sleep and its relationship to pain, dysfunction, and disease activity in juvenile rheumatoid arthritis. Journal of Rheumatology. 2002;29:169–173. [PubMed] [Google Scholar]
- Broderick JE, Stone AA. Paper and electronic diaries: Too early for conclusions on compliance rates and their effects—Comment on Green, Rafaeli, Bolger, Shrout, and Reis (2006) Psychological Methods. 2006;11:106–111. doi: 10.1037/1082-989X.11.1.106. [DOI] [PubMed] [Google Scholar]
- Butz A. Use of health diaries in pediatric research. Journal of Pediatric Health Care. 2004;18(5):262–263. doi: 10.1016/j.pedhc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Germain A, Nofzinger EA, Kupfer DJ. Mood disorders and sleep. In: Stein DJ, Kupfer DJ, Schatzberg AF, editors. The American Psychiatric Publishing Textbook of Mood Disorders. Washington, DC: American Psychiatric Publishing, Inc.; 2006. pp. 717–737. [Google Scholar]
- Chambers C, Corkum PV, Rusak B. Commentary: The importance of sleep in pediatric chronic pain – A wakeup call for pediatric psychologists. Journal of PediatricPsychology. 2008;33:333–334. doi: 10.1093/jpepsy/jsn001. [DOI] [PubMed] [Google Scholar]
- Chorney DB, Detweiler MF, Morriss TL, Kuhn BR. The interplay of sleep disturbance, anxiety and depression in children. Journal of Pediatric Psychology. 2008;33:339–348. doi: 10.1093/jpepsy/jsm105. [DOI] [PubMed] [Google Scholar]
- Connelly M, Anthony KK, Sarniak R, Bromberg MH, Gil KM, Schanberg LE. Parent pain responses as predictors of daily activities and mood in children with juvenile idiopathic arthritis: The utility of electronic diaries. Journal of Pain and Symptom Management. 2010;39:579–590. doi: 10.1016/j.jpainsymman.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL. What good are positive emotions? Review of General Psychology. 1998;2:300–319. doi: 10.1037/1089-2680.2.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL. The role of positive emotions in positive psychology: The broaden-and-build theory of positive emotions. American Psychologist. 2001;56:218–226. doi: 10.1037//0003-066x.56.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller KH, Waters WF, Binks PG, Anderson T. Generalized anxiety and sleep architecture: A polysomnographic investigation. Sleep. 1997;20:370–376. doi: 10.1093/sleep/20.5.370. [DOI] [PubMed] [Google Scholar]
- Gaina A, Sekine M, Chen X, Hamanishi S, Kagamimori S. Validity of Child Sleep Diary Questionnaire among junior high school children. Journal of Epidemiology. 2004;14:1–4. doi: 10.2188/jea.14.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis & Rheumatism. 1997;40:1202–1209. doi: 10.1002/1529-0131(199707)40:7<1202::AID-ART3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Gil KM, Carson JW, Porter LS, Ready J, Valrie CR, Redding-Lallinger R, Daeschner C. Daily stress and mood and their association with pain, health-care use, and school activity in adolescents with sickle cell disease. Journal of Pediatric Psychology. 2003;28:363–373. doi: 10.1093/jpepsy/jsg026. [DOI] [PubMed] [Google Scholar]
- Gil KM, Porter L, Ready J, Workman E, Sedway J, Anthony KK. Pain in children and adolescents with sickle cell disease: An analysis of daily pain diaries. Children's Health Care. 2000;29(4):225–241. [Google Scholar]
- Gonzalez JS, Penedo FJ, Antoni MH, Durán RE, McPherson-Baker S, Ironson G, Schneiderman N. Social support, positive states of mind, and HIV treatment adherence in men and women living with HIV/AIDS. Health Psychology. 2004;23:413–418. doi: 10.1037/0278-6133.23.4.413. [DOI] [PubMed] [Google Scholar]
- Goubert L, Crombez G, Van Damme S. The role of neuroticism, pain catastrophizing and pain-related fear in vigilance to pain: A structural equations approach. Pain. 2004;107:234–241. doi: 10.1016/j.pain.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Hanson MD, Chen E. Daily stress, cortisol, and sleep: The moderating role of childhood psychosocial environments. Health Psychology. 2010;29:394–402. doi: 10.1037/a0019879. [DOI] [PubMed] [Google Scholar]
- Knab B, Engel-Sittenfeld P. The many facets of poor sleep. Neuropsychology. 1983;10:141–147. doi: 10.1159/000118001. [DOI] [PubMed] [Google Scholar]
- Labyak SE, Bourguignon C, Docherty S. Sleep quality in children with juvenile rheumatoid arthritis. Holistic Nursing Practices. 2003;17(4):193–200. doi: 10.1097/00004650-200307000-00006. [DOI] [PubMed] [Google Scholar]
- Lavie P, Nahir M, Lorber M, Scharf Y. Nonsteroidal anti-inflammatory drug therapy in rheumatoid arthritis patients: Lack of association between clinical improvement and effects on sleep. Arthritis and Rheumatism. 1991;34:655–664. doi: 10.1002/art.1780340605. [DOI] [PubMed] [Google Scholar]
- LeBovidge JS, Lavigne JV, Donenberg GR, Miller ML. Psychological adjustment of children and adolescents with chronic arthritis: A meta-analytic review. Journal of Pediatric Psychology. 2003;28(1):29–39. doi: 10.1093/jpepsy/28.1.29. [DOI] [PubMed] [Google Scholar]
- Lewin DS, Dahl RE. Importance of sleep in the management of pediatric pain. Developmental and Behavioral Pediatrics. 1999;20:244–252. doi: 10.1097/00004703-199908000-00007. [DOI] [PubMed] [Google Scholar]
- Lewandowski AS, Palermo TM, De la Mott S, Fu R. Temporal daily associations between pain and sleep in adolescents with chronic pain versus healthy adolescents. Pain. 2010;15:220–225. doi: 10.1016/j.pain.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malleson PN, Oen K, Cabral DA, Petty RE, Rosenberg AM, Cheang M. Predictors of pain in children with established juvenile rheumatoid arthritis. Arthritis and Rheumatism. 2004;51:222–227. doi: 10.1002/art.20238. [DOI] [PubMed] [Google Scholar]
- McGrath PA, de Veber LL, Hearn MT. Multidimensional pain assessment in children. In: Fields HL, Dubner R, Cervero F, editors. Advances in Pain Research and Therapy. New York: Raven Press; 1985. pp. 387–393. [Google Scholar]
- McGrath PA, Seifert CE, Speechley KN, Booth JC, Stitt L, Gibson MC. A new analogue scale for assessing children: An initial validation study. Pain. 1996;64:435–443. doi: 10.1016/0304-3959(95)00171-9. [DOI] [PubMed] [Google Scholar]
- Meltzer LJ, Mindell JA. Sleep and sleep disorders in children and adolescents. Psychiatric Clinics of North American. 2006;29:1059–1076. doi: 10.1016/j.psc.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Murphy PJ, Badia P, Myers BL, Boecker MR, Wright KP. Nonsteroidal anti-inflammatory drugs affect normal sleep patterns in humans. Physiology and Behavior. 1994;55:1063–1066. doi: 10.1016/0031-9384(94)90388-3. [DOI] [PubMed] [Google Scholar]
- O’Brien EM, Waxenberg LB, Atchinson JW, Gremillion HA, Staud RM, McCrae, Robinson ME. Negative mood mediates the effect of poor sleep on pain among chronic pain patients. Clinical Journal of Pain, x. 2010;26:310–319. doi: 10.1097/AJP.0b013e3181c328e9. [DOI] [PubMed] [Google Scholar]
- Passarelli CM, Roizenblatt S, Len CA, Moreira GA, Lopes MC, Guilleminault C, Hilario MOE. A case-controlled sleep study in children with polyarticular juvenile rheumatoid arthritis. Journal of Rheumatology. 2006;33:796–802. [PubMed] [Google Scholar]
- Pressman SD, Cohen S. Does positive affect influence health? Psychological Bulletin. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Ranger M, Campbell-Yeo M. Temperament and pain response: A review of the literature. Pain Management Nursing. 2008;9(1):2–9. doi: 10.1016/j.pmn.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods, Second Edition. Thousand Oaks, CA: Sage Publications, Inc.; 2002. [Google Scholar]
- Sadeh A, Raviv A, Gruber R. Sleep patterns and sleep disruptions in school-agechildren. Developmental Psychology. 2000;36:291–301. doi: 10.1037//0012-1649.36.3.291. [DOI] [PubMed] [Google Scholar]
- Sandstrom MJ, Cillessen AHN, Eisenhower A. Children’s appraisal of peer rejection experiences: Impact on social and emotional adjustment. Social Development. 2003;12:530–550. [Google Scholar]
- SAS Institute. The SAS System, Release 9.2. Cary, NC: Author; 2008. [Computer software] [Google Scholar]
- Schanberg LE, Anthony KK, Gil KM, Maurin EC. Daily pain and symptoms in children with polyarticular arthritis. Arthritis & Rheumatism. 2003;48:1390–1397. doi: 10.1002/art.10986. [DOI] [PubMed] [Google Scholar]
- Schanberg LE, Gil KM, Anthony KK, Yow E, Rochon J. Pain, stiffness, and fatigue in juvenile polyarticular arthritis. Arthritis & Rheumatism. 2005;52(4):1196–1204. doi: 10.1002/art.20952. [DOI] [PubMed] [Google Scholar]
- Schanberg LE, Sandstrom MJ, Starr K, Gil KM, Lefebvre JC, Keefe FJ, Tennen H. The relationship of daily mood and stressful events to symptoms in juvenile rheumatic disease. Arthritis & Rheumatism. 2000;13(1):33–41. doi: 10.1002/1529-0131(200002)13:1<33::aid-art6>3.0.co;2-s. 1<33::AID-ART6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Schwartz JE, Stone AA. Strategies for analyzing ecological momentary assessment. Health Psychology. 1998;17:6–16. doi: 10.1037//0278-6133.17.1.6. [DOI] [PubMed] [Google Scholar]
- Schwartz JRL, Roth T. Neurophysiology of sleep and wakefulness: Basic science and clinical implications. Current Neuropharmacology. 2008;6:367–378. doi: 10.2174/157015908787386050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson JN, Stevens BJ, Feldman BM, Streiner D, McGrath PJ, Dupuis A, Petroz GC. Construct validity of a multidimensional electronic pain diary for adolescents with arthritis. Pain. 2008;136:281–292. doi: 10.1016/j.pain.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Takarangi MKT, Garry M, Loftus EF. Dear diary, is plastic better than paper? I can’t remember: Comment on Green, Rafaeli, Bolger, Shrout, and Reis (2006) Psychological Methods. 2006;11:119–122. doi: 10.1037/1082-989X.11.1.119. [DOI] [PubMed] [Google Scholar]
- Valrie CR, Gil KM, Redding-Lallinger R, Daeschner C. The influence of pain and stress on sleep in children with sickle cell disease. Children's Health Care. 2007a;36(4):335–353. [Google Scholar]
- Valrie CR, Gil KM, Redding-Lassiter R, Daeschner C. Sleep in children with sickle cell disease: An analysis of daily diaries using multilevel models. Journal of Pediatric Psychology. 2007b;32(7):857–861. doi: 10.1093/jpepsy/jsm016. [DOI] [PubMed] [Google Scholar]
- Valrie CR, Gil KM, Redding-Lallinger R, Daeschner C. Daily mood as a moderator of the pain-sleep relationship in children with sickle cell disease. Journal of Pediatric Psychology. 2008;33:317–322. doi: 10.1093/jpepsy/jsm058. [DOI] [PubMed] [Google Scholar]
- van der Brink M, Bandell-Hoekstra ENG, Huijer Abu-Saad H. The occurrence of recall bias in pediatric headache: A comparison of questionnaires and diary data. Headache. 2001;41:11–20. doi: 10.1046/j.1526-4610.2001.111006011.x. [DOI] [PubMed] [Google Scholar]
- Varni JW, Thompson KL, Hanson V. The Varni/Thompson pediatric pain questionnaire. I: Chronic musculoskeletal pain in juvenile rheumatoid arthritis. Pain. 1987;28(1):27–38. doi: 10.1016/0304-3959(87)91056-6. [DOI] [PubMed] [Google Scholar]
- Ward TM, Brandt R, Archbold K, Lentz M, Ringold S, Wallace CA, Landis CA. Polysomnography and self-reported sleep, pain, fatigue, and anxiety in children with active and inactive juvenile rheumatoid arthritis. Journal of Pediatric Psychology. 2008;33:232–241. doi: 10.1093/jpepsy/jsm121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA, Acebo C, Seifer R, Fallone G, Labyak SE, Martin JL. Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003;26:213–216. doi: 10.1093/sleep/26.2.213. [DOI] [PubMed] [Google Scholar]
- Zamir G, Press J, Asher T, Tarasiuk A. Sleep fragmentation in children withjuvenile rheumatoid arthritis. Journal of Rheumatology. 1998;25:1191–1197. [PubMed] [Google Scholar]