Abstract
Background
Cumulative adversity and stress are associated with risk of psychiatric disorders. While basic science studies show repeated and chronic stress effects on prefrontal and limbic neurons, human studies examining cumulative stress and effects on brain morphology are rare. Thus, we assessed whether cumulative adversity is associated with differences in gray matter volume, particularly in regions regulating emotion, self-control, and top-down processing in a community sample.
Methods
One hundred three healthy community participants, aged 18 to 48 and 68% male, completed interview assessment of cumulative adversity and a structural magnetic resonance imaging protocol. Whole-brain voxel-based-morphometry analysis was performed adjusting for age, gender, and total intracranial volume.
Results
Cumulative adversity was associated with smaller volume in medial prefrontal cortex (PFC), insular cortex, and subgenual anterior cingulate regions (familywise error corrected, p <.001). Recent stressful life events were associated with smaller volume in two clusters: the medial PFC and the right insula. Life trauma was associated with smaller volume in the medial PFC, anterior cingulate, and subgenual regions. The interaction of greater subjective chronic stress and greater cumulative life events was associated with smaller volume in the orbitofrontal cortex, insula, and anterior and subgenual cingulate regions.
Conclusions
Current results demonstrate that increasing cumulative exposure to adverse life events is associated with smaller gray matter volume in key prefrontal and limbic regions involved in stress, emotion and reward regulation, and impulse control. These differences found in community participants may serve to mediate vulnerability to depression, addiction, and other stress-related psychopathology.
Keywords: Brain MRI, chronic stress, cumulative adversity, gray matter volume, life trauma, prefrontal cortex, recent adverse life events
Cumulative adversity and repeated exposure to stressful life events are known to increase risk of mood and anxiety disorders (1–6) and addictive disorders (2,5,7). The neurobiology of these disorders identifies the corticostriatal-limbic brain circuits as critical to the pathophysiology of these illnesses (7–9). This circuitry, which includes regions of the prefrontal cortex (PFC), insular cortex, the hippocampus, amygdala, and ventral and dorsal striatum, is also known to mediate the allostatic processes involved in experiencing and coping with stressful experiences (10,11), but the effects of repeated stress exposure or cumulative adversity and chronic stress on changes in brain morphology in humans are not well studied.
Basic science research has shown that repeated and chronic stress changes brain structure, morphology, and function in key regions of the corticostriatal-limbic circuitry, particularly in the PFC (10–12). Stress affects gray matter volume through a number of possible mechanisms, including loss of neurons, decreased dendritic branching, spine density, and/or decreased neurogenesis (12–19). For example, restraint stress in rats resulted in a 16% decrease in dendritic spine density and a 20% decrease in total length and branch number in medial PFC (mPFC) pyramidal neurons (18). In another study, restraint stress in rats resulted in a 20% retraction of apical dendritic arbors in the mPFC, while simultaneously resulting in an increase by 43% in the apical dendritic arborization in the orbitofrontal cortex (15). Loss of synaptic connections, particularly between the medial prefrontal and orbitofrontal cortex regions and amygdala, has been associated with loss of inhibitory affective control and presence of clinical mood disorders (8,20–22). Interestingly, some of these changes may occur in very brief periods of time following a stressor (23,24).
The majority of human research assessing brain changes associated with stress has focused on psychiatric samples of patients with stress-related disorders, such as mood, anxiety, and addictive disorders. For example, a recent meta-analysis (22) of brain volume differences in patients with major depression revealed that the predominant differences in volume occurred in the frontal lobe regions, specifically prefrontal and orbitofrontal cortex, areas associated with emotional processing and stress responsivity. In addition, the subgenual anterior cingulate cortex gray matter volume is significantly reduced in patients with mood disorders (25). Gray matter reductions in the prefrontal regions appear to be similar in the pathophysiology of depression versus posttraumatic stress disorder (26). Among patients with alcohol or substance use disorders, neural changes in frontal, insular, and limbic striatal regions have been reported (27–29). Interestingly, two studies with nonpsychiatric samples also report decreased volume in corticostriatal-limbic regions, such as the hippocampus, orbitofrontal cortex, insula, anterior cingulate, and medial prefrontal cortex, associated with greater perceived stress (30) and greater geographical proximity to events on 9/11 (31). Thus, changes in gray matter volume are associated with stress-related psychiatric disorders but also with a recent adverse life event and perceived stress, suggesting that some stress-related changes in gray matter volume may act as vulnerability markers that precede the presence of stress-related psychiatric disorders.
Therefore, to clarify the role of stress and adversity on differences in brain morphology, the current study assessed effects of cumulative adversity and stress exposure on brain morphology using whole-brain voxel-based morphometric analysis of structural magnetic resonance imaging (MRI) scans in a nonpsychiatric community sample. Cumulative adversity and chronic stress were assessed using the Cumulative Adversity Interview (CAI) (32), a well-established checklist of specific stressful events that have accumulated over the lifetime (recent events, life trauma, major life events) and chronic stress based on the subjects’ perceived appraisal of distress. This assessment has been previously used in large prospective studies of stressful life events and chronic stress examining risk for substance and psychiatric disorders (2,3,5,33–37). Recent research has found that greater cumulative stress, as measured by the CAI, was associated with maladaptive physiological stress processes, such as heart rate variability (38) and greater neural responses to stress in a community sample (39). Based on previous basic science and human research and the known changes in brain regions involved in stress regulation and stress-related psychiatric disorders, we hypothesized that higher cumulative adversity and chronic stress would be associated with lower brain volume in the medial prefrontal cortex and limbic regions, such as the insular cortex, amygdala, and hippocampus. Given that the two prior studies examined specific types of stress, such as recent life events and perceived stress, we also explored whether specific types of stressful life events (e.g., trauma, recent life events, major life events, or perceived chronic stress) and the interaction of cumulative stressful life events with the experience of subjective, chronic stress may have distinct associations with structural morphology.
Methods and Materials
Participants
One hundred three individuals, 70 (68%) men and 33 (32%) women, were recruited from a community sample who responded to advertisements placed either online or in local newspapers and at a community center in and around the New Haven area. All participants were required to be between the ages of 18 and 50 years, able to read and write in English to at least a sixth grade level, and meet stringent health requirements assessed by a specialist research nurse. Exclusion criteria included DSM-IV dependence for any drug or alcohol other than nicotine, head injury, or use of prescribed medications for any psychiatric or medical disorders. All participants gave both written and verbal informed consent and the study was approved by the Human Investigation Committee of the Yale University School of Medicine. Demographics for the sample are reported in Table 1.
Table 1.
Demographics of Sample (n = 103)
| Age | |
| Mean (SD) | 27.71 (7.98) |
| Median | 25 |
| Range | 18–48 |
| Years of Educationa | 15.25 (1.97) |
| Gender (% Male) | 70 (68) |
| Race | |
| African American, n (%) | 20 (19) |
| Caucasian, n (%) | 72 (70) |
| Hispanic, n (%) | 5 (5) |
| Asian, n (%) | 3 (3) |
| Other, n (%) | 3 (3) |
| Cumulative Adverse Life Eventsa | 8.49 (5.10) |
| Major Life Eventsa | 1.57 (1.42) |
| Life Traumasa | 4.61 (3.42) |
| Recent Life Eventsa | 2.30 (2.14) |
| Chronic Stressorsa | 7.79 (4.39) |
SD, standard deviation.
Mean values (standard deviations); all other measures reported in frequency (percents).
Potential subjects completed an initial screening over the telephone to determine eligibility based on inclusion/exclusion criteria. Following initial eligibility screening, participants were scheduled for an assessment session and a scanning session. During the first session, participants met with research assistants to complete informed consent forms and complete medical, substance abuse, and psychiatric health assessments including the Structured Clinical Interview for DSM-IV (40) and the CAI (32). Participants received a physical examination with a research nurse assessing cardiovascular, renal, hepatic, pancreatic, hematopoietic, and thyroid function to ensure all participants were in good health. During a subsequent session, participants underwent MRI data acquisition. Breathalyzer and urine toxicology screens were conducted at each appointment to ensure drug-free status among participants and to verify smoking status.
Cumulative Adversity Interview (CAI)
This 140-item interview is a multifaceted assessment of stressful life events and chronic, subjective stress previously used in prospective, longitudinal research on stress and psychopathology (2–5,32–37). Additional information on the content, reliability, and frequencies of events can be found in Supplement 1. Trained interviewers ask participants about the occurrence and frequency of specific major life events, recent life events, life trauma, and chronic stressors during their lifetime. Prior research supports the use of interviewers to efficiently improve reliability and validity of retrospective reports of stressful life events (41).
MRI Data Acquisition
Subjects were scanned on a Siemens 3 Tesla scanner (Trio; Siemens AG, Erlangen, Germany). Data for each subject consisted of a single sagittally acquired high-resolution T1-weighted magnetization prepared rapid acquisition gradient-echo scan: 176 slices, 1 mm3 isotropic voxels, field of view = 256 × 256 mm, data acquisition matrix = 256 ×256, repetition time = 2530 msec, echo time = 3.66 msec, flip angle = 7°.
Segmentation and Registration
Image segmentation and registration were performed using the VBM8 toolbox (C. Gaser, Department of Psychiatry, University of Jena, Germany; http://dbm.neuro.uni-jena.de/vbm8) in Statistical Parametric Mapping 8 (SPM8; Wellcome Trust Centre for Neuroimaging, University College London, England). After applying a nonlocal means de-noising filter to improve signal-to-noise ratio to the data (42), VBM8 uses an adaptive maximum a posteriori technique (43) and a hidden Markov random field (44) to determine the optimal segmentation. The segmentation procedure also models partial volume effects to account for image voxels that are mixtures of pure tissue types (45). As all model parameters are estimated from the image data, VBM8 does not use tissue probability maps for tissue classification.
As manual segmentation is considered the gold standard for evaluating the quality of automated tissue classification (e.g., [46,47]), the resulting segmentations were validated visually (cf. [48,49]). Particular attention was given to the thickness of the cortical surface, which was compared visually with each subject’s native space image. Gray matter segmentation demonstrated appropriate face validity in all images.
The outputs from the segmentation procedure were rigid-body aligned segmentations for each subject. The gray matter, white matter, and cerebrospinal fluid segmentations were input into DARTEL (diffeomorphic anatomical registration through exponentiated lie algebra) (50) in SPM8. DARTEL is a high-dimensional, diffeomorphic registration algorithm that has performed well in a comparison of registration algorithms (51). DARTEL creates an average template from the data, and the images are registered to this space. Therefore, in an additional step, the DARTEL-registered data were affine-transformed to Montreal Neurological Institute (MNI) space, modulated, and smoothed with a 6-mm Gaussian filter. With modulation, voxel values reflect relative local volume differences between images. Gaussian smoothing reduces the effects of residual misregistration on potential group differences and reduces departures from normality that may occur at some voxels (52). The default parameter settings were also used for all steps of the DARTEL registration. Final outputs were smoothed, modulated gray matter segments (1.5 mm^3 voxels).
Data Analysis
Statistical parametric maps were created in SPM8 to perform between-group comparisons using the smoothed, modulated, and normalized gray matter tissue segmentations output by DARTEL. Separate regression models were created with each scale derived from the CAI as the independent variable and local gray matter volume as the dependent measure. Covariates controlled for subject age, gender, and an estimate of total intracranial volume calculated by voxel-wise summation in a MATLAB script (The MathWorks, Inc., Natick, Massachusetts; Ged Ridgway, http://www.cs.ucl.ac.uk/staff/g.ridgway/vbm/get_totals.m) of the native space gray, white, and cerebrospinal fluid segmentations for each subject.
Whole-Brain Analysis
The whole-brain statistical analysis was conducted using random field-based cluster-size testing (cf. [53]) and familywise error rate (FWE) correction for multiple comparisons. The cluster size test increases sensitivity, relative to voxel-based tests, for spatially extended signals (54), and low thresholds increase the power of these tests for signals of large spatial extent (55). The analysis of cluster extent also better characterizes the spatially distributed nature of group differences within spatially smooth data (56). Clusters were formed from contiguous voxels exceeding an uncorrected one-tailed threshold of p < .025. The FWE corrected threshold for significant cluster size was set at one-tailed p < .05.
Anatomical regions within significant clusters were initially identified in statistical parametric mapping outputs from MNI space coordinates, using the Automated Anatomy Library (57) within the Wake Forest University PickAtlas (ANSIR Laboratory, Wake Forest University School of Medicine, Winston-Salem, North Carolina) (58). Anatomical regions were confirmed and Brodmann areas (BA) determined using the Talairach Atlas (Research Imaging Center, University of Texas Health Science Center, San Antonio, Texas) (after conversion to Talairach coordinates, MNI to Talairach conversion was performed using the Nonlinear Yale MNI to Talairach Conversion Algorithm) (59).
Results
Sample means and standard deviations for CAI scales are displayed in Table 1. Correlations between CAI subscales, age, and education are provided in Supplement 1. Traumatic life events had a modest, but significant, positive association with age. In addition, the combined cumulative adverse life events counts (CALE) score had a modest but significant correlation with chronic stress (r = .23), supporting assertions that both scales may reflect distinct measures of stressful experiences.
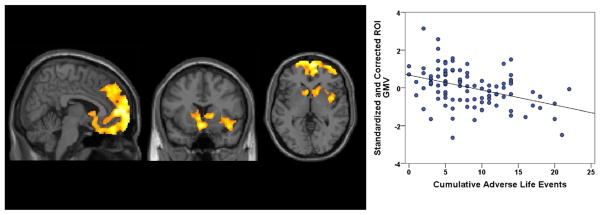
A summary of regions in which smaller gray matter volume (GMV) was observed for each CAI scale is provided in Supplement 1. A negative relationship was found between gray matter volume and the measure of CALE in a large region in the medial prefrontal cortex, anterior cingulate, and right insula (FWE p < .001, cluster extent 20,679 voxels; Figure 1). This cluster further included gyrus rectus and orbitofrontal cortex (BA 11/10) bilaterally and extended in the superior direction to include the dorsomedial prefrontal cortex (BA 10/9). This cluster also extended laterally into the right inferior frontal gyrus (BA 47), the frontopolar cortex (BA 10) bilaterally, the anterior cingulate (BA 24/32), ventral striatum, caudate, and most of the right insular cortex. There were no FWE-corrected associations between CAI scale and regions with increased volume identified in our analyses.
Figure 1.
Using whole-brain voxel-based morphometry, more cumulative adverse life events were associated with lower mean gray matter volume (GMV), after controlling for age, sex, and total intracranial volume in a region (20,679 voxels; familywise error p < .001) of the medial prefrontal cortex, anterior cingulate, and right insula (x = 5.4, y = 13.5, z = 11.5). R2 for regression line = .17. ROI, region of interest identified in the whole-brain voxel-based morphometry.
To examine whether other sociodemographic or psychiatric variables may account for the CAI associations with GMV, we ran additional models examining CALE associations with GMV. The Shipley Institute of Living Scale (60), which is a global measure of cognitive functioning, was used to assess IQ and the Center for Epidemiologic Studies Depression Scale (61) was used to assess depressive symptoms. In addition, smoking status and education level were obtained from intake interviews. All models remained significant (FWE corrected) when smoking (p < .001), depression (p <.001), IQ (p <.001), or education level (p <.001) were covaried. Only small changes in cluster size were observed and regions remained the same.
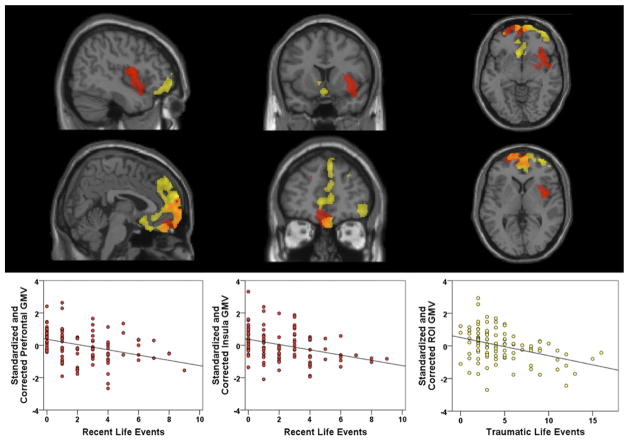
Among the individual CAI subscales, no differences in gray matter volume were identified for the major life events or chronic stress subscales. However, a significant negative relationship was found between gray matter volume and the measure of recent life events in the medial prefrontal cortex (FWE p <.001, cluster extent = 7131 voxels) and the right insula (FWE p = .028, cluster extent = 3893 voxels), as depicted in red in Figure 2. The cluster in medial PFC included gyrus rectus and orbitofrontal cortex bilaterally (BA 11/10); frontopolar cortex (BA 10), mostly in the left hemisphere; and extended into dorsomedial PFC (BA 9). The cluster in the right insula extended across most of this region and also included parts of right orbitofrontal cortex (BA 10/11), putamen, and a small area of the right superior temporal gyrus (including BA 22 and 38).
Figure 2.
Using whole-brain voxel-based morphometry, more frequent recent life events and more traumatic life events were associated with lower mean gray matter volume (GMV) after controlling for age, sex, and total intracranial volume. Regions of interest are displayed (top row: x = 43.5; y = 13.5, z = −6; bottom row: x = 4.0, y = 47.0, z = 3.0) with recent life events regions in red, traumatic life events regions in yellow, and overlapping regions indicated in orange. Recent life events are associated with a region of the medial prefrontal cortex (7131 voxels; familywise error [FWE] p = .001) and the right insula (3893 voxels; FWE p = .028). R2 for the recent life events prefrontal region regression line = .13 and for the insula region = .13. Traumatic life events are associated with a region (12,835 voxels; FWE p <.001) of the medial prefrontal cortex, anterior cingulate, and subgenual areas. R2 for the traumatic life events regression line = .15. ROI, region of interest identified in the whole-brain voxel-based morphometry.
Gray matter volume was also negatively related to the life trauma subscale in the medial prefrontal cortex (FWE p < .001, cluster extent 12,835 voxels), including bilateral gyrus rectus and orbitofrontal cortex (BA 11/10), as well as Brodmann areas 9 and 8 in the dorsomedial PFC. This cluster also extended laterally into the right inferior frontal gyrus (BA 47) and frontopolar cortex bilaterally (BA 10), posteriorly into the rostral anterior cingulate (BA 24/32), and the subgenual area (BA 25), ventral striatum, and caudate. This is depicted in yellow in Figure 2. Overlap in regions between recent life events and trauma regions are indicated in orange.
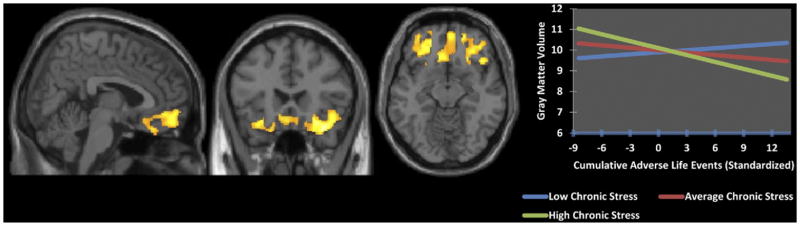
The interaction of CALE × chronic stress was also examined, controlling for the main effects of CALE and chronic stress. Greater accumulation of adverse life events with a greater subjective experience of chronic stress was associated with less gray matter volume within a cluster in the orbitofrontal cortex (FWE p < .001, cluster extent 7175 voxels; Figure 3). This cluster extended bilaterally in the orbitofrontal cortex (BA 11/10) and inferior frontal gyrus (BA 45/47) and the subgenual cingulate region (BA 25), as well as into the left ventral anterior cingulate (BA 32) and right anterior insula.
Figure 3.
Using whole-brain voxel-based morphometry, more cumulative adverse life events and greater chronic stress were associated with lower mean gray matter volume (familywise error p <.001; x = 4.5, y = 22.5, z = −12.5), after controlling for age, sex, and total intracranial volume and the main effects of both scales in a region (7175 voxels) of the orbitofrontal cortex, anterior cingulate, insula, and subgenual areas. The regression interaction is depicted in the graph.
Discussion
In the current study, cumulative adversity over the lifetime was associated with smaller gray matter volume in key frontal and limbic-striatal stress-related brain regions. Smaller gray matter volumes were associated with greater cumulative stressful life events in the frontopolar cortex, ventromedial and orbital frontal cortex, gyrus rectus, inferior frontal gyrus, anterior cingulate, caudate, and subgenual cingulate. Among stressful life events, both traumatic and recent life events (those which occurred in the year before assessment) were associated with regions of smaller gray matter volume. Interestingly, chronic stress, the scale that measures subjects’ experience of ongoing life stressors, was not independently associated with structural changes. However, the interaction of greater chronic stress with greater number of cumulative adverse events was associated with significantly smaller gray matter volume in the orbitofrontal cortex, subgenual area, anterior cingulate, and right anterior insula. On the other hand, individuals who experienced more adverse life events without the experience of ongoing subjective chronic stress showed a positive association between higher adverse life events and increased volume in the orbitofrontal cortex, similar to contrasting mPFC and orbitofrontal cortex findings identified previously in animal models (15). Importantly, these findings demonstrate that accumulation of adversity via exposure to repeated stressful life events, as measured by a sum of adverse life events, was associated with less gray matter in key brain regions involved in emotion regulation, contextual processing, and self control and that such effects were also moderated by higher subjective chronic stress within the orbitofrontal and subgenual cingulate regions. These effects remained even when related demographic and psychiatric variables were included in the model.
Repeated and chronic stress-related changes in the medial prefrontal cortical neuronal synaptic density and spine loss have been well documented in basic science research (10–12). This research is consistent with the current data showing that a history of more cumulative adverse life events was associated with lower gray matter volume in medial prefrontal regions, including the rostral and subgenual anterior cingulate and medial orbitofrontal regions. The mPFC is extensively connected to subcortical limbic structures and is known to regulate stress and emotional arousal (8,62), and reduced brain volume in this key region may partially mediate the vulnerability for depression, addiction, and other stress-related psychopathology. Furthermore, structural and functional changes in the stress-related regions of the medial and lateral prefrontal cortices, amygdala, hippocampus, ventral and dorsal striatum, insula, and midbrain regions also influence reward processing and make up the addiction circuitry (9,63–65). Thus, cumulative adversity and stress and excessive alcohol and drug use each can affect neurobiological processes that promote addictive behavior patterns and ultimately chronic disease risk (7). Smaller brain volume in these regions highlights one potential pathway by which cumulative stress may convey risk for addiction, loss of self-control, and reward-focused behaviors (9,66,67). In addition, the mPFC and the insular cortex are involved in social cognition and processing such that lower volume in this region may also adversely affect functioning involved in regulating interpersonal relationships and negotiating social contexts (68,69). It may also mediate the effects of stress on risk for anxiety disorders (70,71). This may be one pathway by which cumulative adversity compounds itself through difficulties in relationships, loss of socially supportive relations, and increased anxiety.
Despite some prior research pointing to morphological differences in the hippocampus (30), we did not find associations between stress and the hippocampus. Hippocampal reductions have been previously associated with psychiatric disorders and, in more limited research, with experience of stress (72,73). In the context of the current findings, these data would suggest that changes in hippocampal brain morphology may be more clearly related to the pathophysiology of these specific disorders and not necessarily as a result of adversity or stress alone (11,19,74,75). Given that the current sample consisted of community participants without psychiatric disorders, we may speculate that individuals who experience stressful life events but who do not exhibit signs of psychopathology may demonstrate expected morphological changes in the top-down mechanisms of stress processing and not in the frequently accompanying hippocampal regions. On the other hand, stress-related neurochemical changes in limbic regions, such as the amygdala and hippocampal regions, may be more easily detected in region-of-interest morphological analysis and functional neural responses and not easily observable in whole-brain morphological analysis.
Although recent life events were associated with less volume in the mPFC and the right insula, major life events were not associated with any significant morphological changes. Translational research has demonstrated that stress can result in structural alterations in relatively short periods of time (23,24). Findings for the recent life event scale may represent the acute effects of environmental insults on gray matter volume. This finding also fits other research suggesting that some morphological alterations resulting from stress may, given the right circumstances, be overcome over time (76). In the present sample, a history of traumatic life events was associated with smaller volume in the mPFC, including the ventromedial and dorsomedial prefrontal cortex and rostral anterior and subgenual cingulate. Less volume in the subgenual cingulate, gyrus rectus, orbitofrontal cortex, inferior frontal gyrus, left ventral anterior cingulate, and right anterior insula was greatest for those individuals who had a history of more adverse life events and who also reported feeling chronically stressed. Only in the context of cumulative adverse life events was the effect of chronic and subjective distress associated with smaller GMV.
Overall, our findings suggest that cumulative adverse life events, including recent and traumatic life events, are associated with smaller gray matter volume in key regions of the brain involved in emotional, social, and self-regulation, as well as top-down control of limbic and reward-focused processes. The cumulative effects of these environmental exposures on the brain suggest that mediation of future stressful or demanding events may be more challenging for these individuals, particularly if the event requires effortful control, emotion regulation, or integrated social processing. It may be through these stress-induced alterations that individuals with a history of cumulative adversity experience increasing risk for future stressful and adverse life events (2) and concomitant risk of stress-related mental and physical health outcomes. Future research should identify mechanisms of resilience, as well as clinical treatments, which may mitigate the neurotoxic effects of cumulative adversity on brain volume. However, we should also note that lower volumes do not necessarily equate to poorer functioning (11,15,20,77). Stress may promote intense remodeling of mediating pathways and it may be that regions of lower volume represent greater efficiency in functioning or reflect adaptations and resilience. Further research is needed to examine the role that cumulative adversity has in functional responses to stress.
The present study used a nonpsychiatric community sample to examine the effects of cumulative adversity on brain morphology in the absence of psychopathology. Given the region-specific differences identified in our findings in the context of prior research on GMV differences in psychiatric samples, it is likely that these participants are at risk for psychiatric disorders in the absence of protective and resilience factors. Future research should also examine how these structural changes resulting from chronic stress relate to changes in functional and clinical outcomes and how protective factors may moderate risk vulnerability in individuals who have experienced cumulative stress and adversity.
There are several limitations to the current findings. Adverse life events were assessed retrospectively, which may lead to bias or errors in memory. However, test-retest reliability coefficients in a subsample of consecutively seen participants indicate good to excellent reliability of the interview assessment. In addition, it is highly unlikely that gray matter volume associations estimated at the more conservative whole-brain corrected level would result from systematic bias in subject retrospective reporting. It is more likely that memory biases would result in random error and, thus, a null finding. However, individual differences not assessed here may play a role in how an event is experienced or perceived and further research is needed to understand what role they may play. An additional limitation is the examination of gender differences. Disparate sample sizes in gender precluded the examination of gender separately and it may be that moderators of the effects of stress differ by sex. Future research with matched samples is warranted. In addition, this sample had a restricted age range (18 – 48 years), which limits the generalizability of the current findings to older adults and prevents us from examining whether long-term effects of cumulative adversity and chronic stress are associated with different regions than those identified presently. A post hoc analysis of an age and CALE interaction term was not significant, suggesting that this was not a factor in the current sample. On the other hand, the age range in the present sample may also be considered a potential strength by clarifying the significant role stress has on brain morphology independent of aging effects. Further research examining the accumulation of cumulative adversity and chronic stress in older adults is warranted. Finally, further research integrating cumulative adversity and stress effects on multimodal neuroimaging parameters, including voxel-based morphometric analysis and functional MRI, would be useful in understanding the combined morphological and functional effects of stress vulnerability.
Our findings expand upon prior basic science research to demonstrate the impact of accumulating and chronic stress on decreased gray matter volume in key stress, emotion, and behavioral control regions of the brain within nonpsychiatric community individuals. Smaller volumes in these key regions may mediate the increased risk of chronic and cumulative stress on the development of stress-related disorders.
Supplementary Material
Acknowledgments
Funding was provided by National Institutes of Health Grants R01-AA013892, P50-DA016556, UL1-DE019586, PL1-DA024859, and K08-DA029641.
Footnotes
Dr. Guarnaccia has served on the speaker panel and as a consultant for Biogen, Inc; Teva Pharmaceuticals; Accorda Pharmaceuticals; Pfizer, Inc; Serono, Inc; Bayer Pharmaceuticals; and Abbott Pharmaceuticals. Dr. Sinha is on the Scientific Advisory Board for Embera Neurotherapeutics and is also a consultant for Glaxo-Smith Kline Pharmaceuticals. All other authors reported no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 2.Turner RJ, Lloyd DA. Cumulative adversity and drug dependence in young adults: Racial/ethnic contrasts. Addiction. 2003;98:305–315. doi: 10.1046/j.1360-0443.2003.00312.x. [DOI] [PubMed] [Google Scholar]
- 3.Turner RJ, Lloyd DA. The stress process and the social distribution of depression. J Health Soc Behav. 1999;40:374–404. [PubMed] [Google Scholar]
- 4.Turner RJ, Wheaton B. Checklist measurement of stressful life events. In: Cohen S, Kessler R, Underwood GL, editors. Measuring Stress. New York: Oxford University Press; 1995. pp. 29–58. [Google Scholar]
- 5.Lloyd DA, Turner RJ. Cumulative lifetime adversities and alcohol dependence in adolescence and young adulthood. Drug Alcohol Depend. 2008;93:217–226. doi: 10.1016/j.drugalcdep.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wheaton B. Social stress. In: Aneshensel CS, Phelan JC, editors. Handbook of the Sociology of Mental Health. New York: Plenum; 1999. pp. 277–300. [Google Scholar]
- 7.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnsten AF. Prefrontal cortical network connections: Key site of vulnerability in stress and schizophrenia. Int J Dev Neurosci. 2011;29:215–223. doi: 10.1016/j.ijdevneu.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duman RS, Malberg J, Nakagawa S, D’Sa C. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000;48:732–739. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- 14.Duman RS. Depression: A case of neuronal life and death? Biol Psychiatry. 2004;56:140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 15.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 17.Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Res Rev. 2005;4:271–287. doi: 10.1016/j.arr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 19.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 20.Drevets WC. Orbitofrontal cortex function and structure in depression. Ann N Y Acad Sci. 2007;1121:499–527. doi: 10.1196/annals.1401.029. [DOI] [PubMed] [Google Scholar]
- 21.Herwig U, Baumgartner T, Kaffenberger T, Bruhl A, Kottlow M, Schreiter-Gasser U, et al. Modulation of anticipatory emotion and perception processing by cognitive control. Neuroimage. 2007;37:652–662. doi: 10.1016/j.neuroimage.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 22.Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- 24.Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroes MC, Rugg MD, Whalley MG, Brewin CR. Structural brain abnormalities common to posttraumatic stress disorder and depression. J Psychiatry Neurosci. 2011;36:100077. doi: 10.1503/jpn.100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- 28.Rando K, Hong KI, Bhagwagar Z, Li CS, Bergquist K, Guarnaccia J, Sinha R. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: A prospective study. Am J Psychiatry. 2011;168:183–192. doi: 10.1176/appi.ajp.2010.10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- 30.Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage. 2007;35:795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganzel BL, Kim P, Glover GH, Temple E. Resilience after 9/11: Multimodal neuroimaging evidence for stress-related change in the healthy adult brain. Neuroimage. 2008;40:788–795. doi: 10.1016/j.neuroimage.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner RJ, Wheaton B, Lloyd DA. The epidemiology of social stress. Am Sociol Rev. 1995;60:104–125. [Google Scholar]
- 33.Whitesell NR, Beals J, Mitchell CM, Keane EM, Spicer P, Turner RJ AI-SUPERPFP Team . The relationship of cumulative and proximal adversity to onset of substance dependence symptoms in two American Indian communities. Drug Alcohol Depend. 2007;91:279–288. doi: 10.1016/j.drugalcdep.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner RJ, Lloyd DA, Taylor J. Stress burden, drug dependence and the nativity paradox among U.S. Hispanics. Drug Alcohol Depend. 2006;83:79–89. doi: 10.1016/j.drugalcdep.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Turner RJ, Lloyd DA. Stress burden and the lifetime incidence of psychiatric disorder in young adults: Racial and ethnic contrasts. Arch Gen Psychiatry. 2004;61:481–488. doi: 10.1001/archpsyc.61.5.481. [DOI] [PubMed] [Google Scholar]
- 36.Lloyd DA, Turner RJ. Cumulative adversity and posttraumatic stress disorder: Evidence from a diverse community sample of young adults. Am J Orthopsychiatry. 2003;73:381–391. doi: 10.1037/0002-9432.73.4.381. [DOI] [PubMed] [Google Scholar]
- 37.Turner RJ, Lloyd DA. Lifetime traumas and mental health: The significance of cumulative adversity. J Health Soc Behav. 1995;36:360–376. [PubMed] [Google Scholar]
- 38.Lampert RL, Tuit K, Sinha R. Stress, adverse life events and depressed autonomic function as measured by heart rate variability in a community sample. Presented at the American Heart Association Scientific Meeting; November 13-16, 2011; Orlando, Florida. 2011. [Google Scholar]
- 39.Seo D, Tsou KA, Ansell EB, Tuit K, Sinha R. Cumulative adversity is associated with abnormal fMRI neural responses to emotional stress and adverse health outcomes. Presented at the Annual Meeting of the Society for Neuroscience; November 12-16, 2011; Washington, DC. 2011. [Google Scholar]
- 40.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Version (SCID-I/P) New York: New York State Psychiatric Institute; 1996. [Google Scholar]
- 41.Dohrenwend BP. Inventorying stressful life events as risk factors for psychopathology: Toward resolution of the problem of intracategory variability. Psychol Bull. 2006;132:477–495. doi: 10.1037/0033-2909.132.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coupe P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans Med Imaging. 2008;27:425–441. doi: 10.1109/TMI.2007.906087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajapakse JC, Giedd JN, Rapoport JL. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Trans Med Imaging. 1997;16:176–186. doi: 10.1109/42.563663. [DOI] [PubMed] [Google Scholar]
- 44.Cuadra MB, Cammoun L, Butz T, Cuisenaire O, Thiran JP. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Trans Med Imaging. 2005;24:1548–1565. doi: 10.1109/TMI.2005.857652. [DOI] [PubMed] [Google Scholar]
- 45.Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23:84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Anbeek P, Vincken KL, van Osch MJ, Bisschops RH, van der Grond J. Probabilistic segmentation of white matter lesions in MR imaging. Neuroimage. 2004;21:1037–1044. doi: 10.1016/j.neuroimage.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Bouix S, Martin-Fernandez M, Ungar L, Nakamura M, Koo MS, McCarley RW, Shenton ME. On evaluating brain tissue classifiers without a ground truth. Neuroimage. 2007;36:1207–1224. doi: 10.1016/j.neuroimage.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cerasa A, Messina D, Nicoletti G, Novellino F, Lanza P, Condino F, et al. Cerebellar atrophy in essential tremor using an automated segmentation method. AJNR Am J Neuroradiol. 2009;30:1240–1243. doi: 10.3174/ajnr.A1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davies RR, Scahill VL, Graham A, Williams GB, Graham KS, Hodges JR. Development of an MRI rating scale for multiple brain regions: Comparison with volumetrics and with voxel-based morphometry. Neuroradiology. 2009;51:491–503. doi: 10.1007/s00234-009-0521-z. [DOI] [PubMed] [Google Scholar]
- 50.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 53.Hayasaka S, Nichols TE. Validating cluster size inference: Random field and permutation methods. Neuroimage. 2003;20:2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Poline JB, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- 55.Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: Levels of inference and power. Neuroimage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- 56.Chumbley JR, Friston KJ. False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage. 2009;44:62–70. doi: 10.1016/j.neuroimage.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 57.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 58.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 59.Lacadie CM, Fulbright RK, Rajeevan N, Constable RT, Papademetris X. More accurate Talairach coordinates for neuroimaging using non-linear registration. Neuroimage. 2008;42:717–725. doi: 10.1016/j.neuroimage.2008.04.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shipley W. A self-administering scale for measuring intellectual impairment and deterioration. J Psychol. 1940;9:371–377. [Google Scholar]
- 61.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 62.Price JL, Carmichael ST, Drevets WC. Networks related to the orbital and medial prefrontal cortex: A substrate for emotional behavior? Prog Brain Res. 1996;107:523–536. doi: 10.1016/s0079-6123(08)61885-3. [DOI] [PubMed] [Google Scholar]
- 63.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 65.Li CS, Sinha R. Inhibitory control and emotional stress regulation: Neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paulus MP. Neural basis of reward and craving–a homeostatic point of view. Dialogues Clin Neurosci. 2007;9:379–387. doi: 10.31887/DCNS.2007.9.4/mpaulus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn Affect Behav Neurosci. 2007;7:391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- 68.Heatherton TF. Neuroscience of self and self-regulation. Annu Rev Psychol. 2011;62:363–390. doi: 10.1146/annurev.psych.121208.131616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 70.Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Struct Funct. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stein MB, Paulus MP. Imbalance of approach and avoidance: The yin and yang of anxiety disorders. Biol Psychiatry. 2009;66:1072–1074. doi: 10.1016/j.biopsych.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee HY, Tae WS, Yoon HK, Lee BT, Paik JW, Son KR, et al. Demonstration of decreased gray matter concentration in the midbrain encompassing the dorsal raphe nucleus and the limbic subcortical regions in major depressive disorder: An optimized voxel-based morphometry study. J Affect Disord. 2011;133:128–136. doi: 10.1016/j.jad.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 73.Zhang J, Tan Q, Yin H, Zhang X, Huan Y, Tang L, et al. Decreased gray matter volume in the left hippocampus and bilateral calcarine cortex in coal mine flood disaster survivors with recent onset PTSD. Psychiatry Res. 2011;192:84–90. doi: 10.1016/j.pscychresns.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 74.Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: A primer on neuron death. Biol Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- 75.Sapolsky RM. Chickens, eggs and hippocampal atrophy. Nat Neurosci. 2002;5:1111–1113. doi: 10.1038/nn1102-1111. [DOI] [PubMed] [Google Scholar]
- 76.Holzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, et al. Stress reduction correlates with structural changes in the amygdala. Soc Cogn Affect Neurosci. 2010;5:11–17. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McEwen BS. Stress, sex, and neural adaptation to a changing environment: Mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(suppl):E38–E59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.