Abstract
The parabrachial nucleus (PBN) is an area of the brain stem that controls eating and contains endogenous opioids and their receptors. Previously, we demonstrated that acute activation of µ opioid receptors (MOPR) in the lateral PBN increased food consumption. MOPR’s have been divided operationally into µ1 and µ2 receptor subtypes on the basis of the ability of naloxonazine (Nlxz) to block the former but not the latter. We used autoradiography to measure whether Nlxz blocks stimulation by the µ1/µ2 agonist DAMGO (D-Ala2, N-Me-Phe4, Gly5-ol-enkephalin) of the incorporation of [35S]-guanosine 5’(γ-thio)triphosphate ([35S]-GTPγS) into sections of the PBN. In vitro, Nlxz dose dependently inhibited receptor coupling in all areas of the PBN. The 1µM concentration of Nlxz reduced stimulation by 93.1±5% in the lateral inferior PBN (LPBNi) and by 90.5±4% in the medial parabrachial subregion (MPBN). Administration of Nlxz directly into the LPBNi decreased both food intake and agonist stimulated coupling, ex vivo, for the 24h period after infusion. Infusion of Nlxz into the intended area reduced food intake by 42.3% below baseline values. Nlxz infusion prevented DAMGO stimulation of G-protein coupling in LPBNi and markedly reduced this stimulation in the MPBN. The incomplete inhibition of DAMGO stimulated coupling in the MPBN is most likely due to the limited diffusion of Nlxz from the site of infusion (LPBNi) into this brain region. In conclusion, this study demonstrates that the µ1 opioid receptor subtype is present in the parabrachial nucleus of the pons and that these receptors serve to modulate feeding in rats.
Keywords: parabrachial nucleus, naloxonazine, opioids, µ1-opioid receptor, G-protein coupling, obesity
1. Introduction
Mu opioid receptors (MOPR) in the brain mediate physiological processes that increase feeding. Initial evidence suggesting this function for MOPR’s came from studies in which administering exogenous agonists, particularly morphine and the peptide DAMGO (D-Ala2, N-Me-Phe4, Gly5-ol-enkephalin) into the nucleus accumbens (NAC) and other sites in the forebrain, increased food intake in rats (Bakshi and Kelley, 1993; Giraudo et al., 1998; Mann et al., 1988b; Ragnauth et al., 2000; Zhang and Kelley, 2000). Subsequent data demonstrated that infusing DAMGO into the nucleus tractus solitarius (NTS) (Kotz et al., 1997) also increased food intake and led to the view that MOPR’s along the neuraxis, from the deep brainstem to the forebrain, code an opioidergic network that modulates feeding (Glass et al., 1999; Kelley et al., 2005; Levine, 2006).
We have shown that the pontine parabrachial nucleus (PBN) plays a significant, possibly coordinating, role in this network. The PBN relays visceral and gustatory information from the NTS and the spinal cord to the forebrain (Herbert et al., 1990; Hermann and Rogers, 1985; Karimnamazi et al., 2002; Lundy and Norgren, 2005). Discrete infusion of DAMGO into the lateral PBN (LPBN) increased consumption of food and this action was prevented by the nonselective opioid receptor antagonist, naloxone, and also by the selective, competitive MOPR antagonist CTAP (D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2) and the selective, irreversible MOPR antagonist β-FNA (β-funaltrexamine) (Ward and Simansky, 2006; Wilson et al., 2003). Furthermore, each of these antagonists, by themselves, reduced food intake after infusion into the LPBN. By mapping the capacity of MOPR’s to couple to their G-proteins after the irreversible β-FNA, we implicated the lateral inferior subregion (LPBNi) of this area in controlling food intake (Ward and Simansky, 2006).
These data clearly implicated parabrachial MOPR’s in the physiological control of eating. However, pharmacological evidence suggests that functional subtypes of MOPR’s exist. Specifically, whereas CTAP and β-FNA antagonize all DAMGO stimulated actions thought to involve MOPR’s, the drug naloxonazine (Nlxz) appears to inhibit only some of the effects of this agonist. Those Nlxz-sensitive actions have been used operationally to define a µ1 subtype of MOPR’s (Hahn et al., 1982; Hahn and Pasternak, 1982; Ling et al., 1986; Wolozin and Pasternak, 1981). Moreover, molecular studies have also suggested the existence of subtypes of MOPR’s including an alternative splice variant named MOR1-B (Narita et al., 2003; Pan et al., 2001; Pasternak, 2001b). Naloxonazine binds to MOR-1B (which contains exons 1, 2, 3 and 5), indicating that this variant may be the µ1-receptor subtype (Zimprich et al., 1995a). Further, it is relevant to know that the CXBK mice have been engineered with reduced MOR1-B mRNA and, consequently, reduced the analgesic action of endomorphin-1(Narita et al., 2003; Goldberg et al., 1998). It would be interesting to determine whether the PBN of these mice expresses MOPR’s and whether these mice would show a diminished feeding response to opioid stimulation.
The µ1-opioid receptor subtype has been implicated in some, but not all actions of MOPR’s to modulate feeding (Simone et al., 1985). Infusion of the antagonist Nlxz into the ventral tegmental area (VTA) prevented the ability of hedonically positive food-related stimuli to increase dopamine (DA) release in the NAC (Tanda and Di Chiara, 1998). Nlxz did not, however, antagonize the ability of DAMGO to stimulate feeding when this agonist was infused directly into the NAC (Ragnauth et al., 2000). Additionally, intravenous (i.v.) administration of Nlxz, by itself, reduced food intake in adult male rats (Ling et al., 1986; Mann et al., 1988a; Mann et al., 1988b; Simone et al., 1985).
Together, these data strongly suggest regionally specific roles for µ1 and µ2 (i.e.: non-µ1) receptors in feeding. It remains to be determined, however, whether the excitatory influence of parabrachial MOPR’s on food intake involves a particular receptor subclass. For this, we used autoradiography to measure whether Nlxz blocks DAMGO-stimulated incorporation of [35S]-guanosine 5’(γ-thio)triphosphate ([35S]-GTPγS) in coronal sections through the PBN. The action of Nlxz was evaluated first in vitro, to permit characterization of receptor coupling after access of the antagonist to all areas of the PBN. We then administered Nlxz directly into the LPBNi and measured food intake, in vivo, and agonist stimulated coupling, ex vivo, for the 24h period after infusion of the irreversible antagonist (Hahn and Pasternak, 1982; Simone et al., 1985). We demonstrate that the µ1 opioid receptor subtype is present in the parabrachial nucleus of the pons and it serves to control food intake in rats.
2 Results
The selective µ1 antagonist naloxonazine inhibited DAMGO stimulated-[35S] GTPγS incorporation in the PBN, in vitro
Figure 1 shows the inhibition by naloxonazine (Nlxz) of DAMGO stimulated [35S]- GTPγS incorporation in the parabrachial nucleus (PBN), in vitro. In the absence of Nlxz, the selective MOPR agonist DAMGO increased GTPγS incorporation to 195±25 fmol/g above basal in the LPBNi (basal value: 140±9 fmol/g) and to 166±23 fmol/g above basal in the MPBN (basal value: 115±15 fmol/g). Nlxz reduced DAMGO-induced stimulation in a concentration dependent manner. The 1µM concentration of Nlxz reduced stimulation by 93.1±5% in LPBNi and by 90.5±4% in the medial parabrachial subregion to values (fmol/g) that were not significantly different than basal (both p values > 0.10). The MOPR’s in the LPBNi and the MPBN appear to be the µ1 subtype as defined operationally by the ability of Nlxz to interfere with the coupling function.
Fig. 1. The selective µ1 antagonist Nlxz decreased in vitro stimulation of G-protein coupling by DAMGO (1µM) in the PBN.
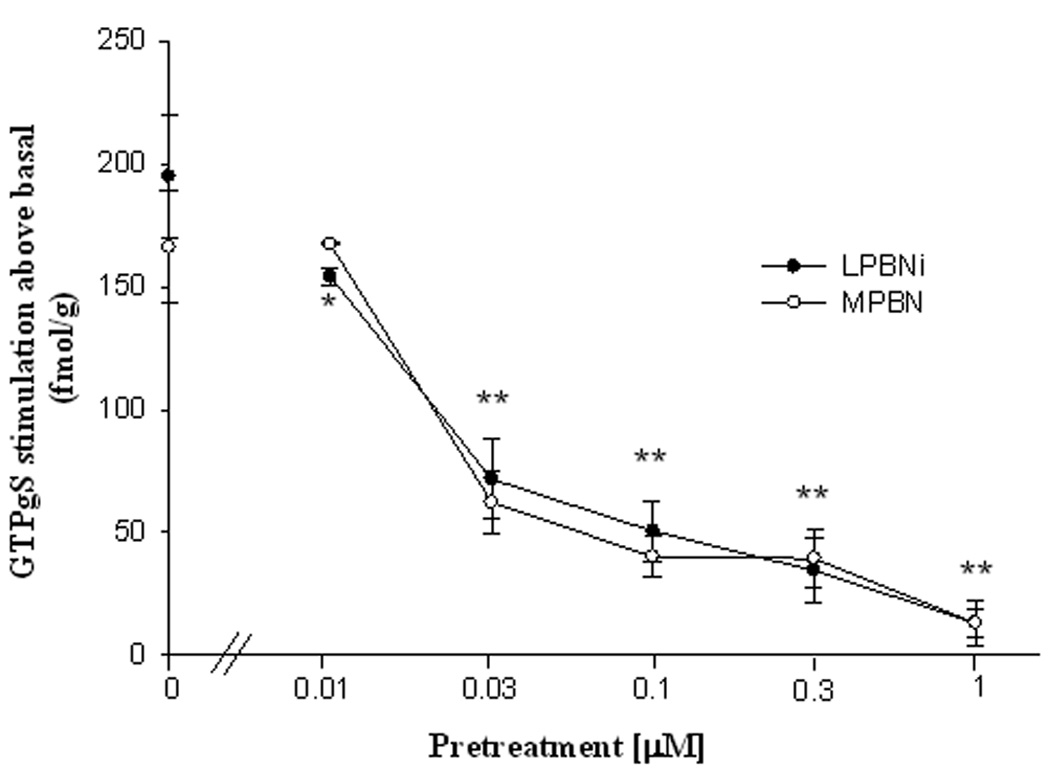
Data represent stimulation by DAMGO above basal values (fmol/g means±SEM). Asterisks indicate difference from value for DAMGO without Nlxz pretreatment: ** p<0.01 for MPBN and LPBNi, *p<0.05 for LPBNi (F(5,5)=13.7). Student Newman-Keuls test after ANOVA (n=7).
Bilateral infusion of naloxonazine into the LPBNi decreased food intake
Twenty rats were infused bilaterally with Nlxz (8nmol/0.5ul per side) or saline (0.5µl) into the LPBNi. Of the ten control rats that were infused with saline, seven were judged histologically (see Fig. 2) to have placements within the LPBNi region of the parabrachial nucleus (Saline-in group) and three outside this region (Saline-out group). The identical distribution was observed for Nlxz infused animals (Nlxz-in, n=7; Nlxz-out, n=3). The 24h consumption of food did not differ among the Saline-in, Saline-out and Nlxz-out groups. By comparison, infusion of Nlxz into the intended area in the LPBNi reduced food intake by 42.3% below baseline values during the 24h after infusion of the drug (Fig. 3).
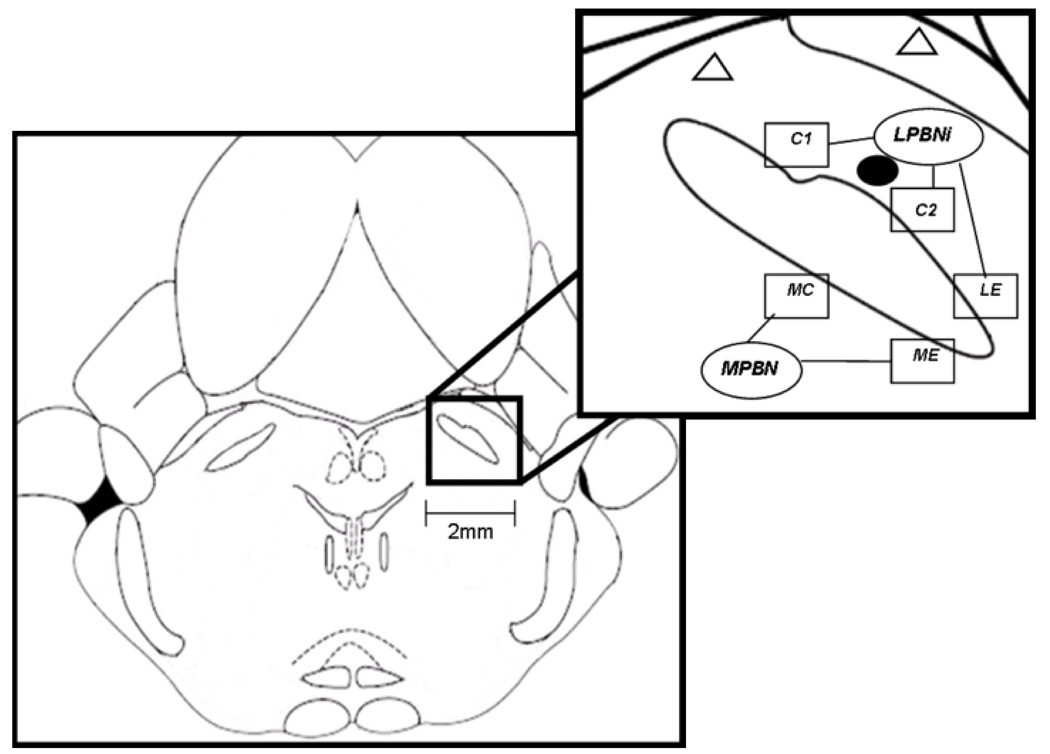
Fig. 2. Schematics showing the location of PBN in dorsal pons (left panel) and subregions sampled for autoradiography (right upper panel).
The line drawing shown in left panel is at the coronal level, approximately 9.8mm caudal to bregma according to the Paxinos and Watson atlas (1998). The upper right panel shows the inferior aspect of the lateral inferior PBN (LPBNi), which includes two regions of interest within the central subregion and one within the external subregion of this nucleus (C1, C2 and LE, respectively). The medial PBN (MPBN) includes a central and external subregion (MC and ME, respectively). Boxes represent the anatomical subregions of the PBN, which were used for densitometric analysis of G-protein coupling, as described in the methods section. Infusions (black filled circle) were made in the LPBNi. Open triangles represent infusions that were outside of the targeted area.
Fig. 3. Infusion of Nlxz reduced consumption of food.
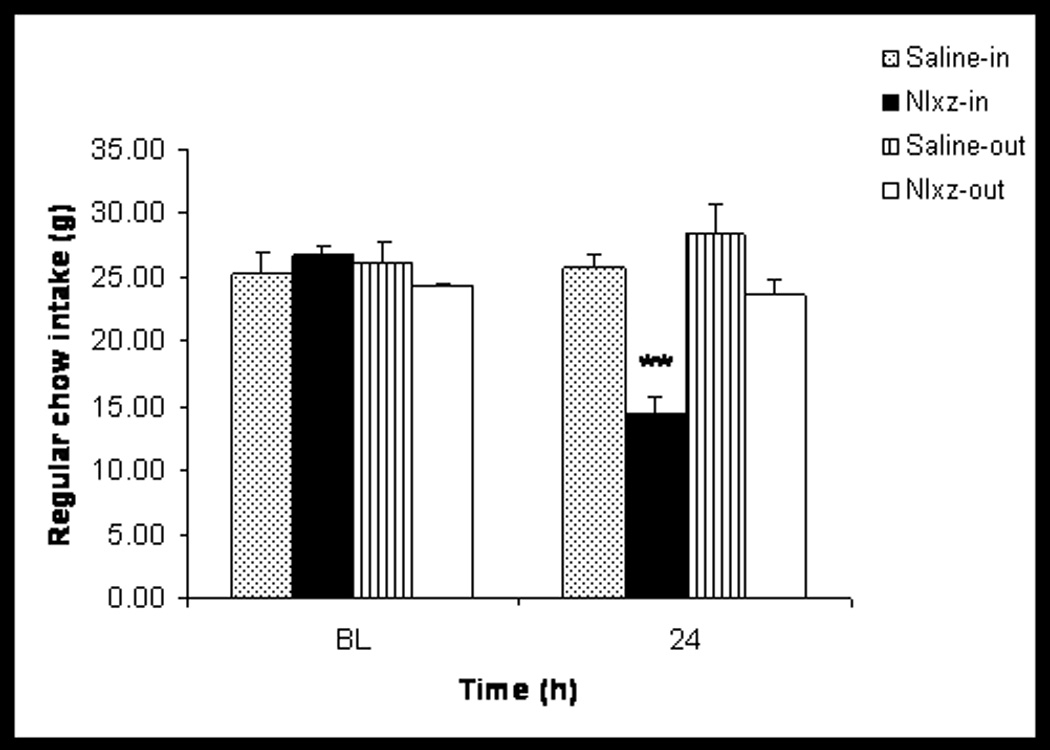
Data separated on the basis of infusions within the intended region of the LPBNi (Saline-in and Nlxz-in, both with n=7) or outside the intended region (Saline-out and Nlxz out, both with n=3). Values are presented as mean±SEM. Asterisks (**) represent difference from their respective baseline (BL) values, F(3,22)= 10.4, p<0.01.
Bilateral infusion of Nlxz into LPBNi decreased DAMGO stimulated G-protein coupling, ex vivo
[35S]GTPγS autoradiography was performed on the two PBN subregions (LPBNi and MPBN) from rat brains obtained 24h after a single infusion of Nlxz or saline into the LPBNi. Autoradiograms of GTPγS stimulation and quantification of the results are shown in Figure 4 and Figure 5, respectively. In saline infused rats (Saline-in), DAMGO increased GTPγS incorporation to 109±12 fmol/g above basal within the LPBNi and to 140±13 fmol/g above basal within the MPBN. Nlxz infusion (Nlxz-in) prevented DAMGO stimulation in LPBNi and reduced this stimulation in the MPBN (4±12 fmol/g and 59±8 fmol/g simulation above basal, respectively). Basal coupling was not affected by Nlxz treatment (e.g., 176±7 fmol/g for Saline-in and 183±14 fmol/g for Nlxz-in, in LPBNi). Animals with placements out of the intended region (saline-out and Nlxz-out) were also analyzed. Rats from the saline-out group displayed increases of 137±21 fmol/g and 138±37 fmol/g above basal for LPBNi and MPBN, respectively. Infusion of Nlxz out of the intended target (Nlxz-out) did not reduce DAMGO stimulated coupling in either the LPBNi (121±41 fmol/g above basal) or the MPBN (166±36 fmol/g above basal).
Fig. 4. GTPγS DAMGO incorporation after saline or Nlxz infusion.
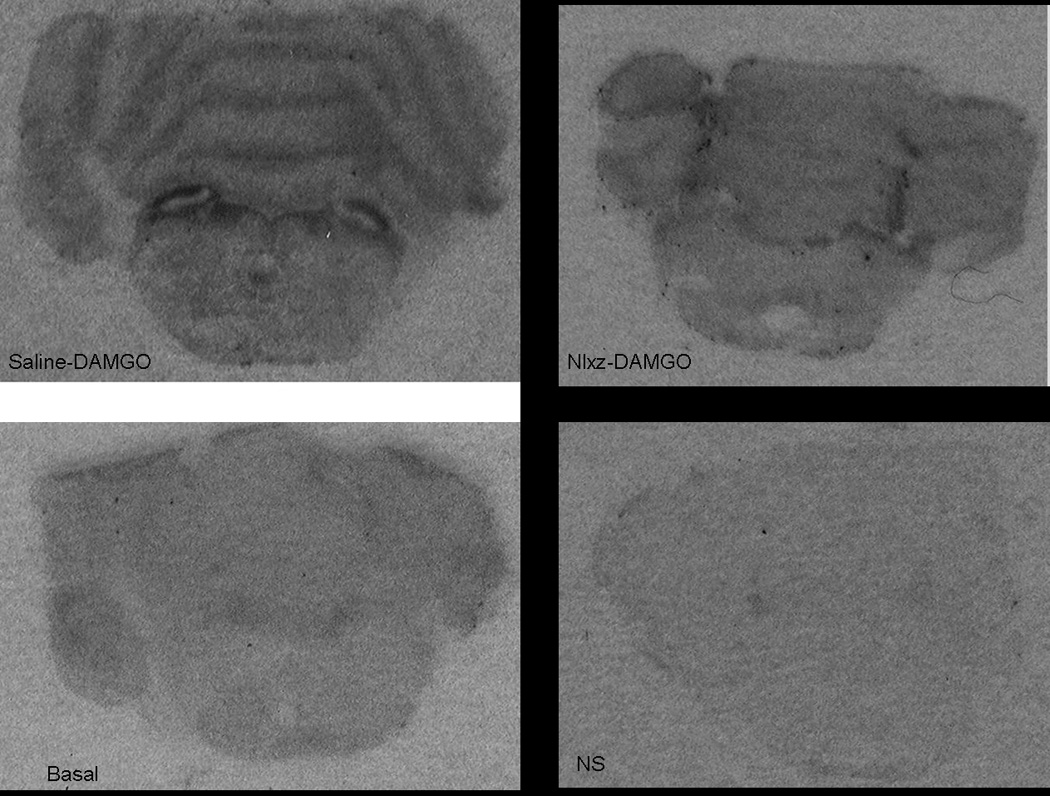
DAMGO increased GTPγS incorporation ex vivo in saline infused rats (DAMGO-saline). Infusion of Nlxz prevented this response (DAMGO-Nlxz). Bottom panels show G-protein coupling when no drug (basal) or unlabeled GTPγS was present (non-specific, NS).
Fig. 5. Naloxonazine-infused animals prevented or reduced GTPγS DAMGO incorporation on the LPBNi and MPBN, respectively.
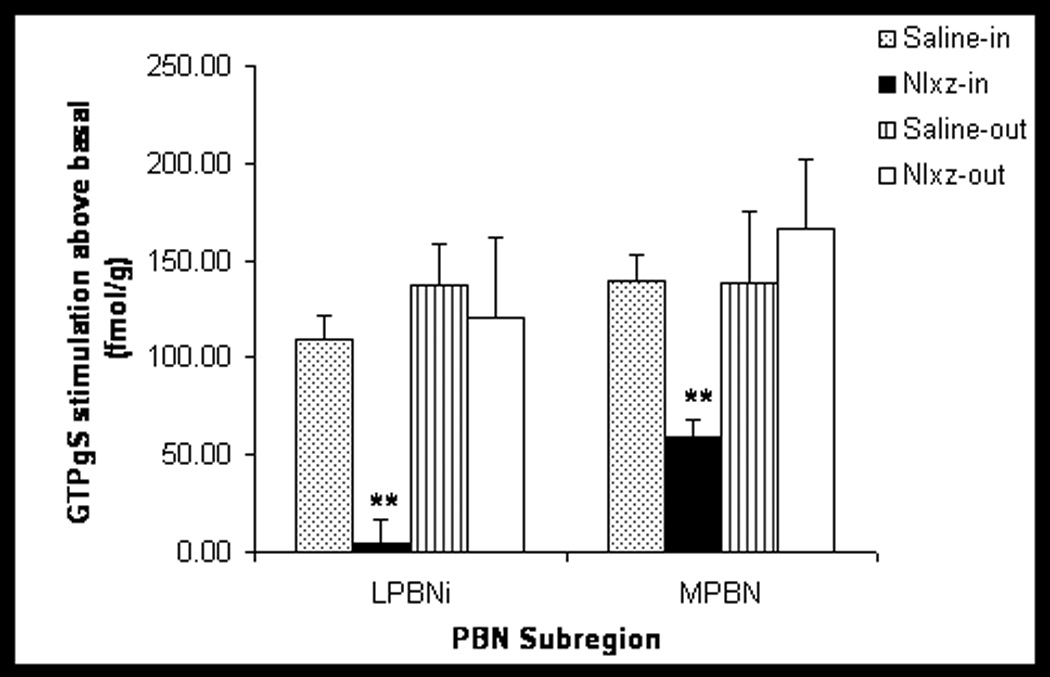
At the end of the 24h measurements of food intake, five rats from Saline-in group, six from Nlxz-in and three from each of the Saline-out and Nlxz-out groups were sacrificed to quantify the effects of Nlxz on the ability of DAMGO to stimulate [35S]GTPγS incorporation into LPBNi and MPBN subregions. Infusion of Nlxz significantly decreased DAMGO stimulated G-protein coupling in LPBNi. A smaller but significant reduction occurred in MPBN. Data expressed as [35S]-GTPγS incorporation in fmol/g stimulation above basal. Asterisks (**) represent difference in DAMGO stimulation between Nlxz or saline groups. F(2,27)=3.2 (MPBN) and F(2,27)=10.6 (LPBNi), both p<0.001.
3. DISCUSSION
This study demonstrates that the µ1 subtype of opioid receptors is present in the parabrachial nucleus of the pons. Furthermore, these receptors mediate processes that modulate food intake in rats. Previous studies from our laboratory have shown that infusion of the selective, irreversible MOPR antagonist β-FNA blocks DAMGO-stimulated G-protein coupling to those receptors in all regions of the PBN (Ward and Simansky, 2006). These results combined with data from other laboratories confirmed the presence of MOPR’s in this region of the brain (Atweh and Kuhar, 1977; Carr et al., 1991; Goodman and Pasternak, 1985; Nicklous and Simansky, 2003; Wilson et al., 2003). Pharmacological evidence suggests that MOPR’s exist in µ1 and µ2 subtypes. The current results in which Nlxz prevented DAMGO stimulated coupling in the PBN in vitro would appear to establish, operationally, that parabrachial MOPR’s are of the µ1 subtype. Although it would be useful to corroborate the absence of functional µ2 opioid receptors in the PBN, there are no readily available pharmacological tools that would help to identify this receptor subtype.
The primary purpose of the present study was to characterize more precisely the MOPR’s mechanism involved in the parabrachial control of feeding. Our data strongly implicate the µ1 subtype in the PBN in this function. It is known that the PBN serves as a relay region of the brain stem, receiving afferent information from the gastrointestinal and gustatory systems, and sending projections to other areas of the brain related to homeostatic regulation (such as the hypothalamus) and to the reward system (such as the nucleus accumbens). Previous studies from our laboratory demonstrated that infusion of DAMGO into the LPBNi increased food intake, whereas infusion of the MOPR antagonists naloxone, CTAP or β-FNA blocked this action (Nicklous and Simansky, 2003; Ward and Simansky, 2006; Wilson et al., 2003). In the present study, we further demonstrated that a single bilateral infusion of the selective µ1 opioid receptor antagonist Nlxz into the LPBNi robustly decreased feeding for at least 24h. Autoradiographic analyses from these animals reveled that Nlxz also prevented DAMGO-stimulated G-protein coupling in the LPBNi, ex-vivo and partially decreased coupling in the MPBN, indicating that the reduction of food intake was essentially mediated through the inactivation of the µ1 MOPR subtype. These results, using a selective MOPR antagonist by itself, suggest that parabrachial µ1 receptors play an important physiological role in modulating consumption of food.
As just noted, Nlxz completely prevented µ1-associated coupling in the LPBNi, but only partially inhibited coupling in the MPBN subregion. Considering that our infusions were targeting the LPBNi, we believe that the difference between subregions is due to the limited diffusion of the drug to the MPBN rather than a residual presence of µ2 MOPR’s in this area. This conclusion is supported by the ability of Nlxz to prevent DAMGO stimulated coupling in the MPBN when the studies were conducted in vitro. Presumably, a smaller volume –or a smaller dose- of infusate could have confined the loss of coupling to the LPBNi. In comparison, larger volume—or a higher dose—would have permitted the drug to diffuse more across the brachium into the MPBN and markedly reduced or eliminated µ1-associated coupling in that subregion. We have reported preliminary data from this laboratory in which a 0.2ul infusion of the irreversible, µ1/µ2-receptor antagonist, β-funaltrexamine (β-FNA) selectively eliminated DAMGO-stimulated coupling within the LPBNi and reduced food intake (Ward and Simansky, 2006). This approach would be useful for further analyzing the actions of Nlxz within the parabrachial region. Future experiments including a dose-response analysis would establish not only the critical anatomical site(s) within the PBN, but also the relationship between the degree of impairment of coupling and the magnitude of reduction of food intake. Nonetheless, our present results establish the foundation for those future experiments, by linking irreversible inhibition (see Hahn et al., 1981; Hahn and Pasternak, 1982) of the capacity of µ1-opioid receptors to couple to their intracellular G-proteins after stimulation by their native neuropeptide ligands to a diminished response for food.
It is possible that our infusions also diffused up the cannula shaft into the ventricular space and/or inferior aspect of the cerebellum. We did take precautions (slow infusion rate and keeping the injector in place after infusion) to prevent the dorsal flow of drug. Also, we reported that infusing Nlxz outside the intended region (“off-target”) did not affect food intake. Those infusions would have provided as much or greater access, compared to “on-target” infusions, to the ventricular lumen and cerebellum. Thus, the on-target infusions implicate parabrachial MOPR’s in control of feeding. A much larger dose of Nlxz has been used in previous studies specifically testing the effects of intracerebroventricular administration of this antagonist on feeding in rats (Leventhal and Bodnar, 1996; Cole et al., 1997). Those experiments used 50µg in 10µl compared to 6µg in 0.5µl in the present study. To our knowledge, data do not exist addressing whether directly infusing Nlxz into the ventricular space caudal to the lateral or third ventricles would reduce food intake. Our current data, combined with previously published work and publicly-reported abstracts from this laboratory, favor a role for µ1-opioid receptors in the LPBNi in modulating feeding.
Our data differ, furthermore, from behavioral data reported for the NAC. In particular, Kelley and her collaborators found that, while the infusion of naloxone, naltrexone or β-FNA into the NAC reduced regular chow intake in rats, a single bilateral infusion of Nlxz did not alter consumption (Kelley et al., 1996). That study infused virtually the same concentration of Nlxz (15nmol/1.0µl) into the NAC (shell or core) as we infused (8nmol/0.5ul/side) in a smaller anatomical area in the present work. The initial conclusion would be that µ1-opioid receptors are important in the role of the PBN, but not of the NAC in controlling feeding. However, they did not assess effects on coupling. Also, they evaluated eating after food deprivation for a test period of only 30 min, beginning 24h after Nlxz infusion. Thus, it is premature to conclude that µ1-opioid receptors do not serve the physiological function of the NAC to control feeding. Notably, in our hands, β-FNA infusions into the LPBN decreased consumption of standard, but not palatable food (Ward and Simansky, 2006), suggesting that the MOPR’s in the PBN are involved in some regulatory aspect of food intake. Clearly, we must be cautious about the extent to which parabrachial µ1-opioid receptors influence feeding, as the role may not extend to conditions involving food deprivation or highly palatable test diet.
Other laboratories have also used Nlxz as a tool to identify the influence of µ1 opioid receptors on feeding behavior. For example, intravenous injection of Nlxz 24h prior to free-feeding or intraventricular infusion of this drug, significantly reduced food consumption (Ling et al., 1986; Mann et al., 1988a; Ragnauth et al., 2000; Simone et al., 1985). Chronic blockade of µ1 receptors by Nlxz produced a rightward shift in the morphine hyperphagia dose-response curve reducing food intake below vehicle values (Mann et al., 1988b). Chronic central administration of Nlxz reduced body weight and food intake in both lean and obese Zucker rats (Cole et al., 1997). The present study, however, is the first to establish µ1 MOPR’s specifically in the parabrachial region in controlling feeding.
The PBN is positioned anatomically to distribute visceral and gustatory information to rostral areas involved in both homeostatic mechanisms that regulate caloric intake and nonhomeostatic mechanisms modulating reward and other responses to positive sensory properties of food (Bodnar, 2004; Fulwiler and Saper, 1984; Hadjimarkou et al., 2004; Herbert et al., 1990; Hermann and Rogers, 1985; Karimnamazi et al., 2002; Zhang et al., 2006). Previous evidence established a role for lateral parabrachial MOPR’s in controlling feeding and, therefore, possibly in gating the distribution of the information along the opioidergic feeding network from the hindbrain to the forebrain. The results of the present study identify the subtype of MOPR more precisely as µ1 and compel analysis of the specific physiological role(s) served by these proteins in ingestion.
4. Experimental Procedure
1- Subjects
Thirty-two male Sprague-Dawley rats (350–400g) purchased from Taconic Farms (Germantown, NY) were housed individually in suspended wire-mesh cages (43 cm length × 22 cm width × 18 cm height). The colony room was maintained at 22–24 °C and at 40–50% humidity under a 12:12-h light—dark cycle (lights on at 0600). Animals had free access to water and standard, pelleted chow (3.34kcal/g; Purina, Mills Laboratory Diet, St. Louis, MO) unless otherwise noted. Subjects were experimentally naïve and allowed one week of adaptation in the colony before surgery or any other procedure. Experimental protocols were carried out in accordance with the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research of the National Research Council” (2003) and were approved by the Institutional Animal Care and Use Committee (IACUC) of Drexel University.
2-[35S] GTPγS Autoradiography
This procedure was based on an established method by Sim et al. (1995), and modified by our laboratory (Ward et al. 2006). After sacrificing the animals and isolating the brains, twenty-micron frozen coronal sections were cut throughout the PBN (Leica cryostat model CM3050, Deerfield, IL), thaw-mounted onto slides coated with chrome-alum and stored at −70°C until use. To initiate this assay, slides were pre-incubated for 40min in Tris-assay buffer (50mM Tris–HCl, 4mM MgCl2, 0.3mM EGTA, 100mM NaCl, pH 7.4) at 25°C, followed by a 20min incubation in guanosine diphosphate (GDP, 2mM in Tris-assay buffer) at 25°C. Sections were then incubated for 2h at 25°C in Tris-assay buffer containing [35S]GTPγS (0.04nM, 1,250 Ci/mmol; Perkin Elmer, Boston, MA) and 2mM GDP (Sigma- Aldrich, St. Louis, MO). Additionally, this incubation was divided into the following conditions: no drug (basal condition); drug corresponding to the specific assay; or unlabeled GTPγS (10µM; guanosine-S′-O-(γ-thio)-triphosphate; MP ICN Biomedicals, Irvine, CA) to determine nonspecific binding. After incubation, slides were rinsed twice in cold 50mM Tris–HCl buffer (4°C, pH 7.4, 2min each rinse). Slides were then rinsed briefly in cold de-ionized water, dried immediately with a cool stream of air and desiccated overnight. Slides were exposed to Kodak Biomax MS film (Eastman Kodak Company, Rochester, NY) for 24h. [14C] standards (31–883nCi/g; Amersham Biosciences, Piscataway, NJ) were placed in each developing cassette. Images from the developed films were scanned and quantified using Image Pro-Plus Version 4.5 software (Media Cybernetics; Newburyport, MA). Densities (optical density units, OD) of [35S]GTPγS incorporated were determined in to the lateral-inferior and the medial parabrachial regions (LPBNi and MPBN, respectively). For every rat, the values from each subregion were averaged and converted to femtomol per gram of tissue (fmol/g) using nonlinear curves generated from the [14C] standards. All OD values (including nonspecific binding) were corrected for background of the film, but not for the different path lengths of isotopic emissions from 35S (tissue sections) and 14C (standards). Each anatomical set of sections per rat contained alternating sections for the nonspecific, basal and the different drug conditions through the PBN. Nonspecific binding was subtracted from each of the conditions.
3- Drugs
Naloxonazine dihydrochloride (Nlxz, M.W:751, Tocris Cookson, Ellisville, MO, USA) was dissolved in deionized water. The MOPR agonist [D-Ala2, N-Me-Phe4, Glycinol5]-Enkephalin (DAMGO, MW: 514, Bachem Bioscience, King of Prussia, PA, USA) was dissolved in saline solution (0.9% w/v). Infusion of saline alone was used as the control. All solutions were prepared on test days.
4- In vitro studies
4-1 Inhibition by naloxonazine of DAMGO-stimulated incorporation of [35S]GTPγS
In order to estimate the concentration range for evaluating the actions of Nlxz in vitro, we first assessed the ability of the classical MOPR antagonist CTAP to inhibit DAMGO-stimulated [35S]GTPγS incorporation in the PBN. CTAP (0.01–1.0µM) antagonized coupling in a concentration-dependent manner, with complete inhibition occurring at 0.1µM (data not shown). We therefore used this concentration range for our studies of Nlxz.
Further, we continued our in vitro inhibition studies by performing assays of agonist stimulated incorporation of [35S]GTPγS. For the anatomical analyses we measured several regions of interest within the LPBNi and MPBN. The LPBNi was chosen because it was targeted by our infusions in the in vivo studies described below. MPBN was chosen because it contains a critical anatomical area involved in relaying gustatory information from the NTS to the forebrain in rats (Lundy & Norgren, 2005). Also, this subregion provided a measure of the extent of diffusion of Nlxz from the LPBNi for the in vivo studies (see section 5). Three regions of interest were analyzed within the LPBNi and two for the MPBN (see Fig. 5). Each animal had five sets of tissue sections through the level of the PBN. Each set was incubated with a different concentration of Nlxz (0µM, 0.01µM, 0.03µM, 0.1µM, 0.3µM, and 1µM), in the presence of DAMGO (1µM). Solutions were prepared immediately before the 2h incubation. Data were analyzed statistically as stimulation above basal in fmol/g of tissue.
5-In vivo studies
5-1 Surgery
Rats were anesthetized with Equithesin (3.5ml/kg i.p.), which was formulated to deliver 36mg/kg pentobarbital sodium and 160mg/kg chloral hydrate. Bilateral stainless steel guide cannulas (26-gauge, 3.8mm center-to-center; Plastics One, Roanoke, VA) were implanted stereotaxically (Kopf Instruments, Tujunga, CA) into the LPBNi with the guide cannula ending at the following coordinates: anteroposterior, - 9.8mm posterior to bregma; mediolateral, ±1.9mm from the midline; and dorsoventral, - 4.8mm below the surface of the skull. After surgery, 33-gauge obturators were inserted into the guides, with the tips flush with the end of the guides, to prevent occlusion. Rats were handled daily and allowed at least seven days for recovery before behavioral testing.
5-2 Drug Infusion
Naloxonazine or saline was delivered bilaterally into the LPBNi of each animal using a stainless steel 33-gauge microinjector (Plastics One), extending 2.5mm below the end of the guide cannula. Infusions were made with a microdrive pump (Harvard Apparatus Model 975, South Natick, MA), connected via polyethylene tubing (PE-10). Drug or vehicle delivery was made in a total volume of 0.5µl per side at a rate of 0.33µl/min. Injectors remained in place for a period of 30s after infusion in order to minimize backflow.
5-3 Action of parabrachial infusion of naloxonazine on food intake
A total of twenty rats were used for the food intake studies. After surgery and recovery, animals were fed with 60–70g of chow. Food remaining the next day was measured and fresh pellets were given at 0900h daily. The amount of food consumed stabilized within five to seven days and the last three consecutive 24h intakes before infusion day were averaged for each rat. These means were taken as the baselines (BL) for the subsequent studies. Animals were then infused once, bilaterally with either Nlxz at a dose equimolar to β-FNA as used in our previous publications (8nmol/0.5µl per side, n=10, Ward and Simansky, 2006) or an equal volume of saline solution (control group, n=10) and food intake was measured for the subsequent 24h. The concentration chosen (8nmol/0.5µl) was also similar to the one used by Kelley and collaborators in which they infused 15nmol/1µl (Kelley et al., 1996). Thus, the local amounts of Nlxz were presumably identical in equal areas of diffusion”.
At the end of the study, rats were sacrificed by guillotine to quantify the effects of Nlxz on the ability of the MOPR agonist DAMGO (1µM) to stimulate [35S]GTPγS incorporation into coronal sections of the PBN as an estimate of the capacity of MOPR to couple to their G-proteins. Brains were rapidly removed and placed in powdered dry ice until the tissue was frozen. After freezing the tissue, the samples were stored at −70°C until use.
5-4 Analysis of cannula placements
Infusion sites from each rat were determined from one to three frozen sections by projecting them onto line-drawn templates with a Camera Lucida (Bausch and Lomb, Rochester, NY, USA). Two observers independently rated the placements without knowledge of the treatments or behavioral results associated with the rat. Seven rats from the Nlxz group and seven saline controls had bilaterally symmetrical placements in LPBNi (Fig. 5). Three rats from each group had cannula placements outside of the targeted region. Data from these rats are presented separately from those that were within the intended target.
6-Statistical analysis
Values for food intake were analyzed by appropriate 2-way ANOVA based upon the actual values. Results from densitometry were analyzed by 2-way ANOVA of the actual values (fmol/g) and after conversion to stimulation above basal. The Student's Newman–Keuls test was used for post hoc comparisons of means. An alpha level of P < 0.05 was the threshold for statistical significance for all tests (Sigma Stat version 3.1, Systat Software Inc., Chicago, IL, USA)
Acknowledgements
This work was supported by USPHS Grant DK067648 from the National Institute of Diabetes and Digestive and Kidney Diseases to KJS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Atweh SF, Kuhar MJ. Autoradiographic localization of opiate receptors in rat brain. III. The telencephalon. Brain Res. 1977;134:393–405. doi: 10.1016/0006-8993(77)90817-4. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. J Pharmacol Exp Ther. 1993;265:1253–1260. [PubMed] [Google Scholar]
- Bodnar RJ. Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides. 2004;25:697–725. doi: 10.1016/j.peptides.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Carr KD, et al. Effects of parabrachial opioid antagonism on stimulation-induced feeding. Brain Res. 1991;545:283–286. doi: 10.1016/0006-8993(91)91298-f. [DOI] [PubMed] [Google Scholar]
- Cole JL, et al. Evaluation of chronic opioid receptor antagonist effects upon weight and intake measures in lean and obese Zucker rats. Peptides. 1997;18:1201–1207. doi: 10.1016/s0196-9781(97)00074-0. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res. 1984;319:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Giraudo SQ, et al. Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the central nucleus of the amygdala and in the paraventricular nucleus in the rat. Brain Res. 1998;782:18–23. doi: 10.1016/s0006-8993(97)01140-2. [DOI] [PubMed] [Google Scholar]
- Glass MJ, et al. Opioids and food intake: distributed functional neural pathways? Neuropeptides. 1999;33:360–368. doi: 10.1054/npep.1999.0050. [DOI] [PubMed] [Google Scholar]
- Goldberg IE, et al. Pharmacological characterization of endomorphin-1 and endomorphin-2 in mouse brain. J. Pharmacol. Exp. Ther. 1998;286:1007–1013. [PubMed] [Google Scholar]
- Goodman RR, Pasternak GW. Visualization of mul opiate receptors in rat brain by using a computerized autoradiographic subtraction technique. Proc Natl Acad Sci U S A. 1985;82:6667–6671. doi: 10.1073/pnas.82.19.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjimarkou MM, et al. Opioid receptor involvement in food deprivation-induced feeding: evaluation of selective antagonist and antisense oligodeoxynucleotide probe effects in mice and rats. J Pharmacol Exp Ther. 2004;311:1188–1202. doi: 10.1124/jpet.104.071761. [DOI] [PubMed] [Google Scholar]
- Hahn EF, et al. Irreversible opiate agonists and antagonists: the 14-hydroxydihydromorphinone azines. J Neurosci. 1982;2:572–576. doi: 10.1523/JNEUROSCI.02-05-00572.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn EF, Pasternak GW. Naloxonazine, a potent, long-lasting inhibitor of opiate binding sites. Life Sci. 1982;31:1385–1388. doi: 10.1016/0024-3205(82)90387-3. [DOI] [PubMed] [Google Scholar]
- Herbert H, et al. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Rogers RC. Convergence of vagal and gustatory afferent input within the parabrachial nucleus of the rat. J Auton Nerv Syst. 1985;13:1–17. doi: 10.1016/0165-1838(85)90002-5. [DOI] [PubMed] [Google Scholar]
- Karimnamazi H, et al. Oral and gastric input to the parabrachial nucleus of the rat. Brain Res. 2002;957:193–206. doi: 10.1016/s0006-8993(02)03438-8. [DOI] [PubMed] [Google Scholar]
- Kelley AE, et al. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kelley AE, et al. Investigation of the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. J Pharmacol Exp Ther. 1996;278:1499–1507. [PubMed] [Google Scholar]
- Kotz CM, et al. Opioids in the nucleus of the solitary tract are involved in feeding in the rat. Am J Physiol. 1997;272:R1028–R1032. doi: 10.1152/ajpregu.1997.272.4.R1028. [DOI] [PubMed] [Google Scholar]
- Levine AS. The animal model in food intake regulation: examples from the opioid literature. Physiol Behav. 2006;89:92–96. doi: 10.1016/j.physbeh.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Ling GS, et al. Naloxonazine actions in vivo. Eur J Pharmacol. 1986;129:33–38. doi: 10.1016/0014-2999(86)90333-x. [DOI] [PubMed] [Google Scholar]
- Lundy R, Norgren R. Gustatory System. In: Paxinos G, editor. The Rat Nervous System. vol. San Diego: Elsevier Academic Press; 2005. pp. 891–921. [Google Scholar]
- Mann PE, et al. Differential sensitivity of opioid-induced feeding to naloxone and naloxonazine. Psychopharmacology (Berl) 1988a;94:336–341. doi: 10.1007/BF00174686. [DOI] [PubMed] [Google Scholar]
- Mann PE, et al. Comparison of effects of chronic administration of naloxone and naloxonazine upon food intake and maintainance of body weight in rats. Neuropharmacology. 1988b;27:349–355. doi: 10.1016/0028-3908(88)90142-6. [DOI] [PubMed] [Google Scholar]
- Narita M, et al. Reduced expression of a novel µ-opioid receptor (MOR) subtype MOR-1B in CXBK mice: Implications of MOR-1B in the expression of MOR-mediated responses. Eur J Neurosci. 2003;18:3193–3198. doi: 10.1111/j.1460-9568.2003.03052.x. [DOI] [PubMed] [Google Scholar]
- Nicklous DM, Simansky KJ. Neuropeptide FF exerts pro- and anti-opioid actions in the parabrachial nucleus to modulate food intake. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1046–R1054. doi: 10.1152/ajpregu.00107.2003. [DOI] [PubMed] [Google Scholar]
- Pan Y-X, et al. Generation of the mu opioid receptor (MOR-1) protein by three new splice variants of the Oprm GENE. Proc. Natl. Acad. Sci. USA. 2001;98:14084–14089. doi: 10.1073/pnas.241296098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak GW. Insights into mu opioid pharmacology: The role of mu opioid receptor subtypes. Life Sci. 2001b;68:2213–2219. doi: 10.1016/s0024-3205(01)01008-6. [DOI] [PubMed] [Google Scholar]
- Ragnauth A, et al. Multiple opioid receptors mediate feeding elicited by mu and delta opioid receptor subtype agonists in the nucleus accumbens shell in rats. Brain Res. 2000;876:76–87. doi: 10.1016/s0006-8993(00)02631-7. [DOI] [PubMed] [Google Scholar]
- Simone DA, et al. Involvement of opioid receptor subtypes in rat feeding behavior. Life Sci. 1985;36:829–833. doi: 10.1016/0024-3205(85)90206-1. [DOI] [PubMed] [Google Scholar]
- Tanda G, Di Chiara G. A dopamine-mul opioid link in the rat ventral tegmentum shared by palatable food (Fonzies) and non-psychostimulant drugs of abuse. Eur J Neurosci. 1998;10:1179–1187. doi: 10.1046/j.1460-9568.1998.00135.x. [DOI] [PubMed] [Google Scholar]
- Ward HG, Simansky KJ. Chronic prevention of mu-opioid receptor (MOR) G-protein coupling in the pontine parabrachial nucleus persistently decreases consumption of standard but not palatable food. Psychopharmacology (Berl) 2006;187:435–446. doi: 10.1007/s00213-006-0463-7. [DOI] [PubMed] [Google Scholar]
- Ward HG, Simansky KJ. Regional specialization of mu-opioid receptors within the parabrachial nucleus of the pons in modulating eating; Society for Neuroscience annual meeting; Atlanta, GA: 2006. [Google Scholar]
- Wilson JD, et al. An orexigenic role for mu-opioid receptors in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1055–R1065. doi: 10.1152/ajpregu.00108.2003. [DOI] [PubMed] [Google Scholar]
- Wolozin BL, Pasternak GW. Classification of multiple morphine and enkephalin binding sites in the central nervous system. Proc Natl Acad Sci U S A. 1981;78:6181–6185. doi: 10.1073/pnas.78.10.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, et al. The mu-opioid receptor subtype is required for the anorectic effect of an opioid receptor antagonist. Eur J Pharmacol. 2006;545:147–152. doi: 10.1016/j.ejphar.2006.06.069. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- Zimprich A, et al. Cloning and expression of an isoform of the rat µ opioid receptor (rMOR1B) which differs in agonist induced desensitization from rMOR1. FEBS Lett. 1995a;359:142–146. doi: 10.1016/0014-5793(95)00028-8. [DOI] [PubMed] [Google Scholar]