Abstract
The genetic, epigenetic and environmental factors may influence the risk for neuropsychiatric disease through their effects on gene transcription. Mechanistically, these effects may be integrated through regulation of methylation of CpG dinucleotides overlapping with single-nucleotide polymorphisms (SNPs) associated with a disorder. We addressed this hypothesis by analyzing methylation of prodynorphin (PDYN) CpG-SNPs associated with alcohol dependence, in human alcoholics. Postmortem specimens of the dorsolateral prefrontal cortex (dl-PFC) involved in cognitive control of addictive behavior were obtained from 14 alcohol-dependent and 14 control subjects. Methylation was measured by pyrosequencing after bisulfite treatment of DNA. DNA binding proteins were analyzed by electromobility shift assay. Three PDYN CpG-SNPs associated with alcoholism were found to be differently methylated in the human brain. In the dl-PFC of alcoholics, methylation levels of the C, non-risk variant of 3′-untranslated region (3′-UTR) SNP (rs2235749; C > T) were increased, and positively correlated with dynorphins. A DNA-binding factor that differentially targeted the T, risk allele and methylated and unmethylated C allele of this SNP was identified in the brain. The findings suggest a causal link between alcoholism-associated PDYN 3′-UTR CpG-SNP methylation, activation of PDYN transcription and vulnerability of individuals with the C, non-risk allele(s) to develop alcohol dependence.
Keywords: alcohol dependence, CpG-SNPs, DNA methylation, epigenetics, prodynorphin, single-nucleotide polymorphisms
INTRODUCTION
The endogenous opioid system (EOS) including dynorphin opioid peptides and κ-opioid receptor plays a critical role in alcohol dependence (Kreek et al. 2005; Shippenberg, Zapata & Chefer 2007; Koob & Volkow 2010; Wee & Koob 2010) Pharmacological and genetic manipulations with the opioid receptors alter alcohol consumption in animals (Kreek et al. 2005; Shippenberg et al. 2007; Koob & Volkow 2010; Wee & Koob 2010). In clinics, the opioid antagonist naltrexone reduces alcohol drinking and relapse rates in subgroups of alcoholics (O’Malley et al. 2002; Anton 2008). Molecular changes in the EOS induced by alcohol may underlie neuroplastic adaptations critical for transition to addiction (Kreek et al. 2005; Shippenberg et al. 2007; Koob & Volkow 2010; Wee & Koob 2010). The EOS regulates neurotransmission in reward circuits and areas involved in cognitive control of addictive behavior (Bencherif et al. 2004; Love, Stohler & Zubieta 2009; Koob & Volkow 2010). Analysis of the EOS in human alcohol-dependent subjects demonstrated upregulation of prodynorphin (PDYN) expression in the dorsolateral prefrontal cortex (dl-PFC), suggesting that EOS maladaptations may contribute to impairment in cognitive control over alcohol-drinking behavior (Bazov et al. unpublished).
Several single-nucleotide polymorphisms (SNPs) in PDYN promoter and exon 4 including 3′-untranslated region (3′-UTR), have been shown to be associated with alcohol dependence (Xuei et al. 2006). Analysis of PDYN demonstrated that three of five PDYN SNPs associated with alcohol dependence with high significance, overlap with CpG dinucleotides (Table 1). These methylation-associated SNPs (mSNPs) are also associated with cocaine and opioid dependence, alcohol/cocaine codependence, and memory in the elderly (Clarke et al. 2009; Kolsch et al. 2009; Yuferov et al. 2009). CpG-SNPs is an important type of polymorphisms that apparently integrates genetic variations, individual variability in epigenome and influences of the environment (Mill & Petronis 2007; Sigurdsson et al. 2009; Xie et al. 2009; Hellman & Chess 2010). Alterations in methylation of PDYN mSNPs under influences of environmental factors acting through epigenetic mechanisms may affect PDYN transcription and vulnerability to develop alcohol dependence.
Table 1.
Three of five prodynorphin SNPs associated with high significance (P < 0.01) with alcohol dependence (selected from (Xuei et al. 2006) form CpG sites. These mSNPs are also associated with cocaine dependence (Yuferov et al. 2009), cocaine/alcohol codependence (Yuferov et al. 2009), opioid dependence (Clarke et al. 2009), or episodic memory in elderly people (Kolsch et al. 2009).
| SNP ID | Position on Chr 20a | SNP location | Sequenceb | P values
|
||||
|---|---|---|---|---|---|---|---|---|
| Alcohol dependence | Cocaine dependence | Cocaine/alcohol codependence | Opioid dependence | Episodic memory | ||||
| rs1022563 | 1954339 | Downstream | TCAGGACTCTCAG/AACACTGCCAGTG | 0.006 | ||||
| rs2235749 | 1959939 | 3′-UTR | TGGCCCAACATAT/CGCACTGGGCATT | 0.007 | 0.014 | 0.003 | ||
| rs6045819 | 1961134 | Exon 4 | TAGCATGGGCCAT/CGAGGACCTGTAC | 0.007 | 0.053 | 0.037 | ||
| rs6035222 | 1963413 | Intron 3 | CCCACTCCAGACT/CTCGCCATCATGG | 0.01 | ||||
| rs1997794 | 1974858 | Promoter | GCCTATTGTGTCG/AGGCCCAGGGAGT | 0.004 | 0.019 | 0.002 | ||
Chromosome positions are based on National Center for Biotechnology Information Human Genome Assembly versus 37.1.
Allelic variations are underlined; SNPs forming CpG, and SNP’s ID are shown in bold. In the CpG-SNPs, the risk allele is shown in italic. mSNPs = methylation-associated SNPs; SNP = single-nucleotide polymorphisms.
The aims of this study were to evaluate whether PDYN mSNPs are methylated in the human brain, whether their methylation levels are altered in alcohol-dependent subjects, and whether there is DNA-binding protein(s) that may selectively target methylated and unmethylated mSNPs, and non-CpG SNP alleles.
METHODS AND MATERIALS
DNA purification, bisulphate treatment, primer design, pyrosequencing, genotyping, RNA quality control and dynorphin RIA are described in supporting information and Tables S1–S3.
HUMANSAMPLES/CASE SELECTION
Tissues were collected at the New South Wales Tissue Resource Centre (TRC), University of Sydney, Australia (Sheedy et al. 2008). Analysis included 14 chronic alcoholics and 14 controls (Table 2). All subjects were male of European descent. Alcohol-dependent subjects met criteria for Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition and National Health and Medical Research Council/World Health Organization criteria, and consumed greater than 80 g of ethanol per day for the majority of their adult lives. Controls had either abstained from alcohol completely or were social drinkers who consumed less than 20 g of ethanol per day on average. Control cases were matched to alcoholic cases by sex, age, race and postmortem interval. Cases with a history of polydrug abuse (with evidence that the individual abused other drugs such as cocaine or heroin) or with medical complications such as liver cirrhosis and the Wernicke–Korsakoff syndrome, or alcoholic cases with concomitant diseases were excluded. Cases with a prolonged agonal life support or cases with a history of cerebral infarction, head injury or neurodegenerative disease (e.g. Alzheimer’s disease) were also excluded. The main body of the population was smokers including 83% of alcoholics and 75% of control subjects. Samples were taken by qualified pathologists under full ethical clearance from the Sydney South West Area Health Service Human Ethics Committee (X03-0074). Informed written consent was obtained from the next of kin.
Table 2.
Demographic data of alcohol-dependent and control subjects.
| Subject No. | Age, years | PMI, hours | Brain pH | Storage time, months | Agonal factor score | Smoking history | Cause of death | Toxicologic findings at time of death |
|---|---|---|---|---|---|---|---|---|
| Controls | ||||||||
| 1 | 34 | 20.5 | 6.73 | 52 | 1 | Yes | Acute exacerbation of asthma | NA |
| 2 | 78 | 6.5 | 6.20 | 30 | 3 | No | Dehydration and adenocarcinoma of the lung and rectum with multiple metastases | None |
| 3 | 63 | 72 | 6.90 | 36 | 2 | Yes | Coronary-artery atherosclerosis | None |
| 4 | 82 | 23.5 | 6.40 | 46 | 3 | NA | Sepsis | None |
| 5 | 38 | 13.5 | 6.26 | 127 | 1 | Yes | ACSVD | None |
| 6 | 69 | 16 | 6.60 | 34 | 2 | Yes | ACSVD | Paracetamol and CO |
| 7 | 56 | 24 | 6.53 | 83 | 1 | Yes | Coronary-artery atheroma | NA |
| 8 | 59 | 20 | 6.56 | 81 | 1 | Yes | Coronary thrombosis | None |
| 9 | 56 | 25 | 6.10 | 32 | NA | NA | CAD | Codeine, morphine, naproxen |
| 10 | 56 | 37 | 6.76 | 38 | 2 | Yes | LV scarring, hypertension and cardiomegaly | None |
| 11 | 82 | 36 | 6.24 | 55 | 2 | No | Myocardial infarction | NA |
| 12 | 44 | 50 | 6.60 | 40 | 1 | Yes | CAD | None |
| 13 | 61 | 24 | 6.52 | 83 | 1 | Yes | CAD | NA |
| 14 | 53 | 16 | 6.84 | 50 | 1 | No | Dilated cardiomyopathy | Lignocaine, sotalol |
| M ± SEM | 59.9 ± 7.1 | 25.3 ± 3.9 | 6.5 ± 0.1 | 59.5 ± 7.1 | 1.6 ± 0.2 | |||
| Alcohol-dependent subjects | ||||||||
| 1 | 34 | 8.5 | 6.61 | 98 | 1 | Yes | Hanging | Alcohol |
| 2 | 77 | 20 | 6.34 | 97 | 2 | Yes | Lobular pneumonia | None |
| 3 | 79 | 48 | 6.34 | 87 | 1 | Yes | CAD | Temazepam |
| 4 | 39 | 24 | 6.56 | 78 | 1 | Yes | Aortic sclerosis | NA |
| 5 | 70 | 33.5 | 6.24 | 74 | 3 | Yes | Respiratory failure | None |
| 6 | 56 | 45 | 6.51 | 73 | 1 | NA | BEV | Alcohol |
| 7 | 59 | 24 | 6.57 | 71 | 1 | No | Cardiomyopathy | None |
| 8 | 56 | 22 | 6.52 | 71 | 1 | Yes | CAD and UGIH | None |
| 9 | 56 | 15 | 6.66 | 67 | 1 | NA | CAD and emphysema | Nordiazepam |
| 10 | 81 | 36 | 6.44 | 58 | 1 | Yes | Sepsis | None |
| 11 | 44 | 15 | 6.48 | 51 | 1 | No | CAD | Diazepam, noridazepam |
| 12 | 52 | 45.5 | 6.78 | 45 | 1 | Yes | Lobar pneumonia | None |
| 13 | 62 | 49 | 6.49 | 44 | NA | Yes | CAD | Sertraline |
| 14 | 53 | 57 | 6.75 | 40 | 1 | Yes | CAL | NA |
| M ± SEM | 58.4 ± 3.6 | 29.7 ± 3.5 | 6.5 ± 0.1 | 68.1 ± 4.7 | 1.2 ± 0.2 | |||
ACSVD = atherosclerotic cardiovascular disease; BEV = bleeding esophageal varices; CAD = ischemic heart disease; CAL = chronic airflow limitation; CO = carbon monoxide; LV = left ventricular; NA = not available; PMI = postmortem interval; SEM = standard error of the mean; UGIH = upper gastrointestinal hemorrhage.
DNA-binding proteins were analyzed in extracts prepared from postmortem human brain tissues collected from the Department of Forensic Medicine, Karolinska Institute, Stockholm, Sweden, with the consent of relatives. The study was approved by the local ethical committee of the Karolinska Institute. Demographic data are shown in Table S4.
Preparation of nuclear extract
Nuclear extracts from human dl-PFC from three subjects (see Table S4 in Supporting information for demographic data of human subjects whose postmortem tissues were used) and Sprague-Dawley GD-20 rat fetal brain (RFB) were prepared using a protocol (Bakalkin, Yakovleva & Terenius 1994) adapted from Dignam et al. (Dignam et al. 1983). Briefly, tissues were homogenized in Dignam’s buffer A, supplemented with protease inhibitors. The homogenate was centrifuged for 5 minutes at 4500× g, the pellet was extracted in buffer C supplemented with 0.2% Nonidet P-40 and protease inhibitors and was centrifuged twice at 20 000× g for 10 minutes. The resulting supernatant was designated as the ‘nuclear’ extract and kept at −80°C until studied. DC assay (Bio-Rad, Hercules, CA) was used for measuring protein concentrations.
Electromobility shift assay (EMSA)
The EMSA was performed essentially as described previously (Bakalkin, Yakovleva & Terenius 1993). Nuclear extracts (dl-PFC: 25 μg; RFB: 5 μg) were added to the binding mixture [(20 mM HEPES, pH 7.5; 50 mm NaCl, 1 mm Na-EDTA, 37.5% glycerol, and 1.5 mm dithiothreitol (DTT), with 20 μg bovine serum albumin (BSA; Rosh Diagnostics, Mannheim, Germany), 0.3 μg poly(dI–dC)–poly(dI–dC)] and 90 000 cpm 32P–labeled oligonucleotide in 20 μl reaction medium, incubated for 20 minutes at room temperature, and resolved on a 5% native polyacrylamide gel in 0.5 × TGE (25 mM Tris-HCl, 0.19 M glycine, 1 mM EDTA, pH 8.5) buffer. After the electrophoresis, gels were fixed in 15% methanol containing 5% acetic acid for 15 minutes, dried and analyzed by autoradiography or by Phosphoimager BAS 1500 (Fuji Film, Kanagawa, Japan) using Fuji Film Image Gauge software for quantification. Polyclonal rabbit anti-c-Myc, USF2 and NeuroD antibodies, or rabbit IgG (5 μg) all obtained from Santa Cruz Biotechnology (Santa Cruz, CA), were incubated with extracts for 40 minutes at 4°C and then for 20 minutes at 22°C before EMSA. In pilot experiment, one of three nuclear extracts characterized by high DNA-binding activity compared with extracts prepared from two other subjects was selected for further EMSA studies. Three experiments were performed with these human and RFB extracts.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) molecular mass determination
Analysis of molecular mass of DNA-binding protein in RFB extract was performed as described elsewhere (Ossipow, Laemmli & Schibler 1993). Briefly, RFB nuclear extract (125 μg) was denatured in SDS loading buffer for 5 minutes at 95°C, and was subjected to SDS-PAGE on 10% SDS-polyacrylamide Tricine gel at 240 V. The gel strips with resolved proteins were sliced uniformly into molecular mass intervals. Gel slices were crushed into 1.5 volumes of renaturation buffer (3% Triton X-100, 20 mm Hepes, 100 mm NaCl, 5 mg/ml BSA, 3 mm ZnCl2, 3 mm MgCl2, 2 mM DTT, 0.1 mm phenylmethyl-sulfonyl fluoride, 0.1 mm benzamidine-HCl) and incubated overnight at 4°C. The polyacrylamide was pelleted by centrifugation, and the supernatant was then assayed for DNA binding activity by EMSA. Molecular mass standards (Amersham International, Amersham, UK) were used to determine the molecular mass intervals of the excised gel slices.
STATISTICAL ANALYSIS
Data normality was analyzed using Kolmogorov–Smirnov test. Normally distributed datasets were analyzed using one or two-way analysis of variance (ANOVA) followed by post hoc test. Covariate influence of demographic parameters was assessed with analysis of covariance using general regression model. In the absence of data on the linearity between DNA methylation and PDYN expression, Spearman’s rank correlations were analyzed to determine the association between these variables. Significance was set at P < 0.05, and trend at 0.05 < P < 0.1. Statistica 9.0 package (StatSoft Scandinavia, Sweden) was used for statistical analysis unless otherwise mentioned.
RESULTS
Analysis of the demographic characteristics (Table 2) showed no significant differences in age (t28 = 0.35, P = 0.72), postmortem interval (PMI) (t28 = −1.33, P = 0.19), storage time (t28 = −0.44, P = 0.66), agonal factor score (U-test, P = 0.2), and proportions of smokers and nonsmokers (Fisher’s test, P = 0.5) between controls and alcohol-dependent subjects. The brain pH (t28 = −0.71, P = 0.48) and RNA quality indicator (t26 = 1.02, P = 0.3) values did not significantly differ between the two groups.
Three of five PDYN SNPs that are associated with alcoholism with high significance (Xuei et al. 2006), form a CpG dinucleotide (Table 1). The Pearson’s χ2 test did not reveal a significant association of these SNP variants with alcohol dependence (Table S3) in the analyzed sample that consisted of 14 control and 14 alcoholic subjects, and was much smaller than a sample size required for reliable analysis of genetic associations.
The PDYN promoter mSNP (rs1997794; T > C; the risk G allele forms CpG) was methylated in the dl-PFC of controls and alcoholics at low levels (15–23%; Table 3). Higher levels of methylation (66–79%) were detected for the exon 4 mSNP (rs6045819; T > C; the risk C allele forms CpG). A limited number of subjects with this mSNP (3 controls and 2 alcoholics; Table 3) precluded further comparison of the two groups.
Table 3.
Methylation levels of three prodynorphin methylation-associated SNPs associated with alcohol dependence in the dl-PFC of controls and alcoholics. Analysis of postmortem human brain specimens.
| ID | Genotype | SNP location | Number of subjects
|
Methylation levels %
|
P values | ||
|---|---|---|---|---|---|---|---|
| Controls | Alcoholics | Controls (Mean ± SD) | Alcoholics (Mean ± SD) | ||||
| rs1997794 | CC + CT | Promoter | 10 | 10 | 15.0 ± 10.0 | 23.0 ± 11.0 | 0.11c |
| rs6045819 | CC + CT | Exon 4 | 3 | 2 | 79.0 ± 13.0 | 66.0 ± 4.0 | |
| rs2235749 | CC + CT a, b | 3′-UTR | 10b | 12 | 60.9 ± 3.0 | 66.7 ± 3.9 | 0.001 |
| CC | 5 | 8 | 61.4 ± 2.1 | 65.5 ± 3.4 | <0.05 | ||
| CT | 5b | 4 | 60.4 ± 3.9 | 69.0 ± 4.0 | <0.02 | ||
Two-way analysis of variance with alcoholism and brain area (dl-PFC and MC) as factors, followed by post hoc Student’s t-test was used to evaluate differences between methylation levels of the 3′-UTR SNP (rs2235749; CC and CT genotypes were pooled) between control and alcoholics. A significant group effect [F(1.40) = 4.7, P < 0.05], a significant region effect [F(1.40) = 7.2, P = 0.01] and a significant group × region interaction [F(1.40) = 10.56, P < 0.01] were identified. Analysis of covariance failed to reveal significant influence of age, postmortem index, brain pH, agonal factor score, smoking history or storage time on the methylation differences. Data for the MC are shown in Table S5.
A statistical outlier (CT genotype, controls, dl-PFC) with methylation level exceeding two SDs from the mean value in the group was excluded from the analysis.
Student’s t-test was used for comparison. dl-PFC = dorsolateral prefrontal cortex; MC = ••; SD = standard deviation; SNP = single-nucleotide polymorphisms
The C variant of the 3′-UTR mSNP (rs2235749; C > T) is the non-risk, major allele (Xuei et al. 2006; Yuferov et al. 2009). Data on methylation of this SNP were analyzed by two-way ANOVA with group (controls and alcoholics) and region [dl-PFC and motor-cortex (MC)] as independent between-group factors. A signifi-cant group effect [F(1.40) = 4.7, P < 0.05], a significant region effect [F(1.40) = 7.2, P = 0.01] and a significant group × region interaction [F(1.40) = 10.56, P < 0.01] were revealed (Fig. 1a; Table 3). A post hoc Student’s t-test showed significant differences in the methylation status in the dl-PFC for pooled CC and CT genotypes (P = 0.001) between controls and alcoholics, and for each genotype separately (CC genotype: P < 0.05; CT genotype: P < 0.02). Analysis of covariance failed to reveal significant influence of age, PMI, brain pH, smoking history or storage time on the methylation differences. No differences were evident in the MC (P = 0.44; Table S5).
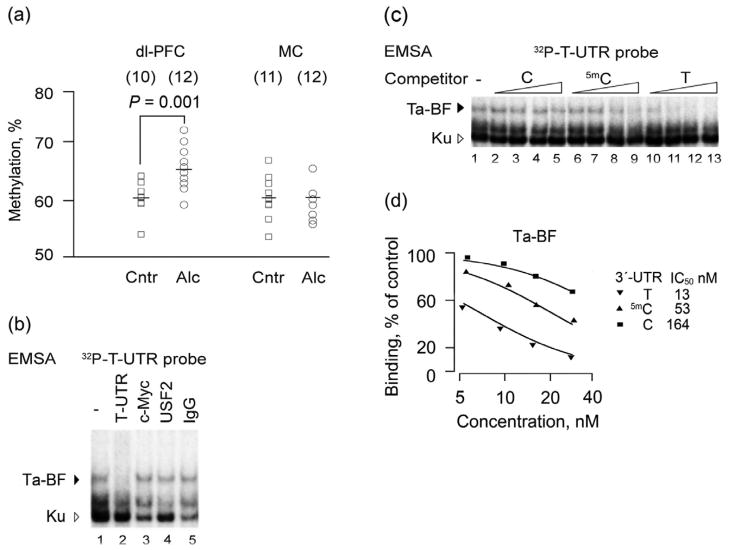
Figure 1.
Methylation of the exon 4, 3′-UTR mSNP (rs2235749) of the prodynorphin gene in the dorsolateral prefrontal cortex (dl-PFC) and MC of alcohol-dependent and control subjects. Identification of a DNA-binding factor with differential binding affinity for the risk, T allele, and the methylated and unmethylated non-risk, C allele in human dl-PFC. (a) Scatter plot of individual methylation levels of controls (Cntr) and alcoholics (Alc); mean values for each group are shown as horizontal lines. The number of subjects in the group is shown in parentheses. A statistical outlier with dl-PFC methylation value greater than two standard deviations from the mean value was excluded. (b) Electromobility shift assay of DNA-binding factors in nuclear extracts from human dl-PFC with the 32P-labeledT-UTR probe (seeTable 4) in the presence (lanes 2–13; concentrations of 3.1, 6.2, 12.5 and 25 ng/20 μl) and the absence of the C-, 5mC- and T-UTR oligonucleotides as unlabeled competitors. T allele binding factor (Ta-BF), the T allele DNA-binding factor. Unspecific complexes are shown by a triangle; they apparently formed by protein Ku, a ds-DNA-end binding factor abundant in human brain. (c) Displacement curves for T-, 5mC-, and C-UTR oligonucleotides and IC50 values from curve fitting shown for Ta-BF. 100% was defined as binding in the absence of unlabeled competitors. (d) Experiments with antibodies against c-Myc or USF2 showed no supershift or depletion of Ta-BF. IgG, rabbit immunoglobulin used as negative control. Only the upper complex was specific; its formation was blocked by addition of unlabeled T-UTR oligonucleotide. Data in b–d are shown for one representative experiment out of three experiments performed with nuclear extract prepared from tissues obtained from subject # 2 (Table S4). Extracts prepared from two other subjects showed the same pattern but weaker DNA-binding activities. dl-PFC = dorsolateral prefrontal cortex; MC = motor cortex
Our previous analysis demonstrated that PDYN mRNA and dynorphins A and B are upregulated (1.7-, 2.3- and 2.5-fold, respectively) in the dl-PFC but not in the MC in alcoholics compared with controls (Bazov et al. unpublished). Differences between alcoholic and control groups both consisting of individuals whose DNA methylation was assessed (pooled CC + CT genotypes) are still significant for PDYN mRNA (P < 0.04) and dynorphin A (P < 0.001) (Table S6). Because of low number of subjects with the TT genotype (n = 5), statistical analysis failed to reveal influence of the 3′-UTR mSNP genotype on PDYN expression. Using data for the CC and CT sub-populations of controls and alcoholics, we analyzed correlations between the PDYN 3′-UTR mSNP methylation and PDYN mRNA, and mature PDYN derived opioid peptides dynorphin A and dynorphin B (Table S7). In the dl-PFC, methylation of the 3′-UTR mSNP was significantly correlated with dynorphins A (P < 0.02; r = 0.49) and B (P < 0.03; r = 0.44), but not with PDYN mRNA (CC and CT genotypes were pooled). The CT genotype showed significant or trend correlations between (1) methylation and PDYN mRNA (P < 0.06; r = 0.64); (2) methylation and dynorphin A (P < 0.04; r = 0.68); and (3) methylation and dynorphin B (P < 0.08; r = 0.61).
To assess whether there is molecular mechanism of selective recognition of unmethylated and methylated C allele, and the risk, T allele of the 3′-UTR mSNP, we used EMSA. Nuclear extracts prepared from human dl-PFC (Fig. 1b–d) and RFB (Fig. 2), enriched in transcription factors, were analyzed. Incubation of the PDYN T allele (T-UTR oligonucleotide; Table 4) used as a labeled probe, with the human dl-PFC nuclear extract produced three main retarded complexes. In competition experiments, the upper complex showed high affinity for the T-UTR oligonucleotide (Fig. 1c, lanes 10–13); two lower complexes were not sequence specific, and were apparently formed by the Ku protein, a ds-DNA-end-binding factor that is abundant in the human brain (Bakalkin et al. 1998). The C allele (C-UTR)—oligonucleotide (Table 4) demonstrated lower affinity (12-fold) compared with that of the T-UTR oligonucleotide as evident from the displacement curves (Fig. 1c,d). Methylation of the C allele resulted in threefold increase in binding affinity. However, methylated C allele still had lower fourfolds affinity than that of the T allele.
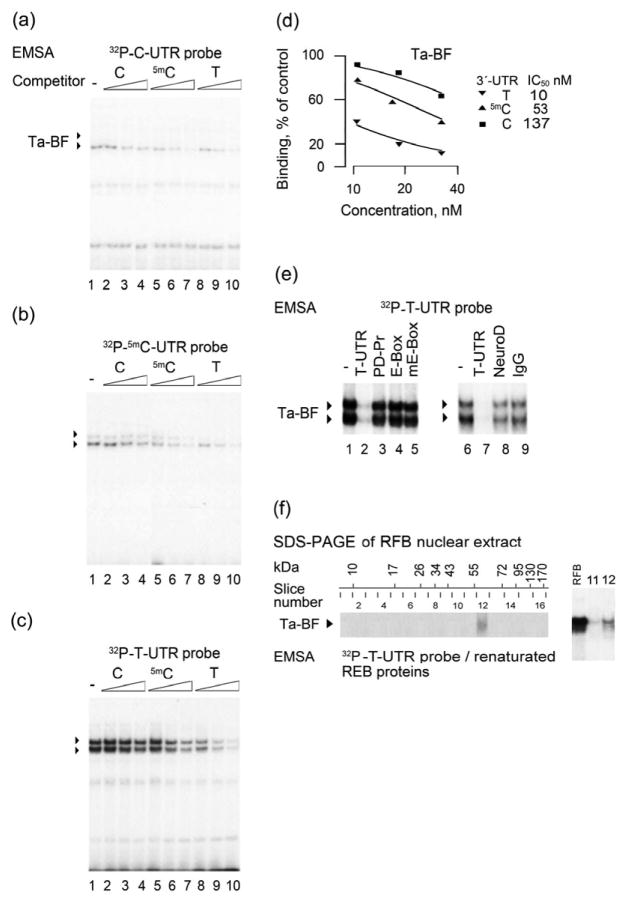
Figure 2.
Electromobility shift assay (EMSA) of rat T allele binding factor (Ta-BF). (a) C- (b) 5mC- or (c–f) T-UTR oligonucleotides used as labeled probes were incubated with nuclear extract from rat fetal brain (RFB). (a–d) Competition experiments with unlabeled C-, 5mC-, and T-UTR oligonucleotides in concentrations 6.2, 12.5 and 25 ng/20 μl. Formation of the two complexes shown by filled triangles was more prominent in (c), and the ratio of the upper to lower complexes was dependent on RFB nuclear protein concentrations. (d) Displacement curves forT-, 5mC- and C-UTR oligonucleotides. IC50 values are shown for the upper Ta-BF complex. The lower complex demonstrated a similar rank order of affinities for the three oligonucleotides. (e) Competition experiments with unlabeled T-UTR, PD-Pr and E-box oligonucleotides and anti-NeuroD-antibody. Formation of both the upper and lower complexes was blocked by addition of unlabeled T-UTR oligonucleotide, but not by any of the E-box oligonucleotides (for sequences, see Table 4). (f) Molecular weight of rat Ta-BF. Rat brain nuclear proteins were resolved by Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The gel strips were sliced into 16 pieces, and proteins were extracted, renatured and analyzed by EMSA with the 32P-T-UTR probe (left panel). Molecular size intervals in kDa and 16 gel slices are shown on the top of the image. EMSA identified Ta-BF as a protein with a molecular weight of approximately 63 kDa. The mobility of the complexes formed by 32P-T-UTR oligonucleotide with the RFB nuclear extract proteins, and renatured proteins extracted from the gel slice no. 12 in an EMSA were identical (right panel)
Table 4.
The T and C variants of 3′-UTR single-nucleotide polymorphisms (rs2235749) oligonucleotides, and E-box oligonucleotides used in an EMSA. E-box consensus sequence is CANNTG, where N denotes any nucleotide (Seo et al. 2007; Kee 2009). T- and G-boxes are variants of E-box.
| Sequence | DNA targetelement | |
|---|---|---|
| T-UTRa | TGGCCCAACATA-T-GCACTGGGCAT | T-box |
| C-UTR | TGGCCCAACATA-C-GCACTGGGCAT | mutant T-box |
| 5mC-UTR | TGGCCCAACATA-5mC-GCACTGGGCAT | |
| PD-Prb | CGGGCCATGCACGTGCCTGCTGAC | G-box |
| E–boxc | CGTGCGGCCTGACAGGTGCTTTGA | E-box |
| mE-box d | CGTGCGGCCTGCACACTGCTTTGA | mutant E-box |
E-box and its variant are underlined. T, C and 5mC alleles of the 3′-UTR are shown in bold.
T- and G-boxes are present in the T allele 3′-UTR and PDYN promoter (PD-Pr) oligonucleotides.
E-box sequence was taken from the SMU gene (Kataoka et al. 2000).
Nucleotides destroying E-box are shown in italic.
Similar results were produced with the RFB nuclear extract (Fig. 2). However, two sequence-specific complexes instead of one were formed with the T-UTR labeled probe (Fig. 2c). The ratio of the upper to lower complexes was dependent on extract concentration. At low concentrations, the lower complex was dominant, whereas at the high concentrations, the upper complex was predominantly formed (not shown) suggesting its oligomeric nature. Experiments with the labeled T-, 5mC- and C-UTR probes, and the displacement experiment with the same unlabeled oligonucleotides demonstrated that the rank order of affinity (Fig. 2a–d) for binding to these DNA variants, was similar for (1) both RFB complexes, and (2) two RFB complexes and the specific complex in the human dl-PFC. The DNA-binding factor with high affinity for the T allele was named as the T allele binding factor (Ta-BF).
The T allele forms the T-box (CATATG), a variant of E-box (CANNTG, where N is any nucleotide) that both are DNA targets for several transcription factors (Seo et al. 2007; Kee 2009) (Table 4). In the displacement experiments, oligonucleotides with canonical wild type (CAGGTG), or mutant (ACACTG) E-box sequences, or with the E-box variant known as G-box (CACGTG; PD-Pr) did not compete with the labeled T-UTR probe for binding to Ta-BF (Fig. 2e; lanes 1–5). Furthermore, antibodies against c-Myc and USF2, two dominant E-box–binding proteins in human dl-PFC, and against NeuroD that targets T-box, did not affect Ta-BF–DNA binding (Fig. 1b lanes 3–5 and Fig. 2e, lanes 6–9). These antibodies depleted or supershifted the respective transcription factors in control experiments. Thus, Ta-BF may be a new binding protein, which does not belong to the family of E-box transcription factors.
Molecular weight of Ta-BF from RFB nuclear extract was determined by combination of SDS-PAGE and EMSA (Fig. 2f). The RFB nuclear proteins were resolved by SDS-PAGE, the gel strips with resolved proteins were sliced into pieces, proteins were extracted, renatured and analyzed by EMSA with the labeled T-UTR probe. EMSA identified Ta-BF as a protein with a molecular weight of approximately 63 kDa (Fig. 2f). The mobility of the complexes formed by the unfractionated RFB nuclear extract proteins, or renatured proteins extracted from the gel slice no. 12, with the labeled T-UTR oligonucleotide were virtually identical in EMSA (Fig. 2f, the right panel).
DISCUSSION
The first principal finding of this study is the increase in methylation levels of C, non-risk variant of the PDYN 3′-UTR mSNP (rs2235749 SNP: C > T) in the dl-PFC in alcohol-dependent subjects. This area of the brain is involved in cognitive control of alcohol-drinking behavior, and shows upregulation of PDYN expression in alcoholics (Table S6) (Bazov et al. unpublished). The elevated methylation of the 3′-UTR mSNP may be a consequence of chronic alcohol consumption or an inherent property of alcoholics.
The second finding is the positive correlation of the PDYN 3′-UTR mSNP methylation with dynorphins that suggests a functional link between the methylation and gene expression. Immunohistochemical analysis with anti-PDYN antibodies demonstrated that approximately 15% of cells in the dl-PFC express PDYN (unpublished observations). Therefore, the 6% increase in methylation levels in alcoholics when all DNA molecules are taken as 100%, may correspond to de novo methylation of both alleles in 40%, or one allele in 80% of PDYN expressing cells.
The third is the finding of Ta-BF that has differential binding affinity for the T, and methylated and unmethylated C alleles of the 3′-UTR mSNP, and that may be involved in regulation of PDYN transcription through binding to the T allele or methylated 3′-UTR mSNP C allele. A positive correlation between PDYN expression and 3′-UTR mSNP methylation may be explained if binding of Ta-BF to the methylated C allele results in transcriptional activation. It would be essential to identify this 63 kDa protein and establish its role in PDYN regulation relevant for alcoholism.
Animal data suggest that adaptations in dynorphins and κ-opioid receptor play a role in alcohol dependence (Shippenberg et al. 2007; Walker, Zorrilla & Koob 2010; Wee & Koob 2010). Ethanol, similarly to addictive drugs, has been reported to increase the activity of the dynorphin/κ-opioid receptor system. Upregulated dynorphins may contribute to increased ethanol self-administration during withdrawal following chronic alcohol exposure. Thus, the blockade of the κ-opioid receptors decreased ethanol self-administration in ethanol-dependent animals, with no effect in non-dependent animals (Walker et al. 2010). This may occur if dynorphins are elevated in the dependent animals and oppose alterations in dopamine neurotransmission in the NAc in response to ethanol administration (Shippenberg et al. 2007). The PDYN/κ-opioid receptor system has not been studied yet in the striatum of human alcoholics. However, analysis of a functional haplotype implicated in vulnerability to develop cocaine dependence and cocaine–alcohol co-dependence, has been reported to be related to lower PDYN transcription in human striatum (Yuferov et al. 2009). The apparent contradiction between animal and human data may be resolved if PDYN expression depends on the phase of the addiction cycle; the upregulation during the development of dependence may be followed by the decrease in activity of this system at the phase of maintenance. In this scenario, human individuals with non-risk PDYN genotype may have the elevated striatal dynorphins (Yuferov et al. 2009) that may play a protective function by preventing both the development and maintenance of substance dependence.
In contrast to the striatum, PDYN and dynorphins have been found to be upregulated in the dl-PFC in alcohol-dependent subjects (Table S6) (Bazov et al. unpublished). In this area of the brain, dynorphins may be involved in the regulation of cognitive functions, while their upregulation may contribute to impairment in cognitive control over alcohol-drinking behavior. Our preliminary animal study supports this hypothesis by showing that several ethanol binges may upregulate dynorphins in the frontal cortex and impair behavior of rats in cognitive tasks, while κ-opioid antagonists normalize the behavior (unpublished data). Evidence for impairment of cognitive processes by upregulated dynorphins has been presented in several animal and human studies (Jiang et al. 1989; Sandin et al. 1998; Nguyen et al. 2005; Yakovleva et al. 2007; Bakalkin et al. 2010). Thus, spatial learning and memory were impaired by synthetic dynorphin (Sandin et al. 1998). PDYN expression was increased in aged mice and rats that perform worse than young animals in learning and memory tests (Jiang et al. 1989), while the aged PDYN-deficient mice showed a better acquisition and retention of spatial performance compared with wild-type animals (Nguyen et al. 2005). In postmortem human study, elevated dynorphin levels that correlated with neuropathological score were found in the PFC of patients with Alzheimer’s disease (Yakovleva et al. 2007). Three human mutations in dynorphin A that result in excessive generation of this peptide causing a neurodegenerative disorder spinocerebellar ataxia type 23 associated with cognitive problems, have been recently reported and provide evidence for the concept of a pathogenic role of elevated dynorphins in human brain (Bakalkin et al. 2010).
Because PDYN 3′-UTR mSNP methylation positively correlates with dynorphins, we may speculate that methylation of this CpG may contribute to the elevation of PDYN transcription, and consequently to the impairment in cognitive function including control over alcohol-drinking behavior. This may occur in subjects with the C, non-risk variant, who along with the subjects having the T, risk allele, develop alcohol dependence. In other words, the adaptive increase in methylation of the C, non-risk allele of 3′-UTR mSNP may make this allele to be similar to the T, risk allele in its influences on vulnerability to develop alcohol dependence. A low number of subjects with the T, risk allele (n = 5) hampered statistical analysis of influence of the 3′-UTR mSNP genotype on PDYN gene expression in frames of the present study that is a limitation of the work. The findings should be verified in a larger independent sample. Special attention should be drawn to comparative analysis of influences of PDYN 3′-UTR mSNP variant on PDYN expression in the dl-PFC and striatum; it is still unclear whether the T or C allele has activatory influences on PDYN transcription in the dl-PFC. However, such analysis may be complicated because of methylation of the C allele that may impede the comparison between genotypes.
Altogether, these findings suggest that the genetic, epigenetic and environmental factors associated with a risk for alcohol dependence may mechanistically converge on the PDYN 3′-UTR CpG–SNP and that the resulting methylation signals may be translated into disease predisposition via alterations in PDYN transcription by such factors as Ta-BF. The HabMap database identified 2 252 113 C/T and G/A SNPs in the autosomal chromosomes. Of those, 34% are located within a CpG dinucleotide (Sigurdsson et al. 2009). Some of these mSNPs may be associated with a disease, and alterations of their methylation under environmental influences may be a general phenomenon affecting gene expression and contributing to disease vulnerability (Mill & Petronis 2007; Sigurdsson et al. 2009; Xie et al. 2009; Hellman & Chess 2010).
Acknowledgments
We thank Dr Alexander Kuzmin for help with statistical analysis. This work was supported by grants from the Swedish Council for Working Life and Social Research (FAS), Research Foundation of the Swedish Alcohol Retail Monopoly (SRA), AFA Forsäkring and Swedish Science Research Council to GB. The Australian Brain Donor Programs NSW Tissue Resource Centre was supported by The University of Sydney, National Health and Medical Research Council of Australia, National Institute of Alcohol Abuse and Alcoholism and NSW Department of Health.
Footnotes
Author Contributions
GB designed the research; MMT, IB, HW, PW and TY performed the research; DS, CH, KA and HD collected human brain samples; MMT, IB, FN, TY and GB, analyzed and discussed the data; and MMT, IB, TY and GB wrote the paper. All authors have critically reviewed content and approved final version submitted for publication.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
Appendix S1 Materials and methods.
Table S1 Primers and PCR conditions for methylation analysis by pyrosequencing.
Table S2 Primers and PCR conditions for genotyping analysis.
Table S3 PDYN variants in control and alcoholic dependent subject.
Table S4 Demographic data of human subjects whose dl-PFC tissues were used in EMSA.
Table S5 Methylation levels of PDYN SNPs associated with alcohol dependence in the MC in controls and alcoholics.
Table S6 Levels of PDYN mRNA, and dynorphin A and dynorphin B peptides in the dl-PFC of control and alcohol-dependent subjects with the CC + CT genotypes of the 3′-UTR SNP (rs2235749).
Table S7 Correlations of the 3′-UTR mSNP methylation with PDYN mRNA and dynorphins A and B in dl-PFC of controls and alcoholics.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Anton RF. Naltrexone for the management of alcohol dependence. N Engl J Med. 2008;359:715–721. doi: 10.1056/NEJMct0801733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakalkin G, Watanabe H, Jezierska J, Depoorter C, Verschuuren-Bemelmans C, Bazov I, Artemenko KA, Yakovleva T, Dooijes D, Van de Warrenburg BP, Zubarev RA, Kremer B, Knapp PE, Hauser KF, Wijmenga C, Nyberg F, Sinke RJ, Verbeek DS. Prodynorphin mutations cause the neurodegenerative disorder spinocerebellar ataxia type 23. Am J Hum Genet. 2010;87:593–603. doi: 10.1016/j.ajhg.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakalkin G, Yakovleva T, Hurd YL, Nussenzweig A, Li GC, Terenius L. Autoantigen Ku in the brain. Developmentally regulated expression and subcellular localization. Neuroreport. 1998;9:2147–2151. doi: 10.1097/00001756-199806220-00044. [DOI] [PubMed] [Google Scholar]
- Bakalkin G, Yakovleva T, Terenius L. NF-kappa B-like factors in the murine brain. Developmentally-regulated and tissue-specific expression. Brain Res Mol Brain Res. 1993;20:137–146. doi: 10.1016/0169-328x(93)90119-a. [DOI] [PubMed] [Google Scholar]
- Bakalkin G, Yakovleva T, Terenius L. Prodynorphin gene-expression relates to Nf-Kappa-B factors. Mol Brain Res. 1994;24:301–312. doi: 10.1016/0169-328x(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Bencherif B, Wand GS, McCaul ME, Kim YK, Ilgin N, Dannals RF, Frost JJ. Mu-opioid receptor binding measured by [11C]carfentanil positron emission tomography is related to craving and mood in alcohol dependence. Biol Psychiatry. 2004;55:255–262. doi: 10.1016/j.biopsych.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Clarke TK, Krause K, Li T, Schumann G. An association of prodynorphin polymorphisms and opioid dependence in females in a Chinese population. Addict Biol. 2009;14:366–370. doi: 10.1111/j.1369-1600.2009.00151.x. [DOI] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucl Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman A, Chess A. Extensive sequence-influenced DNA methylation polymorphism in the human genome. Epigenetics Chromatin. 2010;3:11. doi: 10.1186/1756-8935-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HK, Owyang VV, Hong JS, Gallagher M. Elevated dynorphin in the hippocampal formation of aged rats: relation to cognitive impairment on a spatial learning task. Proc Natl Acad Sci USA. 1989;86:2948–2951. doi: 10.1073/pnas.86.8.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka H, Murayama T, Yokode M, Mori S, Sano H, Ozaki H, Yokota Y, Nishikawa S, Kita T. A novel snail-related transcription factor Smuc regulates basic helix-loop-helix transcription factor activities via specific E-box motifs. Nucl Acids Res. 2000;28:626–633. doi: 10.1093/nar/28.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee BL. E and ID proteins branch out. Nat Rev Immunol. 2009;9:175–184. doi: 10.1038/nri2507. [DOI] [PubMed] [Google Scholar]
- Kolsch H, Wagner M, Bilkei-Gorzo A, Toliat MR, Pentzek M, Fuchs A, Kaduszkiewicz H, van den Bussche H, Riedel-Heller SG, Angermeyer MC, Weyerer S, Werle J, Bickel H, Mosch E, Wiese B, Daerr M, Jessen F, Maier W, Dichgans M. Gene polymorphisms in prodynorphin (PDYN) are associated with episodic memory in the elderly. J Neural Transm. 2009;116:897–903. doi: 10.1007/s00702-009-0238-5. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction (vol 35, pg 217, 2010) Neuropsychopharmacology. 2010;35:1051. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Love TM, Stohler CS, Zubieta JK. Positron emission tomography measures of endogenous opioid neurotransmission and impulsiveness traits in humans. Arch Gen Psychiatry. 2009;66:1124–1134. doi: 10.1001/archgenpsychiatry.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill J, Petronis A. Molecular studies of major depressive disorder: the epigenetic perspective. Mol Psychiatry. 2007;12:799–814. doi: 10.1038/sj.mp.4001992. [DOI] [PubMed] [Google Scholar]
- Nguyen XV, Masse J, Kumar A, Vijitruth R, Kulik C, Liu M, Choi DY, Foster TC, Usynin I, Bakalkin G, Bing G. Prodynorphin knockout mice demonstrate diminished age-associated impairment in spatial water maze performance. Behav Brain Res. 2005;161:254–262. doi: 10.1016/j.bbr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Ossipow V, Laemmli UK, Schibler U. A simple method to renature DNA-binding proteins separated by SDS-polyacrylamide gel electrophoresis. Nucl Acids Res. 1993;21:6040–6041. doi: 10.1093/nar/21.25.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin J, Nylander I, Georgieva J, Schott PA, Ogren SO, Terenius L. Hippocampal dynorphin B injections impair spatial learning in rats: a kappa-opioid receptor-mediated effect. Neuroscience. 1998;85:375–382. doi: 10.1016/s0306-4522(97)00605-2. [DOI] [PubMed] [Google Scholar]
- Seo S, Lim JW, Yellajoshyula D, Chang LW, Kroll KL. Neurogenin and NeuroD direct transcriptional targets and their regulatory enhancers. EMBO J. 2007;26:5093–5108. doi: 10.1038/sj.emboj.7601923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedy D, Garrick T, Dedova I, Hunt C, Miller R, Sundqvist N, Harper C. An Australian brain bank: a critical investment with a high return! Cell Tissue Bank. 2008;9:205–216. doi: 10.1007/s10561-008-9076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson MI, Smith AV, Bjornsson HT, Jonsson JJ. HapMap methylation-associated SNPs, markers of germline DNA methylation, positively correlate with regional levels of human meiotic recombination. Genome Res. 2009;19:581–589. doi: 10.1101/gr.086181.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, Koob GF. Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol. 2010;16:116–119. doi: 10.1111/j.1369-1600.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie HH, Wang M, Bischof J, Bonaldo MD, Soares MB. SNP-based prediction of the human germ cell methylation landscape. Genomics. 2009;93:434–440. doi: 10.1016/j.ygeno.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Xuei X, Dick D, Flury-Wetherill L, Tian HJ, Agrawal A, Bierut L, Goate A, Bucholz K, Schuckit M, Nurnberger J, Jr, Tischfield J, Kuperman S, Porjesz B, Begleiter H, Foroud T, Edenberg HJ. Association of the kappa-opioid system with alcohol dependence. Mol Psychiatry. 2006;11:1016–1024. doi: 10.1038/sj.mp.4001882. [DOI] [PubMed] [Google Scholar]
- Yakovleva T, Marinova Z, Kuzmin A, Seidah NG, Haroutunian V, Terenius L, Bakalkin G. Dysregulation of dynorphins in Alzheimer disease. Neurobiol Aging. 2007;28:1700–1708. doi: 10.1016/j.neurobiolaging.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Yuferov V, Ji F, Nielsen DA, Levran O, Ho A, Morgello S, Shi R, Ott J, Kreek MJ. A functional haplotype implicated in vulnerability to develop cocaine dependence is associated with reduced PDYN expression in human brain. Neuropsychopharmacology. 2009;34:1185–1197. doi: 10.1038/npp.2008.187. [DOI] [PMC free article] [PubMed] [Google Scholar]