Abstract
Background
Intrathecal baclofen (ITB) is an effective treatment for secondary dystonia. However, in many patients with dystonia, placement of an intrathecal catheter is difficult due to anatomic anomalies or spinal fusion. Intraventricular baclofen (IVB) has been shown to be an effective alternate route for drug delivery in a small series of patients.
Objective
To present the largest series of IVB cases to date, and compare the complication rate to that of a concurrent cohort of ITB cases.
Methods
The senior author’s series of IVB cases were reviewed. All contemporaneous cases of ITB for dystonia were reviewed as a control group. Data were collected by retrospective medical records review.
Results
Thirty IVB patients and 33 ITB patients were identified. Mean follow up was 15 and 16 months, respectively. IVB patients were younger, more commonly underweight, and had more severe dystonia, though no difference between groups was significant. Eleven patients (37%) experienced complications in the IVB group, and 16 (48%) in the ITB group. Kaplan-Meier survival analysis showed an odds ratio of 0.67 (95% CI 0.30–1.48, p=0.31) in favor of IVB. Adjusting for age and underweight status yielded an odds ratio of 0.64 (95% CI 0.29–1.42, p=0.27) in favor of IVB. There were 7 catheter or leak-related complications in the ITB group and 2 in the IVB group (p=0.15).
Conclusion
Intraventricular baclofen is as safe as intrathecal baclofen. There may be a lower risk of catheter or leak-related complications with IVB, though this study was too small to show significance.
Keywords: baclofen, dystonia, infusion (intraventricular), injection (spinal), postoperative complications
Introduction
Intrathecal baclofen (ITB) has been used for treatment of secondary dystonia since it was first reported by Narayan, et al. in 1991.1 Several publications have reported success with ITB for treatment of primary and secondary dystonia.2–10 The largest study to date of ITB for dystonia was an observational study of 77 patients who had significant improvement in their Barry-Albright dystonia (BAD) scale scores.11 In that study, patients whose intrathecal catheter was placed at T4 or higher had a better response to therapy that those whose catheters were positioned at T6 or lower. However, the high rate of response was accompanied by a 38% complication rate (CSF leak, infection, mechanical problem with catheter or pump being most common).
The use of intraventricular baclofen (IVB) as an alternative to ITB was first employed in a patient in whom placement of an intrathecal catheter was deemed too technically difficult due to a short and lordotic neck and existence of a previous spinal fusion. The second case was a patient with severe dystonia who had not responded to intrathecal baclofen.12 In both cases, FDA authorization was obtained. The first patient responded well while the second had minimal response. After systematically examining the safety of IVB in animals, this technique was employed in several more cases.13 Examination of the first ten cases raised several interesting observations.14 Eight of 10 patients had a response to baclofen with significant decrease in dystonia scores. The dose of baclofen required to achieve satisfactory control appeared to be lower than typical ITB doses. However, the two patients who did not have satisfactory response to IVB also had poor response to ITB. There were three complications in these ten patients including 2 infections and 1 CSF leak.
Given the high reported rate of complication with ITB and the apparent satisfactory response to IVB, we postulated that IVB might be a more appropriate method of baclofen delivery for many patients with dystonia. Placement of the intraventricular catheter can often be much simpler and less technically demanding than placement of the spinal intrathecal catheter, particularly in patients with spine fusions. In addition, wound leaks and infections that are prevalent in all reported series of ITB may be less common when IVB is utilized.
The purpose of this report is to provide details of the largest series of patients to date treated with IVB for dystonia and to compare results and complications of IVB treatment with those of a contemporaneous series of patients treated with ITB for dystonia. In addition, by examining this large series of patients, we further evaluate some of the interesting observations from the initial small series of IVB patients.
Methods
We performed a retrospective study to compare intraventricular baclofen (IVB) with spinal intrathecal baclofen (ITB) for treatment of dystonia. All information for this study was collected through retrospective medical records review. This study was considered exempt from review by the University of Wisconsin Health Sciences IRB (protocol number 2010-0462). Intrathecal baclofen is approved by the FDA for treatment of spasticity, but use for dystonia is considered “off label.” The same is true of the pump and catheter system.
All patients treated with IVB by the senior author were identified and served as the experimental group. The control group consisted of all patients treated for dystonia with ITB during the time period during which IVB cases were also being performed. Patients whose primary movement disorder was spasticity were excluded from analysis. Presence of spasticity in addition to predominant dystonia was not a reason for exclusion. One additional exclusion criterion was follow up duration of less than one month, though if a complication occurred at any time, including before one month, the patient was not excluded.
Technique for placement of ITB and IVB pump and catheter has been previously described.14, 15 Briefly, the intraventricular catheter was placed via a coronal or parietal burr hole using standard cranial landmarks without neuronavigation. Coronal burr hole was used in all cases except those patients with excessive folding/corrugation of the skin in this area such that obtaining asepsis in the depths of the folds would be difficult. An endoscope was then inserted into the sheath and used to guide the sheath into an optimal position, with the goal being the third ventricle if possible. The Medtronic 8709SC catheter, a single piece catheter that connects to a short stem attached to the side port of a Synchromed pump, was then tunneled from the pump site to the cranial incision. The catheter was then placed through the sheath and the sheath peeled away. This method resulted in a single connection, with the 8709SC catheter running from the pump connection into the ventricle without interruption. Postoperative imaging was not routinely obtained.
The formulation of baclofen used was the clinically available form, Lioresal, with concentration of 500, 1000 or 2000 mcg/ml depending on the planned initial dose of medication, with no difference in concentration between ITB and IVB patients.
The primary outcome was presence of a complication. Because the vast majority of the studied patients received all pump-related care at our institution, we believe that our ability to capture all pump-related complications was good. The choice of a complication as a primary outcome allowed us both to compare the safety of ITB versus IVB and assured that we capture the true incidence of the primary outcome.
Secondary outcomes were an absolute decrease in BAD score from pretreatment to post treatment values, and baclofen dose. Additionally, we collected covariates likely to reflect either the severity of dystonia or the likelihood of a complication. These variables included pretreatment severity of dystonia as indicated by BAD score, patient age, and whether or not the patient was underweight. Underweight was defined as weight less than the 5th percentile for age for pediatric patients and BMI less than 19 for adult patients.
The primary outcome of presence of a complication was analyzed using both univariate and multivariate methods. The Kaplan-Meier method with Mantel-Haenzel test of significance was used to compare pump survival without a complication over time in a univariate fashion. Cox proportional hazards analysis was used to perform multivariate analyses of the rate of complications. Two-sided t-test was used to compare secondary outcome variables.
Results
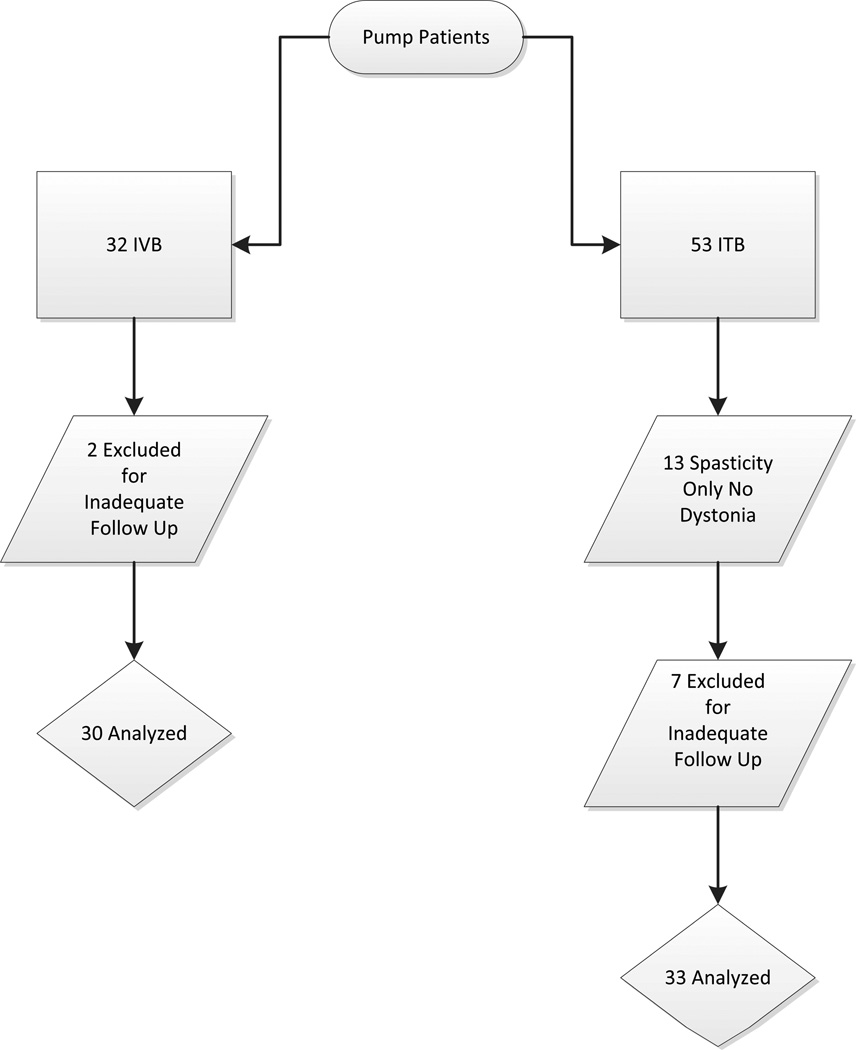
A total of 32 patients who had undergone IVB therapy for dystonia were identified. Pump placement operations occurred between January 2005 and July 2010 (all but one between July 2007 and July 2010). A total of 53 patients undergoing ITB pump placements were identified, occurring between July 2007 and May 2010. Of these, 13 underwent ITB therapy for spasticity without dystonia and were therefore excluded. Two patients from the IVB group and 7 patients from the ITB group were excluded due to follow up duration less than one month. No patient in whom a complication was identified was excluded due to inadequate follow up. In total, 30 patients made up the IVB group with 33 patients in the ITB group. Figure 1 shows the patients included in each group.
Figure 1.
Summary of patients evaluated and included from each group
Patient ages in the IVB group ranged from 2 to 35 years, with a mean of 16 years. Ages in the ITB group ranged from 9 months to 44 years, with a mean of 19 years, (16 v. 19 years, p=0.34). The IVB group also had a higher percentage of underweight patients (14 of 25 patients for whom weight is documented, 56% v. 10 of 19 patients, 53%; p=0.75 by Chi-squared test) and a higher pretreatment mean BAD score (16 v. 14, p=0.41). Mean follow up duration was 15–16 months for both groups. None of these differences was statistically significant. Etiology of dystonia was similar in the two groups as well, with cerebral palsy as the leading cause in both groups. Other underlying causes were cerebral anoxic injury, traumatic brain injury, pantothenate kinase deficiency and other metabolic syndromes. Table 1 shows a comparison of the two groups.
TABLE.
Group Characteristics
| ITB | IVB | p-value | |
|---|---|---|---|
| Number of Patients Included | 33 | 30 | |
| Cerebral palsy as etiology for dystonia | 26/33* | 21/30** | |
| Age, years, mean (range) | 19 (9 mo – 44 yrs) | 16 (2 – 35 yrs) | 0.34 |
| Underweight, n (%) | 10 (53%) | 14 (56%) | 0.75 |
| Pre-treatment BAD, mean (SD) | 14 (8) | 16 (8) | 0.41 |
| Follow Up, mean | 16 mo | 15 mo | |
| Other etiologies | |||
ITB: traumatic brain injury, pantothenate kinase deficiency
IVB: methylmalonic aciduria, anoxic injury, pantothenate kinase deficiency, traumatic brain injury, Duane syndrome
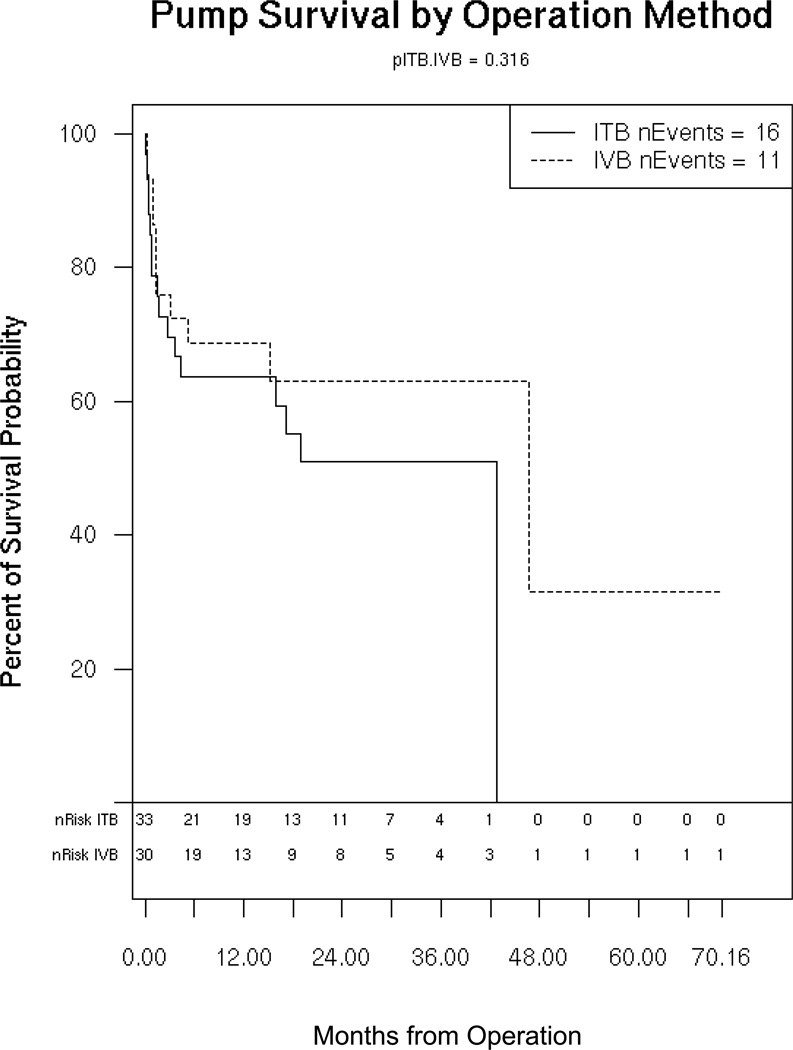
In the ITB group, there were 16 total complications, 8 infections and 7 CSF leaks or catheter malfunctions, and one death from respiratory failure, yielding a complication rate of 48%. In the IVB group, there were a total of 11 complications, including 5 infections, 2 CSF leak or catheter malfunctions, 2 cases of newly diagnosed hydrocephalus requiring shunt, and one death from respiratory failure. The overall complication rate for IVB was 37%. The odds ratio for occurrence of a complication was 0.67 (95% CI 0.30–1.48, p=0.32 by Mantel-Haenzel). Figure 2 shows the Kaplan-Meier curve depicting pump survival without a complication comparing IVB to ITB. Multivariate analysis controlling for age showed an odds ratio favoring IVB of 0.64 (95% CI 0.29–1.42, p=0.27 by Cox PH). Controlling for both age and underweight status required removal of 19 patients for whom information about weight was not available. The multivariate analysis of only the remaining 44 patients showed an odds ratio in favor of IVB of 0.64 (95% CI 0.25–1.66, p=0.36).
Figure 2.
Kaplan-Meier survival curve comparing IVB to ITB for presence of a complication
When considering only CSF leak or catheter malfunctions, the IVB group had 2 out of 30 patients for a rate of 6.7%. The ITB group had 7 out of 33 patients, for a rate of 21% (p=0.15).
Analysis of secondary outcome variables showed mean decrease in BAD score of 7 points in the IVB group versus 9 points in the ITB group (p=0.61). However, pre and post treatment BAD scores were only available for 12 patients in the IVB group and 11 patients in the ITB group. Mean baclofen dose was 595 micrograms per day for IVB and 476 micrograms per day for ITB (p=0.27). Final baclofen dosing was available for 23 patients in the IVB group and 22 patients in the ITB group.
Discussion
We report the largest series to date of intraventricular baclofen therapy for dystonia. Infusion of baclofen into the CSF space has previously been shown to be an effective treatment for dystonia, and preliminary studies indicate that intraventricular baclofen may be equally effective. The primary objective of the present study is to compare the rate of complication of ITB and IVB in this patient population. Complications of intrathecal baclofen, such as infection and CSF leak, are common in patients with generalized dystonia.14 Many patients are undernourished due to the high metabolic demands of dystonic posturing. Additionally, dystonic movements often place pressure on pump and catheter insertion sites. However, given the lack of effective intervention for these patients, a high rate of complications may be acceptable, as the benefit for these patients may outweigh the risk. The present report shows that IVB has a similar complication rate as ITB. Our cohort of patients who received IVB was younger, had more severe dystonia, and was more commonly underweight. Though the differences in these factors were not statistically significant, they might be thought to lead to an increase in the rate of complications. That a slightly lower, though again insignificant, rate of complication was seen with IVB strongly supports the null hypothesis that IVB is as safe as ITB.
Considering only CSF leak or catheter-related complications, there were 2 such problems in the IVB group compared to 7 in the ITB group. While this difference also does not reach statistical significance, it was an expected finding. Placement of the IVB catheter is less technically demanding that placement of the ITB catheter, particularly in patients who have had previous spinal fusion or who require laminotomy for catheter placement. This was one of the initial indications for IVB, and may be a major reason that IVB was chosen for some patients included in this analysis. Therefore, one might expect an even higher rate of CSF leak or catheter complications had this selected group of patients undergone ITB therapy. No patient in the IVB group experienced a catheter disconnection, nor did any require a revision for a malpositioned catheter.
Unlike the report cited earlier, this series does not show that baclofen doses were lower in patients with IVB. Rather, doses of IVB were slightly higher than ITB, though not statistically significant. This may be due to the fact that IVB patients had somewhat higher initial BAD scores, and thus might be expected to have higher drug requirements. Based on experience with this group of patients, we postulate that the initial baclofen dosing and dose titration can be done in a similar fashion whether an intraventricular catheter or an intrathecal catheter at a high cervical level is used. With either catheter placement, dose titration to 1000 mcg/day or higher was tolerated without obtundation or lethargy in patients with severe dystonia.
Two patients in the IVB group developed new hydrocephalus requiring a shunt. Both of these patients had undergone preoperative imaging of the brain; one had prominent ventricles prior to IVB catheter placement, and the other did not. We believe that the first patient likely had underlying hydrocephalus, and a shunt was placed after leakage from the cranial incision following pump implantation. The second patient had a small amount of hemorrhage at the time of catheter insertion, and we believe that this likely contributed to the development of hydrocephalus. However, in this patient, a shunt was placed after hydrocephalus and bilateral subdural hygromas developed, without incision leakage or clinical deterioration. Both patient had shunts placed within 2 weeks of pump placement. While the association between IVB and hydrocephalus is not strong enough to draw any conclusions, it will be important to keep this in mind in the evaluation of these patients and in future studies. Furthermore, there appears to be little difference in the dose requirement of patients with or without CSF shunts.
This retrospective study has several limitations. The primary outcome was chosen largely because the authors are confident that complete follow up information was available with regards to the presence of complications. However, because of the dependence on the medical record, only those patients in whom BAD scores and baclofen dose were meticulously documented at the time of the most recent follow up visit were included in the evaluation of the secondary outcomes. Despite this being the largest series of patients treated with IVB reported to date, it remains a small study with conclusions limited by size. Based on the observed difference in complication rate between IVB and ITB, a prospective study would require over 400 patients to have adequate power for this difference to be statistically significant. Additionally, the mean follow up of 15 months is short, and no information about long-term efficacy of IVB is provided here. This technique has been in use at our institution since 2007, and long term follow up studies will be forthcoming. It should also be noted that the patients included in the previous reports of IVB treatment of dystonia were included in this analysis.
Conclusion
We report a comparison of the rate of complications of treatment of dystonia with IVB versus ITB. This is the largest series of patients treated with IVB to date. Based on these data, IVB appears to be as safe as ITB in this population. Pump-based therapies for dystonia continue to have a high rate of complications.
Acknowledgments
This project was supported in part by the University of Wisconsin Institute for Clinical and Translational Research (UW ICTR), funded through an NIH Clinical and Translational Science Award (CTSA), grant number 1 UL1 RR025011.
Footnotes
Disclosure: Dr. Albright served as a consultant for Medtronic Corporation, but received no support or remuneration.
References
- 1.Narayan RK, Loubser PG, Jankovic J, Donovan WH, Bontke CF. Intrathecal baclofen for intractable axial dystonia. Neurology. 1991 Jul;41(7):1141–1142. doi: 10.1212/wnl.41.7.1141. [DOI] [PubMed] [Google Scholar]
- 2.Albright AL, Barry MJ, Painter MJ, Shultz B. Infusion of intrathecal baclofen for generalized dystonia in cerebral palsy. J Neurosurg. 1998 Jan;88(1):73–76. doi: 10.3171/jns.1998.88.1.0073. [DOI] [PubMed] [Google Scholar]
- 3.Awaad Y, Munoz S, Nigro M. Progressive dystonia in a child with chromosome 18p deletion, treated with intrathecal baclofen. J Child Neurol. 1999 Feb;14(2):75–77. doi: 10.1177/088307389901400202. [DOI] [PubMed] [Google Scholar]
- 4.Paret G, Tirosh R, Ben Zeev B, Vardi A, Brandt N, Barzilay Z. Intrathecal baclofen for severe torsion dystonia in a child. Acta Paediatr. 1996 May;85(5):635–637. doi: 10.1111/j.1651-2227.1996.tb14109.x. [DOI] [PubMed] [Google Scholar]
- 5.Siebner HR, Dressnandt J, Auer C, Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve. 1998 Sep;21(9):1209–1212. doi: 10.1002/(sici)1097-4598(199809)21:9<1209::aid-mus15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Penn RD, Gianino JM, York MM. Intrathecal baclofen for motor disorders. Mov Disord. 1995 Sep;10(5):675–677. doi: 10.1002/mds.870100524. [DOI] [PubMed] [Google Scholar]
- 7.Dalvi A, Fahn S, Ford B. Intrathecal baclofen in the treatment of dystonic storm. Mov Disord. 1998 May;13(3):611–612. doi: 10.1002/mds.870130344. [DOI] [PubMed] [Google Scholar]
- 8.Dressler D, Oeljeschlager RO, Ruther E. Severe tardive dystonia: treatment with continuous intrathecal baclofen administration. Mov Disord. 1997 Jul;12(4):585–587. doi: 10.1002/mds.870120416. [DOI] [PubMed] [Google Scholar]
- 9.Diederich NJ, Comella CL, Matge G, Becker G, Schiltz F, Metz H. Sustained effect of high-dose intrathecal baclofen in primary generalized dystonia: a 2-year follow-up study. Mov Disord. 1997 Nov;12(6):1100–1102. doi: 10.1002/mds.870120649. [DOI] [PubMed] [Google Scholar]
- 10.Ford B, Greene P, Louis ED, et al. Use of intrathecal baclofen in the treatment of patients with dystonia. Arch Neurol. 1996 Dec;53(12):1241–1246. doi: 10.1001/archneur.1996.00550120049016. [DOI] [PubMed] [Google Scholar]
- 11.Albright AL, Barry MJ, Shafton DH, Ferson SS. Intrathecal baclofen for generalized dystonia. Dev Med Child Neurol. 2001 Oct;43(10):652–657. doi: 10.1017/s0012162201001190. [DOI] [PubMed] [Google Scholar]
- 12.Albright AL. Intraventricular baclofen infusion for dystonia. Report of two cases. J Neurosurg. 2006 Jul;105(1 Suppl):71–74. doi: 10.3171/ped.2006.105.1.71. [DOI] [PubMed] [Google Scholar]
- 13.Albright AL. Long-term intraventricular baclofen infusion in beagles. J Neurosurg. 2007 Sep;107(3 Suppl):225–227. doi: 10.3171/PED-07/09/225. [DOI] [PubMed] [Google Scholar]
- 14.Albright AL, Ferson SS. Intraventricular baclofen for dystonia: techniques and outcomes. Clinical article. J Neurosurg Pediatr. 2009 Jan;3(1):11–14. doi: 10.3171/2008.10.PEDS0847. [DOI] [PubMed] [Google Scholar]
- 15.Albright AL, Turner M, Pattisapu JV. Best-practice surgical techniques for intrathecal baclofen therapy. J Neurosurg. 2006 Apr;104(4 Suppl):233–239. doi: 10.3171/ped.2006.104.4.233. [DOI] [PubMed] [Google Scholar]