Abstract
Background
This study compares young adult survivors of childhood cancer (YASCC) and young adults without a history of serious illness/injury on physical activity levels and examines psychological predictors of physical activity in survivors over a two month period.
Procedure
YASCC participants (n = 117) and healthy controls (n = 148), ages 18–30, recruited during cancer survivorship clinic or primary care clinics completed self-report measures of physical activity, health problems, psychological distress, and health beliefs (Health Perceptions, Satisfaction with Healthcare, Cognitive Competence, Autonomy). Survivorship providers completed ratings of health problems and treatment intensity for survivors.
Results
Survivors had significantly lower levels of physical activity than controls. Family income, survivor-reported health problems and less positive health beliefs were associated with lower rates of physical activity. Provider-reported survivor health problems and ratings of cancer treatment intensity were not related to survivor physical activity. Less positive survivor beliefs about their cognitive competence predicted survivor physical activity two months later after accounting for other pertinent demographic, medical and psychological variables.
Conclusions
YASCC were significantly less active than healthy controls. YASCC with more self-identified health problems and negative beliefs about their cognitive competence were less physically active. Beliefs about their health and cognitive competencies may be viable areas for assessment and intervention in order to promote increased engagement in physical activity.
Keywords: survivorship, long-term survival, psychology, psychosocial health behaviors
With childhood cancer survival rates exceeding 80% [1], there is greater emphasis on survivor long-term health and wellbeing. Data from the Childhood Cancer Survivor Study (CCSS) indicate that the majority of young adult survivors of childhood cancer (YASCC) experience at least one significant health condition [2], including cardiovascular problems[2–4] and obesity [5], and are at risk for subsequent cancers in adulthood [6]. This increased risk for health problems highlights the importance of health-promoting behaviors. Regular physical activity, in particular, may mitigate the severity of late effects and enhance quality of life [7].
Increasing evidence, however, suggests that YASCCs do not adequately take responsibility for their health and that young adults in general engage in suboptimal levels of health-promoting behaviors. Similar to the typical age-related trajectory of physical activity [8], survivor physical activity declines in late childhood and young adulthood [9,10]. In general, YASCCs do not meet physical activity recommendations and exercise less than controls [11,12]. However, the current literature on YASCC physical activity is limited by studies with small sample sizes [e.g., 4,13,14], no control group [e.g., 15,16,17] or the lack of a non-sibling control group [e.g., 9]. For example, a study from the CCSS found that 52% of survivors did not meet CDC activity guidelines and were more likely to be inactive than sibling controls [9]. Although this finding provides foundational evidence for physical activity deficits in YASCCs, additional studies are needed that compare survivor physical activity against non-sibling, healthy controls since physical activity among siblings is positively correlated due to known family influences on exercise [18]. Studies using sibling controls may underestimate the impact of a history of childhood cancer on physical activity levels in young adulthood since the magnitude of difference between survivors and siblings may be reduced due to shared family factors that influence physical activity. The only study comparing YASCC physical activity to healthy controls found similar rates of physical activity among groups, but was limited by a small sample [14].
Research exploring predictors of YASCC physical activity has identified demographic, medical, and psychosocial factors in cross-sectional studies. Younger survivor age, higher socioeconomic status (SES), and male gender [9] are related to higher rates of physical activity. Cancer type and treatment-related variables (e.g., cranial radiation) also have been associated with YASCC physical activity [9]. Late effects, including fatigue and cancer-related pain, have been cited as significant barriers to physical activity [19].
Investigations also have found strong associations between psychological variables and YASCC physical activity. Cancer-related anxiety, negative affect [19], and depression [9] have been associated with inactivity. Social withdrawal and antidepressant use during adolescence also has been related to increased risk of survivor inactivity in adulthood [20].
Oeffinger [21] proposed that examining the health beliefs of YASCC may offer a clinically useful framework for understanding the health behaviors of this vulnerable population. The health belief model [22] conceptualizes engagement in health promoting behaviors as, in part, the result of beliefs related to perceived vulnerability to health problems, perceived benefits and costs associated with the health behavior, and self-efficacy and locus of control. Consistent with this model [22], more positive beliefs about ability to engage in physical activity and fewer perceived negatives associated with exercise have been positively related with YASCC physical activity [15]. Few studies have examined YASCC psychological functioning and beliefs as predictors of physical activity while accounting for demographic and medical variables. Such research is important in order to establish these factors as viable targets for exercise-promoting interventions as demographic and medical variables are not readily modifiable.
Given the limitations of the existing studies on YASCC physical activity, specifically the lack of non-sibling comparisons and the absence of prospective studies, additional research is needed that addresses these shortcomings. The objectives of this study were to compare levels of physical activity in YASCC and healthy controls two months after (Time 2) a medical appointment (Time 1) and to identify the psychological and belief factors associated with physical activity in YASCC over this two-month period. The two-month period is relevant given evidence for this length of time in forming and maintaining health-promoting habits such as exercise [23]. Specific hypotheses were: 1) YASCC participants would have lower levels of physical activity than the comparison group at Time 2; 2) within the YASCC group, medical factors, cancer treatment intensity and survivor health problems, will have individual negative associations with physical activity at Time 2; and 3) increased psychological distress and less adaptive beliefs at Time 1 would predict lower rates of YASCC physical activity at Time 2 after adjusting for demographic and medical variables.
Methods
Participants
The present study is a secondary analysis of data collected for an institutional review board-approved study of adolescent and young adult long-term survivors of childhood cancer and healthy comparison young adults [24]. The current sample includes 117 YASCCs and 148 comparison group participants between the ages of 18 and 30 (M = 21.91, SD = 2.78). YASCC younger than 18 were excluded due to potential developmental influences on physical activity (e.g., younger children and teenagers more influenced by parents [25]). YASCC inclusion criteria included cancer diagnosis occurring before age 21 and at least 5 years prior to study participation, completing cancer treatment at least 2 years prior, English-speaking, and being able to read independently at the fifth grade level. Survivors of brain tumors or those with severe cognitive impairment were excluded. YASCC diagnoses included leukemias (43%), solid tumors (37%) and lymphomas (20%). YASCCs were on average 12.3 years (SD = 4.91) from diagnosis and 25.6% of YASCC participants self-reported receiving cranial radiation as part of their cancer treatment. Inclusion criteria for the comparison group were no history of a chronic or life-threatening illness or injury, English-speaking, and reading at the fifth grade level. Comparison group participants were excluded if they had a history of a psychiatric hospitalization or were currently pregnant.
Procedures
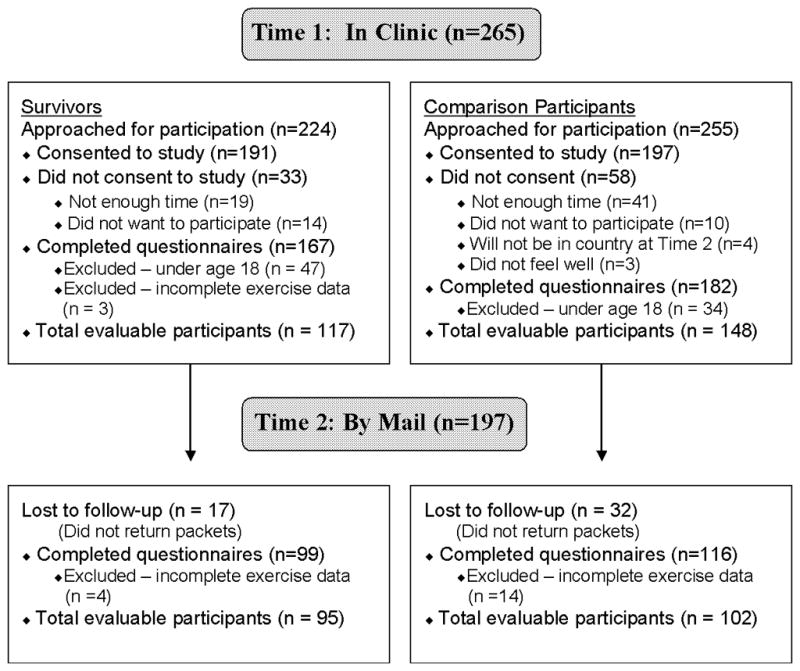
YASCC participants were recruited during survivorship clinic appointments at a large pediatric medical center. Comparison group participants were recruited from an urban family practice office, an adolescent medicine clinic, or a university student health center. Recruitment of the comparison group was targeted to the anticipated demographic characteristics of the survivor population. Participants provided consent and completed study measures during their clinic appointment (Time 1) and again 2 months later via mail (Time 2; see Figure I).
Figure 1.
Measures
Demographic Information
Participants completed a basic demographic questionnaire. This questionnaire assessed participant race, educational achievement, and family income.
Physical Activity
The Godin Leisure-Time Exercise Questionnaire [GLTEQ; 26] is a valid and reliable measure [27] of physical activity during a “typical 7-day period.” The GLTEQ has been used in a variety of populations, including survivors of breast [28] and ovarian cancer [29] and adults treated for brain tumors [30]. Each participant reported the average number of times he/she engages in at least 15 minutes of activity across three exercise categories: vigorous (e.g., running, swimming), moderate (e.g., dancing, fast walking), and mild(e.g., yoga, fishing). Responses to the three categories are weighted and summed to create a modified Leisure Score Index (LSI). The LSI score served as the measure of physical activity for each time point.
Health Status and Treatment Intensity
YASCC and comparison group participants completed the self-report version of The Health Knowledge Inventory [HKI; 31], which lists 35 different health problems. Health problems may be either organic/major (I.e., related to an organ or a significant late effect) or constitutional/other (i.e., unspecific symptoms, such as fatigue/pain, or less severe problems). The total number of health problems endorsed is summed. Cancer survivorship providers also completed the provider-version of the HKI for YASCC participants.
The Intensity of Treatment Rating Scale 2.0 [ITR-2; 32] was used to classify YASCCs’ cancer treatment intensity on a four-point scale based on data abstracted from the medical record related to diagnosis, stage, and treatment modality. Inter-rater reliability for the entire YASCC sample is rs=0.96 [24]. Due to the limited number of survivors whose treatments were classified into the lowest level, the four levels of treatment intensity were collapsed into two levels (least/moderate intensity and very/most intensity).
Psychological Distress
The Brief Symptom Inventory 18 [BSI-18; 33] is an 18-item self-report measure of psychological distress. The Global Severity Index (GSI) score, which has demonstrated reliability and validity, was used as a global measure of psychological distress.
The Posttraumatic Stress Checklist – Civilian Version [PCL-C; 34] is a valid 17-item self-report questionnaire that assesses DSM-IV symptoms of posttraumatic stress disorder (PTSD) [35]. The total scale of the PCL-C was used for the current study.
The Brief Mood Rating Scale [BMRS; 36] lists adjectives describing positive (happy, joyful, enjoyment/fun, pleased) and negative affect (depressed/blue, unhappy, angry/hostile, frustrated, worried/anxious). Participants rated how well the adjectives described them during the previous week. Only the negative affect subscale was used in analyses.
Belief Measures
The selected measures of beliefs assess relevant aspects of the health belief model [22], including perceived health vulnerability, locus of control and self-efficacy. The Health Competence Beliefs Inventory [HCBI; 37] is a 21-item measure of beliefs about health and well-being. The HCBI has four factors: 1) Health Perceptions measures beliefs about health vulnerabilities (e.g., I have a reason to worry about my health); 2) Satisfaction with Healthcare assesses beliefs about satisfaction with and confidence in healthcare providers (e.g., My doctor understands my concerns); 3) Cognitive Competence addresses beliefs about one’s ability to concentrate, remember and learn information (e.g., I learn new things as easily as other people); and 4) Autonomy examines beliefs related to independence in one’s healthcare and in general (e.g., I feel comfortable going to the doctor by myself). HCBI factors are associated with relevant psychological outcomes including posttraumatic stress symptoms and quality of life [37].
The Perceived Health Competence Scale [PHCS; 38] is an 8-item instrument assessing perceived ability to carry out health behaviors and influence personal health outcomes. The PHCS has strong psychometric properties and validity [38].
Statistical Analyses
Measures of central tendency, variability and association were computed for all variables, with an emphasis on physical activity and psychological variables. Comparisons were conducted on relevant demographic and psychological distress variables at Time 1 using two-sample t-tests and chi-square analyses, to estimate the equivalence of the groups. Survivor HCBI Cognitive Competence was compared between survivors with and without a history of cranial radiation using two-sample t-tests due to its potential impact on neurocognitive functioning [39]. Due to the non-normality of Time 2 physical activity scores, a new Time 2 physical activity variable was derived using a Box-Cox transformation exponent of 0.60, for which normality was achieved.
The first hypothesis, that YASCC would have lower levels of physical activity than the comparison group, was examined using a regression-based analysis of covariance model after adjusting for physical activity at Time 1, family income and race based on group differences obtained during baseline comparisons. The second hypothesis, that medical factors would have negative associations with YASCC Time 2 physical activity levels, was assessed two ways. Physical activity levels were compared within survivors based on whether they received more or less intense cancer treatment regimens using a regression-based analysis of variance model. Second, the association between survivor-reported health problems and Time 2 physical activity levels and between provider-reported health problems and Time 2 physical activity levels, individually, were specified and tested using two separate simple linear regression models.
The third hypothesis, that Time 1 distress and belief variables would significantly predict Time 2 YASCC physical activity after adjusting for baseline physical activity, demographic and medical variables, was tested using a multiple linear regression model. To identify the most influential covariates to include in the model, a series of nine adjusted single covariate regression models were specified and tested. Covariates with p values < 0.15 were entered into the multiple regression model. Due to the strong intercorrelations among the psychological distress variables, only global distress, as measured by the BSI GSI, was included in the subsequent model. Models were tested for survivors only due to this paper’s focus on survivors. Effect sizes were calculated using Cohen’s f2. Model assumptions were tested prior to analysis with no substantive violations identified. The criterion for statistical significance was set at α = 0.05 for all analyses. All data were analyzed using SPSS version 19 (IBM Inc., Armonk, NY).
Results
Descriptive Analyses
Demographic characteristics are presented in Table I. The two groups did not differ in age, gender or education level at Time 1. However, the comparison group had more African-American participants and more participants from the lowest income group. Therefore, comparisons of physical activity between YASCC and comparison group participants controlled for race and family income. YASCC and comparison group participants did not differ in global psychological distress, symptoms of posttraumatic stress or negative affect at Time 1. Associations between levels of physical activity and the psychological variables are presented in Table II for YASCC participants. Although not hypothesized, increased levels of psychological distress were significantly related to lower rates of physical activity in YASCC participants but not for the comparison group. There were no differences on HCBI Cognitive Competence between survivors with and without a history of cranial radiation, t(136) = −0.06, p = 0.95.
TABLE I.
Demographic Characteristics by Group
| Survivors (n = 117) | Compariso (n = 148) | pa | |||
|---|---|---|---|---|---|
|
| |||||
| M | SD | M | SD | ||
| Age (years) | 21.62 | 2.57 | 22.13 | 2.93 | 0.14 |
| Frequency | % | Frequency | % | ||
| Female Gender | 64 | 55 | 77 | 52 | 0.66 |
| Ethnicity/Race | 0.03 | ||||
| African-American | 5 | 4 | 16 | 11 | |
| Asian | 1 | 1 | 5 | 3 | |
| Caucasian | 106 | 90 | 119 | 80 | |
| Hispanic | 3 | 3 | 5 | 3 | |
| More than one race | 2 | 2 | 3 | 2 | |
| Education | 0.06 | ||||
| < HS graduate | 3 | 3 | 4 | 3 | |
| HS graduate | 23 | 20 | 37 | 25 | |
| Some college | 57 | 48 | 47 | 32 | |
| Graduated college | 34 | 29 | 58 | 39 | |
| Annual Family Income ($) | 0.04 | ||||
| <35,000 | 17 | 15 | 39 | 26 | |
| 35,000–74,999 | 40 | 34 | 44 | 30 | |
| 75,000–124,999 | 38 | 32 | 30 | 20 | |
| >125,000 | 15 | 13 | 22 | 15 | |
Based on chi-squares for all variables except age, which was tested using a t-test.
TABLE II.
Correlations between Primary Variables for Young Adult Survivors of Childhood Cancer
| Variables | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. T1 LSI | 0.57** | −0.20* | −0.30** | 0.17 | 0.00 | 0.26** | 0.15 | 0.19* | −0.36** | −0.22* | −0.23* |
| 2. T2 LSI | - | −0.10 | −0.25* | 0.10 | 0.18 | 0.36** | 0.07 | 0.13 | −0.32** | −0.19 | −0.24* |
| 3. Age | - | - | 0.04 | −0.21* | 0.00 | 0.02 | 0.11 | −0.03 | 0.02 | 0.14 | 0.05 |
| 4. Patient HKI | - | - | - | −0.45** | 0.13 | −0.40** | −0.12 | −0.34** | 0.44** | 0.43** | 0.29** |
| 5. T1 HCBI HP | - | - | - | - | 0.16 | 0.34** | 0.21* | 0.48** | −0.44** | −0.42** | −0.25** |
| 6. T1 HCBI Sat | - | - | - | - | - | 0.19* | 0.11 | 0.41** | −0.12 | −0.22* | −0.04 |
| 7. T1 HCBI Cog | - | - | - | - | - | - | 0.19* | 0.35** | −0.54** | −0.49** | −0.49** |
| 8. T1 HCBI Aut | - | - | - | - | - | - | - | 0.21* | −0.10 | −0.11 | −0.01 |
| 9. T1 PHCS | - | - | - | - | - | - | - | - | −0.47** | −0.46** | −0.28** |
| 10. T1 BSI GSI | - | - | - | - | - | - | - | - | - | 0.72** | 0.71** |
| 11. T1 PCL-C total | - | - | - | - | - | - | - | - | - | - | 0.51** |
| 12. T1 Negative affect | - | - | - | - | - | - | - | - | - | - | - |
BSI GSI, Brief Symptom Inventory Global Severity Index; HCBI, Health Competence Beliefs Inventory; HKI, Health Knowledge Inventory; LSI, Leisure Score Index; PCL-C, Posttraumatic Stress Checklist – Civilian; PHCS, Perceived Health Competence Scale;
p < .05;
p < .01.
Comparing Physical Activity by Group (Hypothesis 1)
As predicted, YASCC participants reported significantly lower levels of physical activity than comparison group participants at Time 2 when controlling for Time 1 physical activity, race and family income F(5, 185) = 5.46, p = 0.02. YASCC participants reported engaging in moderate-to-vigorous physical activity 2.1 fewer times per week than comparison group participants at Time 2 (Table III).
Table III.
Frequencya of Physical Activity by Group (n=265)
| Time 1 Survivors (n = 117) | Time 1 Comparison (n = 148) | Time 2 Survivors (n = 95) | Time 2 Comparison (n = 102) | |||||
|---|---|---|---|---|---|---|---|---|
| M | (SD) | M | (SD) | M | (SD) | M | (SD) | |
| Strenuous exercise frequency | 1.81 | (1.85) | 2.30 | (2.18) | 1.56 | (1.72) | 2.69 | (2.20) |
| Moderate exercise frequency | 2.98 | (2.36) | 3.78 | (3.09) | 3.07 | (2.29) | 3.99 | (3.48) |
| Mild exercise frequency | 4.13 | (3.45) | 4.02 | (3.11) | 4.44 | (3.25) | 5.40 | (4.81) |
| Summary exercise scoreb | 43.57 | (27.34) | 51.68 | (31.42) | 42.73 | (28.32) | 60.16 | (41.81) |
Frequency refers to the number of times during a typical 7-day period in which the participant endorsed engaging in each particular type of exercise for more than 15 minutes;
Summary exercise score = (9 x Strenuous) + (5 x Moderate) + (3 x Mild).
Examining group differences by sex reveals similar results. Male YASCC participants had significantly lower rates of physical activity than comparison group participants at Time 2 when controlling for Time 1 physical activity F(3, 81) = 4.23, p < 0.05. For female participants, there were not any significant differences between groups at Time 2 when controlling for Time 1 physical activity levels.
Medical Factors Associated with YASCC Physical Activity (Hypothesis 2)
There were no differences in physical activity based on treatment intensity F(1, 90) = 1.72, p = 0.19. More survivor-reported F(1, 91) = 5.73, p = 0.02, but not provider-reported F(1, 85) = 1.17, p = 0.28, health problems were significantly associated with less physical activity.
Psychological Factors Associated with YASCC Physical Activity (Hypothesis 3)
Within the single covariate regressions adjusting for Time 1 YASCC physical activity (Table IV), more positive HCBI Cognitive Competence (p < 0.01) was associated with higher levels of Time 2 exercise. Based on their p values in the adjusted single covariate models, the following predictors were included in the multiple regression model examining YASCC Time 2 physical activity: Time 1 physical activity, family income, overall survivor distress and HCBI Cognitive Competence. This model accounted for a significant amount of variance in Time 2 YASCC physical activity [F(4, 81) = 12.00, p < 0.001, Cohen’s f2 = 0.59]. In addition to higher levels of Time 1 physical activity (t[84] = 4.73, p < 0.001), more positive beliefs about one’s cognitive abilities at Time 1 was significantly related to higher Time 2 exercise levels while accounting for the other variables (t[84] = 2.46, p = 0.02).
Table IV.
Linear Regression Models Predicting Survivor Time 2 Physical Activity
| Covariates | Adjusted Single-covariate models
|
Multiple-covariate modela
|
||||
|---|---|---|---|---|---|---|
| β | SE | P-value | β | SE | P-value | |
| T1 Physical Activity | 0.08 | 0.01 | <0.01 | 0.07 | 0.01 | <0.01 |
| Family Incomeb | 0.75 | 0.40 | 0.06 | 0.63 | 0.41 | 0.12 |
| Survivor Ageb | −0.05 | 0.14 | 0.73 | |||
| Survivor Genderb | −0.38 | 0.72 | 0.60 | |||
| Survivor-Reported HKIb | −0.11 | 0.09 | 0.24 | |||
| Cancer Treatment Intensityb | −0.23 | 0.36 | 0.52 | |||
| T1 BSI GSIb | −0.07 | −0.05 | 0.14 | 0.02 | 0.06 | 0.72 |
| T1 PHCSb | 0.02 | 0.06 | 0.73 | |||
| T1 HCBI Health Perceptionsb | 0.03 | 0.09 | 0.74 | |||
| T1 HCBI Cognitive Competenceb | 0.33 | 0.11 | <0.01 | 0.36 | 0.15 | 0.02 |
| Constant | −2.19 | 4.99 | ||||
BSI GSI, Brief Symptom Inventory Global Severity Index; HCBI, Health Competence Beliefs Inventory; HKI, Health Knowledge Inventory; PHCS, Perceived Health Competence Scale;
Overall multiple covariate model is statistically significant [F(4, 81) = 12.00, p < 0.001, R2 = 0.37 Cohen’s f2 = 0.59];
Single-covariate model adjusted for influence of T1 Physical Activity.
Discussion
Despite the potential benefits of physical activity to offset medical late effects in cancer survivors (e.g., cardiovascular disease, obesity), YASCCs had lower levels of physical activity than comparison group participants. Differences in cancer treatment intensity did not distinguish YASCCs in terms of their exercise rates and provider ratings of health problems were not associated with physical activity. Higher levels of self-reported health problems were associated with lower rates of survivor physical activity. Survivors’ beliefs about their cognitive capabilities significantly predicted survivor physical activity two months later when accounting for other pertinent variables.
Consistent with previous studies of YASCC physical activity [9,11,12], young adult survivors in this study were less physically active than controls. These results underscore the increased risk for chronic health problems in childhood cancer survivors. While the CCSS found higher levels of survivor inactivity compared to sibling controls [9], the participants were older than the present sample, with many patients and siblings, respectively, over age 30 (59%, 65%) or age 40 (17%, 27%). The current study contributes to the literature by focusing on young adults and documenting their lower activity levels in comparison to healthy controls and provides further evidence for their developmental vulnerabilities as they assume greater responsibility for their health.
The findings regarding the medical variables provide insight into areas for assessment to identify survivors at greater risk for physical inactivity. In contrast to some studies showing associations between physical activity and treatment-related factors [9], provider ratings of the intensity of cancer treatment were not related to survivor physical activity, possibly due to the restricted range of treatment intensities in the current sample (e.g., few treatments were classified as least intense). Increased levels of survivor-reported health problems were significantly associated with lower levels of exercise while provider ratings of survivor health problems were not associated with YASCC physical activity. These discrepant findings may be due to differences in awareness regarding constitutional symptoms on the HKI, such as fatigue or pain, and suggests the importance of assessing YASCCs’ perceptions of their health problems.
More positive YASCC beliefs about cognitive abilities (e.g., perceptions of one’s concentration, memory, learning) as measured by the HCBI at baseline emerged as the only significant predictor of higher levels of physical activity at follow-up when adjusting for baseline levels of exercise and demographic and psychological variables. This study did not address whether HCBI Cognitive Competence reflects actual neurocognitive functioning. Since these beliefs did not differ based on cranial radiation history, they are likely distinct from actual function. Such beliefs may be the result of an interaction between survivors’ actual cognitive abilities and their self-efficacy or confidence in their cognitive abilities. YASCCs who feel more positive about their cognitive abilities may more effectively incorporate regular physical activity into their routines. Surprisingly, survivor’s perceptions regarding their likelihood of future illness and health uncertainty (HCBI Health Perception) and their self-efficacy for engaging in health-promoting behaviors (PHCS) were not associated with physical activity. These factors are key components of the health belief model and have been related to physical activity in both adolescent and young adult survivors[15,40].
An interesting incidental finding was the differential nature of the associations between baseline levels of psychological distress and physical activity for the YASCC and comparison group participants. Consistent with previous studies [9,19], there were strong independent associations between higher survivor psychological distress and lower levels of physical activity. This association was not apparent for comparison group participants despite there being no differences between groups on these psychological variables. However, the influence of psychological distress on YASCC physical activity faded when also accounting for survivor beliefs. This may be because it is the negative effects of psychological distress on self-efficacy for cognitive functions that is responsible for the decreased levels of physical activity. Future research should more carefully explore the interactions between beliefs and psychological distress and this potential vulnerability of YASCCs to the detrimental effects of psychological distress on health behaviors.
This study is one of the first to compare YASCC physical activity with a non-sibling control group and to prospectively examine predictors of physical activity. The findings are concerning for YASCC long-term health and quality of life and indicate areas for clinical and research efforts. Results suggest that assessing beliefs may provide an innovative and critical avenue for understanding and promoting YASCC engagement in health behaviors. Health care providers should regularly assess survivors’ physical activity levels and screen for potential cognitive factors that may negatively influence engagement in exercise, such as survivor beliefs about cognitive competence and self-efficacy. Due to the modifiable nature of beliefs, interventions that seek to enhance survivor beliefs about cognitive abilities may benefit many facets of survivor functioning, including levels of physical activity. Such interventions also could address survivors’ potentially overly-negative perceptions about their health given the association between survivor-reported, but not provider-reported, health problems and exercise. Future research should examine whether positive intervention-related changes in survivor beliefs regarding cognitive competence or their health mediate increases in survivor physical activity.
Despite the strengths of this study, there are some limitations. First, although this study’s measure of physical activity is widely-used and validated, it relies on self-report and recall of participants’ rates of physical activity. Second, YASCC participants in this study were seeking survivorship care and may not be representative of the larger population of survivors.
Overall, this study contributes to the literature on YASCC physical activity by addressing two limitations of previous investigations. The findings underscore the need for increased YASCC engagement in physical activity and highlight the importance of beliefs and perceptions in health-promoting behaviors of young adult survivors.
Acknowledgments
This research was supported by the National Cancer Institute (CA 106928, CA128805). The authors thank all those who participated in this study.
Footnotes
There are no conflicts of interest to disclose.
References
- 1.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2008. Bethesda, MD: National Cancer Institute; 2011. [Google Scholar]
- 2.Oeffinger KC, Mertens A, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. New England Journal of Medicine. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 3.Geenen MM, Bakker PJ, Kremer LC, et al. Increased prevalence of risk factors for cardiovascular disease in long-term survivors of acute lymphoblastic leukemia and Wilms tumor treated with radiotherapy. Pediatric Blood and Cancer. 2010;55:690–697. doi: 10.1002/pbc.22518. [DOI] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Buchanan GR, Eshelman DA, et al. Cardiovascular risk factors in young adult survivors of childhood acute lymphoblastic leukemia. Journal of Pediatric Hematology and Oncology. 2001;23:424–430. doi: 10.1097/00043426-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Mertens AC, Sklar CA, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: A report from the Childhod Cancer Survivor Study. Journal of Clinical Oncology. 2003;21:1359–1365. doi: 10.1200/JCO.2003.06.131. [DOI] [PubMed] [Google Scholar]
- 6.Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2001;13:3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 7.Paxton RJ, Jones LW, Rosoff PM, et al. Associations between leisure-time physical activity and health-related quality of life among adolescent and adult survivors of childhood cancers. Psycho-Oncology. 2010;19:997–1003. doi: 10.1002/pon.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sallis JF, Taylor WC, Dowda M, et al. Correlates of vigorous physical activity for children in grades 1 through 12. Comparing parent-reported and objectively measured physical activity. Pediatric Exercise Science. 2002;14:30–44. [Google Scholar]
- 9.Ness KK, Leisenring WM, Huang S, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer. A report from the Childhood Cancer Survivor Study. Cancer. 2009;115:1984–1994. doi: 10.1002/cncr.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyc VL, Hadley W, Crockett G. Prediction of health behaviors in pediatric cancer survivors. Medical and Pediatric Oncology. 2001;37:42–46. doi: 10.1002/mpo.1161. [DOI] [PubMed] [Google Scholar]
- 11.Stolley MR, Restrepo J, Sharp LK. Diet and physical activity in childhood cancer survivors: A review of the literature. Annals of Behavioral Medicine. 2010;39:232–249. doi: 10.1007/s12160-010-9192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winter C, Muller C, Hoffmann C, et al. Physical activity and childhood cancer. Pediatric Blood and Cancer. 2010;54:501–510. doi: 10.1002/pbc.22271. [DOI] [PubMed] [Google Scholar]
- 13.Reeves M, Eakin E, Lawler S, et al. Health behaviours in survivors of childhood cancer. Australian Family Physician. 2007;36:95–96. [PubMed] [Google Scholar]
- 14.Jarvela LS, Niinikoski H, Lahteenmaki PM, et al. Physical activity and fitness in adolescent and young adult long-term survivors of childhood acute lymphoblastic leukaemia. Journal of Cancer Survivorship. 2010;4:339–345. doi: 10.1007/s11764-010-0131-0. [DOI] [PubMed] [Google Scholar]
- 15.Finnegan L, Wilkie DJ, Wilbur J, et al. Correlates of physical activity in young adult survivors of childhood cancers. Oncology Nursing Forum. 2007;34:E60–E69. doi: 10.1188/07.ONF.E60-E69. [DOI] [PubMed] [Google Scholar]
- 16.Robien K, Ness KK, Klesges LM, et al. Poor adherence to dietary guidelines among adult survivors of childhood acute lymphoblastic leukemia. Journal of Pediatric Hematology and Oncology. 2008;30:815–822. doi: 10.1097/MPH.0b013e31817e4ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demark-Wahnefried W, Werner C, Clipp EC, et al. Survivors of childhood cancer and their guardians: Current health behaviors and receptivity to health promotion programs. Cancer. 2005;103:171–180. doi: 10.1002/cncr.21009. [DOI] [PubMed] [Google Scholar]
- 18.Pugliese J, Tinsley B. Parental socialization of child and adolescent physical activity: A meta-analysis. Journal of Family Psychology. 2007;21:331–343. doi: 10.1037/0893-3200.21.3.331. [DOI] [PubMed] [Google Scholar]
- 19.Cox CL, Montgomery M, Oeffinger KC, et al. Promoting physical activity in childhood cancer survivors. Results from the Childhood Cancer Survivor Study. Cancer. 2009;115:642–654. doi: 10.1002/cncr.24043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krull KR, Huang S, Gurney JG, et al. Adolescent behavior and adult health status in childhood cancer survivors. Journal of Cancer Survivorship. 2010 doi: 10.1007/s11764-010-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oeffinger KC. Longitudinal risk-based health care for adult survivors of childhood cancer. Current Problems in Cancer. 2003;27:143–167. doi: 10.1016/s0147-0272(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 22.Strecher VJ, Rosenstock IM. The health belief model. San Francisco: Jossey-Bass; 1997. [Google Scholar]
- 23.Lally P, Van Jaarsveld CHM, Potts HWW, et al. How are habits formed: Modelling habit formation in the real world. European Journal of Social Psychology. 2010;40:998–1009. [Google Scholar]
- 24.Kazak AE, DeRosa B, Schwartz L, et al. Psychological outcomes and health beliefs in adolescent and young adult (AYA) survivors of childhood cancer and controls. Journal of Clinical Oncology. 2010;28:2002–2007. doi: 10.1200/JCO.2009.25.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilliam MB, Madan-Swain A, Whelan K, et al. Social, demographic, and medical influences on physical activity in child and adolescent cancer survivors. Journal of Pediatric Psychology. 2011 doi: 10.1093/jpepsy/jsro85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian Journal of Applied Sports Science. 1985;10:141–146. [PubMed] [Google Scholar]
- 27.Godin G, Shephard RJ. Godin Leisure-Time Exercise Questionnaire. Medicine and Science in Sports and Exercise. 1997;29 (Supplement):S36–S38. [Google Scholar]
- 28.Valenti M, Porzio G, Aielli F, et al. Physical exercise and quality of life in breast cancer survivors. International Journal of Medical Sciences. 2008;5:24–28. doi: 10.7150/ijms.5.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevinson C, Faught W, Steed H, et al. Associations between physical activity and quality of life in ovarian cancer survivors. Gynecologic Oncology. 2007;106:244–250. doi: 10.1016/j.ygyno.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 30.Jones LW, Guill AB, Keir ST, et al. Using the theory of planned behavior to understand the determinants of exercise intention in patients diagnosed with primary brain cancer. Psycho-Oncology. 2007;16:232–240. doi: 10.1002/pon.1077. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz L, Mao J, Werba BE, et al. Self-reported health problems of young adults in clinical settings: Survivors of childhood cancer and healthy controls. Journal of the American Board of Family Medicine. 2010;23:306–314. doi: 10.3122/jabfm.2010.03.090215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werba BE, Hobbie WL, Kazak AE, et al. Classifying the intensity of pediatric cancer treatment protocols: The Intensity of Treatment Rating Scale 2.0 (ITR-2) Pediatric Blood and Cancer. 2007;48:673–677. doi: 10.1002/pbc.21184. [DOI] [PubMed] [Google Scholar]
- 33.Derogatis LR. Brief Symptom Inventory (BSI) Minneapolis, MN: NCS Pearson Inc; 2000. [Google Scholar]
- 34.Weathers FW, Ford J. Psychometric review of the PTSD Checklist. In: Stamm BH, editor. Measurement of Stress, Trauma, and Adaptation. Lutherville, MD: Sidran Press; 1996. pp. 250–251. [Google Scholar]
- 35.Ruggiero KJ, Ben KD, Scotti JR, et al. Psychometric properties of the PTSD Checklist -Civilian version. Journal of Traumatic Stress. 2003;16:495–502. doi: 10.1023/A:1025714729117. [DOI] [PubMed] [Google Scholar]
- 36.Diener E, Emmons RA. The independence of positive and negative affect. Journal of Personality and Social Psychology. 1985;47:1105–1117. doi: 10.1037//0022-3514.47.5.1105. [DOI] [PubMed] [Google Scholar]
- 37.DeRosa BW, Kazak AE, Doshi K, et al. Development and validation of the Health Competence Beliefs Inventory in young adults with and without a history of childhood cancer. Annals of Behavioral Medicine. 2011;41:48–58. doi: 10.1007/s12160-010-9228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith MS, Wallston KA, Smith CA. The development and validation of the Perceived Health Competence Scale. Health Education Research. 1995;10:51–64. doi: 10.1093/her/10.1.51. [DOI] [PubMed] [Google Scholar]
- 39.Moore BD., III Neurocognitive outcomes in survivors of childhood cancer. Journal of Pediatric Psychology. 2005;30:51–63. doi: 10.1093/jpepsy/jsi016. [DOI] [PubMed] [Google Scholar]
- 40.Keats MR, Culos-Reed SN, Courneya KS, et al. Understanding physical activity in adolescent cancer survivors: An application of the theory of planned behavior. Psycho-Oncology. 2007;16:448–457. doi: 10.1002/pon.1075. [DOI] [PubMed] [Google Scholar]


