Abstract
Background and Purpose
The blood supply to the medulla oblongata is distinct from that of other areas of the brainstem, and thus the mechanism underlying medullary infarctions may be distinct. However, few studies have investigated this.
Methods
Of 3833 stroke patients who were on the stroke registry between February 1999 and April 2008, those with medullary infarctions demonstrated on diffusion-weighted magnetic resonance imaging were enrolled. We analyzed the topography, the involved arterial territories, and the etiologic mechanisms of the lesions.
Results
In total, 142 patients were enrolled in the study. Bilateral medullary infarctions were rare (2.2%). Lesions involving the anteromedial or lateral territories were common in the upper medulla oblongata, whereas lateral territorial involvements were common in the middle and lower regions of the medulla oblongata. Significant stenosis (>50%) or occlusion of the vertebral artery was common (52.2%). Among stroke subtypes, large-artery atherosclerosis was most common (34.5%), while lacunae and cardioembolism were rare (3.5% and 4.2%, respectively). Vertebral artery dissection was frequent. The stroke mechanisms differed with the involved vascular territories. Large-artery atherosclerosis produced lesions in the lateral, anteromedial, and posterior territories. None of the cardioembolisms or other etiologies involved anteromedial or anterolateral territories, but all involved the lateral and/or posterior territories. Lacunar infarction was found only in the anteromedial and anterolateral territories.
Conclusions
The topography and mechanisms of infarctions involving the medulla oblongata are different with the involved arterial territories. These findings may be associated with the distinct pattern of arterial supply to the medulla oblongata.
Keywords: cerebral infarction, MRI, medulla oblongata
Introduction
Infarctions involving the medulla oblongata are rare. Previous studies have revealed characteristic clinicolesion correlations in patients with medullary infarctions, and particularly those between the medial and the lateral medulla.1-5 The brainstem is typically supplied by the circumferential arteries and the small direct perforating arteries from the basilar or vertebral arteries;6 however, the blood supply of the medulla oblongata is distinct from that of the other brainstem areas. Its unique arterial supply arises from the anterior and posterior spinal arteries (ASA and PSA, respectively), in addition to the perforating arteries and the long circumferential artery.7 Therefore, the mechanisms underlying medullary infarctions may be distinct from infarctions in other brainstem areas. However, few studies have investigated the mechanism underlying medullary infarctions.8
We investigated the mechanisms underlying medullary infarctions - in particular relative to the arterial territories involved - in patients whose lesions were demonstrated on diffusion-weighted magnetic resonance imaging (DWI). We also investigated the clinical features of medullary infarctions relative to the arterial territory involved.
Methods
Enrolled patients
The patient pool for this study comprised consecutive patients with an infarction involving the medulla oblongata who were registered in the Yonsei Stroke Registry (YSR) between February 1999 and April 2008. The YSR is a prospective hospital-based stroke registry for acute brain infarction or transient ischemic attack that occurs within 7 days after symptom onset.9,10 Patients were evaluated according to their medical history, neurologic examination, standard blood tests, brain imaging studies [computed tomography and/or magnetic resonance imaging], cerebral angiographic studies, and a 12-lead electrocardiogram. Magnetic resonance imaging was conducted with the aid of a 1.5-tesla system (Signa Horizon, GE Medical Systems, Milwaukee, WI, USA, or Intera or Achieva, Philips Medical Systems, Best, The Netherlands) or a 3.0-tesla system (Achieva, Philips Medical Systems). Transesophageal echocardiography was performed in all patients except those with poor cooperation due to decreased consciousness, impending brain herniation, poor systemic conditions, swallowing difficulty, lack of informed consent, or intubation.11
Of the 3833 patients with an acute stroke registered in the YSR, 182 (4.7%) were identified with infarctions involving the medulla oblongata during the study period. After excluding 32 patients who had not undergone DWI and 8 patients for whom a lesion was not found on DWI, 142 patients were enrolled in this study; angiography was performed in 136 (95.8%) of these patients. Digital subtraction angiography was performed in 54 patients (38.0%), while magnetic resonance angiography was conducted in 82 (58.8%). The degree of stenosis was measured based on the North American Symptomatic Carotid Endarterectomy Trial method,12 or the Warfarin vs. Aspirin for Symptomatic Intracranial Disease method.13 This study was approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System.
Topography of the lesions
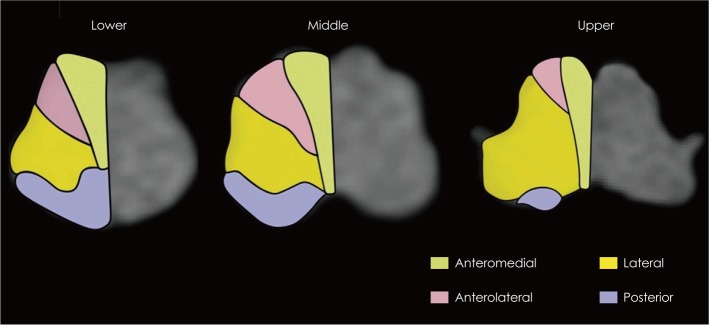
The infarctions were categorized into four groups (anteromedial, anterolateral, lateral, and posterior) based on previously published templates for arterial territories (Fig. 1).6,7 The lesions were further categorized into those involving the upper, middle, or lower medulla oblongata. The upper, middle, and lower medulla oblongata were defined as the areas with massive bulging of the dorsolateral area due to the restiform body, with bulging of the lateral surface due to the inferior olive, and with a relatively round shape without bulging of the lateral surface, respectively.14
Fig. 1.
Diagrams showing the arterial territory of the medulla oblongata.
Risk factors and etiologic classification of stroke
Hypertension was diagnosed when a patient had high blood pressure (systolic ≥140 mm Hg or diastolic ≥90 mm Hg) on repeated measurements during admission, or when the patient had been treated with any antihypertensive medication. Diabetes mellitus was diagnosed when a patient had a high fasting plasma glucose level (≥7 mmol/L) or had been treated with any oral hypoglycemic agents and/or insulin. Hypercholesterolemia was defined as a high lipid profile (fasting serum total cholesterol level ≥6.2 mmol/L or low-density lipoprotein cholesterol >4.1 mmol/L), or a history of treatment with lipid-lowering drugs after a diagnosis of hypercholesterolemia. Current smokers were those who had smoked within the 3 months prior to admission. Etiologic mechanisms of stroke were determined using the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification,15 in which vascular dissection (categorized into the subtype of stroke of other determined etiology) was determined only when typical imaging features such as 'double lumen', 'intimal flap', or 'pearl and string sign' were present.16,17
Statistical analysis
All statistical analyses were performed using the Windows SPSS package (version 18.0, SPSS, Chicago, IL, USA). Symptoms relative to the arterial territory were compared using the χ2 test. The generalized estimating equation was used to analyze the etiologic mechanism of stroke, the difference in rostrocaudal distribution, and the difference in clinical features, because variables such as the vascular territory and the rostrocaudal distribution were allowed multiple checks.18 The results of the generalized estimating equation are expressed as the estimated probability of an event (EPE), which represents the average probability of the observed responses that is expected to occur. A probability value of p<0.05 was considered to be indicative of statistical significance.
Results
Topography of the lesions
The infarction in the medulla oblongata was unilateral in 139 patients and bilateral in 3 patients (2 involved bilateral anteromedial territories, and 1 involved bilateral anteromedial and anterolateral territories). The infarctions were isolated to the medulla oblongata in 87 patients. Coexisting infarctions outside of the medulla oblongata were present in the remaining 55 patients. There were 43 patients with concomitant lesions in the cerebellum. Involvement of the cerebellar hemisphere was seen in 42 patients (97.7%) and a vermis infarction was seen in 29 patients (67.4%). Cerebellar lesions involved the posterior inferior cerebellar artery (PICA) territory in all 43 patients (100%). The anterior inferior cerebellar artery territory was also involved in three patients (7.0%), and the superior cerebellar artery was involved in another three (7.0%).
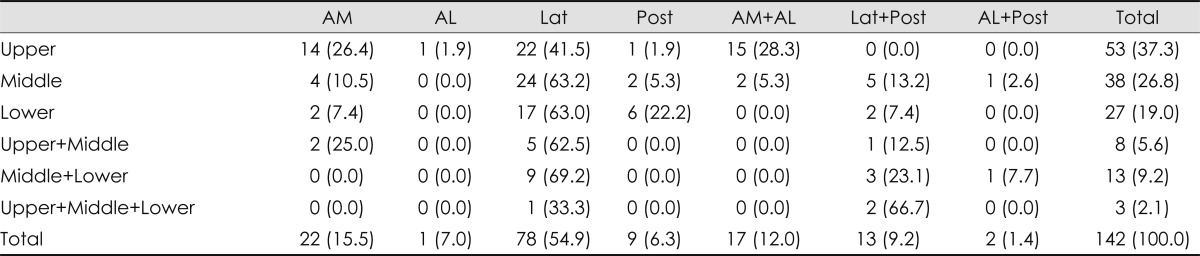
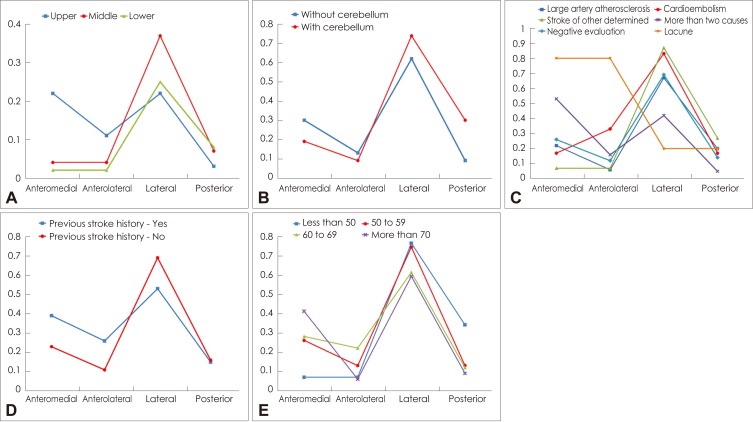
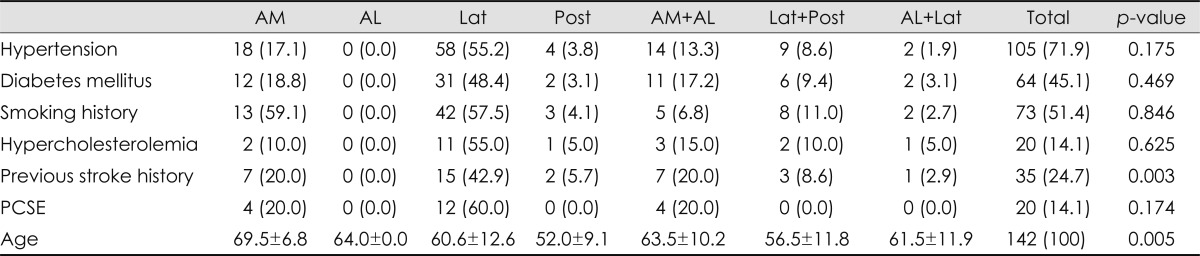
Lesions were most common in the lateral territory, followed by the anteromedial and the anteromedial plus anterolateral territories (Table 1). Involvement of the lateral territory (n=93) was most common, followed by the anteromedial (n=39) and posterior (n=22) territories. The involved vascular territories differed with the rostrocaudal topographical location of the infarction (Table 1; p<0.001). In the upper medulla, lesions involving the anteromedial or lateral territories were frequent (EPE=0.22 for each), while those of the posterior territory were rare (EPE=0.03). However, lesions involving the lateral territory were seen predominantly in the middle and lower medulla (EPE=0.37 and 0.25, respectively) (Fig. 2A). Those patients with coexisting cerebellar involvements exhibited different arterial territory involvements in the medulla oblongata. Posterior territory involvement was more common in patients with (EPE=0.30) than without (EPE=0.09) coexisting cerebellar lesions (Fig. 2B, Table 1; p=0.043). There were 13 patients with lesions in the posterior territory with concomitant cerebellar involvement; all of them had lesions in the cerebellum involving the PICA territory, nine had vertebral artery (VA) stenosis, and the remaining four had stenosis of the PICA.
Table 1.
Topographical differences among vascular territories involved
Numbers in parentheses are percentages. p-value was less than 0.001.
AL: anterolateral, AM: anteromedial, Lat: lateral, Post: posterior.
Fig. 2.
Comparison between the groups based on generalized estimating equations analysis. Each graph represents comparisons between the arterial territory and rostrocaudal topographical involvements (A), cerebellar involvement (B), stroke mechanism (C), previous stroke history (D), and age (E). The Y-axis is the estimated probability of an event, which is the average probability of the observed responses that is expected to occur. The levels of statistical significance were as follows: p<0.001 (A), p=0.043 (B), p<0.001 (C), p=0.003 (D), and p=0.005 (E).
Etiology of infarction
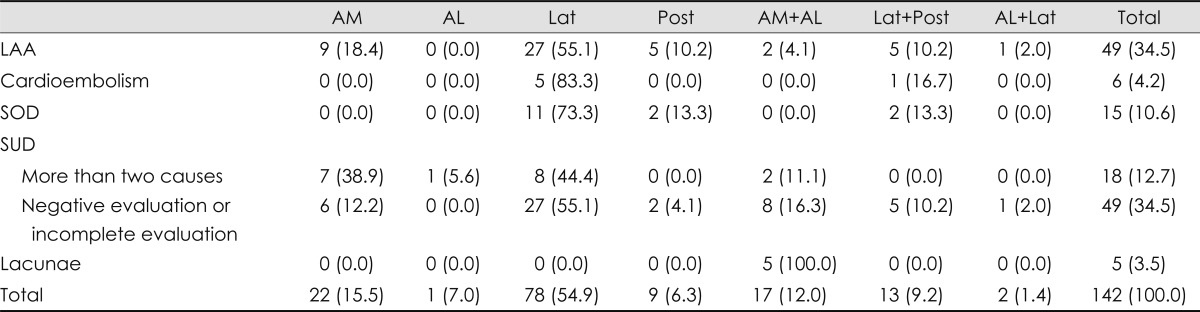
Large-artery atherosclerosis (LAA) was the most common stroke subtype (34.5%), whereas lacunae and cardioembolisms were rare or uncommon (3.5% and 4.2%, respectively). Stroke of other determined etiologies comprised 10.6% of medullary infarctions, comprising VA dissection in 13 patients (9.2%) and antiphospholipid antibody syndrome in 2 patients (1.4%). The mechanism of infarction differed significantly with the vascular territories (Table 2, p<0.001). LAA was common in the lateral, anteromedial, and posterior territories (EPE= 0.67, 0.22, and 0.2, respectively) (Fig. 2C). None of the cardioembolisms or other etiologies involved the anteromedial or anterolateral territories, but all involved the lateral and/or posterior territories. Lacunar infarction was only found in the anteromedial and anterolateral territories (Table 2). The mechanism differed neither in groups categorized according to the rostrocaudal topography of the lesions (p=0.499) nor between patients with and without coexisting cerebellar lesions (p=0.201). However, only one of the patients with VA dissection had a lesion in the upper medulla, while the other patients had lesions in the middle or lower medulla (upper : middle : lower=1 : 9 : 4, three of four patients with lesions in the lower medulla also had lesions in the middle medulla).
Table 2.
Etiologic mechanisms of medullary infarction
Numbers in parentheses are percentages. p-value was less than 0.001.
AL: anterolateral, AM: anteromedial, LAA: large artery atherosclerosis, Lat: lateral, Post: posterior, SOD: stroke of other determined etiology, SUD: stroke of undetermined etiology.
Arterial lesions
A lesion was found in the VA in 95 of all the patients with medullary infarction (73.6%). Occlusion of the VA was seen in 36 patients (25.4%), and significant stenosis (≥50%) was found in 38 patients (26.8%). Thirteen patients (10.1%) had lesions in the PICA. The frequency of vertebral arterial lesions did not differ with the vascular territories (p=0.547). Eight patients had lesions involving the anteromedial territory and they also had lesions involving the cerebellum. All of them demonstrated infarctions in the PICA territory of the cerebellum, and three of them showed additional lesions in the superior cerebellar artery territory. All but one patient (who did not undergo an angiographic study) exhibited atherosclerotic lesions in the VA, and the stenosis was significant in five of them, which suggests arterial embolism as the probable mechanism.
Demographic characteristics
Approximately three-quarters of the 142 patients (n=107, 75.4%) were men. The median age at symptom onset was 63 years (range 33-83 years). The most common risk factor was hypertension, followed by smoking and diabetes mellitus (Table 3). Among 35 patients with a previous ischemic stroke, 14 (46.7%) had strokes in the territory of the posterior circulation. Twenty patients (14.1%) had potential cardiac sources of embolism, which included atrial fibrillation (11 patients), atrial flutter (1 patient), valvular heart disease (1 patient), and a patent foramen ovale (7 patients). Comparison of demographic factors according to the vascular territories revealed that age and previous history of stroke differed significantly with the vascular territories (Table 3). A previous history of stroke was more frequent in patients with lesions in the anteromedial or anterolateral areas (Fig. 2D). Patients with a lesion in the lateral or posterior territory were younger (EPE=0.76 and 0.34, respectively) than those with a lesion in the anteromedial or the anterolateral territory (EPE=0.07 for each) (Fig. 2E).
Table 3.
Demographic characteristics according to arterial territories involved
Numbers in parentheses are percentages.
AL: anterolateral, AM: anteromedial, Lat: lateral, PCSE: Potential cardiac sources of embolism, Post: posterior.
Clinical features
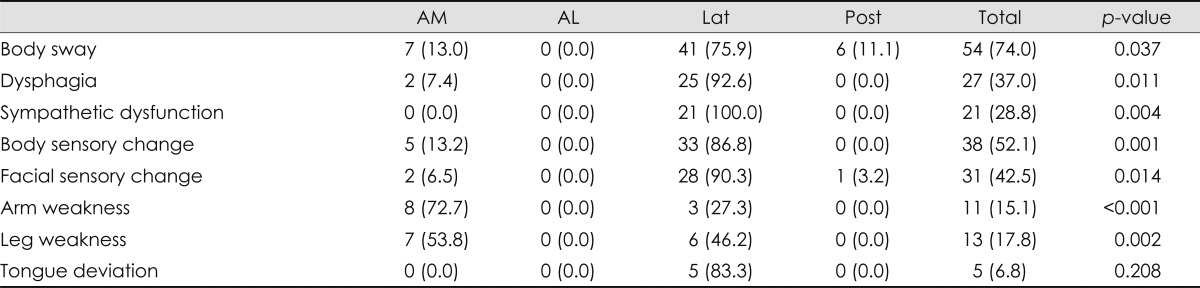
Of the 87 patients who had an infarction involving only the medulla oblongata, a single arterial territory was involved in 73 patients; none had an isolated infarction of the anterolateral territory. The clinical features in those 73 patients are listed in Table 4. Body sway, dysphagia, facial sensory change, and body sensory change were frequent among those with a lesion in the lateral territory, and all patients with sympathetic dysfunction had a lesion in the lateral territory. Limb weakness was frequent in the anteromedial territory. Body sway was a predominant symptom of a lesion involving the posterior territory. Tongue deviation was observed infrequently (5 patients, 6.8%: ipsilateral in 3 and contralateral in 2), and all patients had a lesion in the lateral territory. Two patients with contralateral tongue deviation had lesions in the upper medulla oblongata.
Table 4.
Symptoms and signs according to involved arterial territories in 73 patients with a lesion in the single arterial territory
Numbers in parentheses are percentages.
AL: anterolateral, AM: anteromedial, Lat: lateral, Post: posterior.
Discussion
Several topographical characteristics of medullary infarctions are described herein. Bilateral involvement of the medulla oblongata was rare, and none of the patients with infarctions in the lateral or posterior territory exhibited any bilateral lesions. Furthermore, concomitant involvement of the medial medulla (anteromedial and anterolateral territories) and the lateral or posterior medulla was seen in only two patients (anterolateral and lateral territories). These topographical characteristics may be explained by the arterial system supplying the medulla oblongata. Most parts of the medulla oblongata are supplied by paired arteries (VA, PICA, ASA, and PSA), which may prevent bilateral infarctions. The arteries supplying the medial medulla (usually the ASA) originate distant from those supplying the lateral medulla (usually the PICA).
The patterns of involvement also differed among the upper, middle, and lower regions of the medulla oblongata. Involvement of the anteromedial territory was seen predominantly in the upper medulla, whereas that of the posterior territory was more frequent in the middle or lower medulla. These differences may also be ascribed to the different arteries supplying the upper and middle-lower medulla. The paired ASAs, which originate from the VA, supply the upper medulla and then merge into a single artery, which supplies the middle-lower medulla.6 Therefore, occlusion of the ASA upstream of the mergence might not produce infarction in the middle and lower medulla. The higher frequency of posterior territory involvement in the middle and lower medulla may be associated with the area of the posterior territory being larger in the middle and lower medulla than in the upper medulla.
LAA was the most common etiology of medullary infarctions in our study. Cardioembolism and stroke of other determined etiology developed infarctions only in the lateral and posterior territories. The lateral territory is supplied by the PICA or the VA. The posterior territory of the medulla oblongata is usually supplied by the PSA, which usually originates from the VA. The PSA originates from the PICA in cases where the PICA originates extradurally from the VA.19 Most of the patients with stroke of other determined etiology had VA dissection. Concomitant cerebellar involvement was more frequent in patients with a lesion in the posterior territory, and most of them had an atherosclerotic lesion in the proximal relevant artery. These findings suggest that emboli from the heart or the proximal arterial lesion produce infarctions more commonly in the territories supplied by the PSA and long circumferential arteries. Lacunar infarction was an infrequent mechanism in patients with medullary infarctions, and all patients with lacunar infarctions involved the anteromedial and anterolateral territories in our study. The most common mechanism of medial medullary infarction in a recent study was small-vessel occlusion,20 which is consistent with our findings. Lacune typically account for 20-25% of stroke patients.9,21-23 Infrequent lacune in medullary infarctions may be attributable to the large areas of the medulla oblongata that are supplied by the PICA, ASA, and PSA rather than perforating arteries from the VA. Infrequent classic lacunar syndrome in lateral or posterior territory involvement, which is a prerequisite for the diagnosis of a subtype of lacune in the Trial of Org 10172 in Acute Stroke Treatment classification, might also contribute to the low frequency of lacune in medullary infarctions.
In this study we compared the clinical symptoms and signs relative to the four vascular territories involved, while most previous clinicotopographical correlation studies were based on a dichotomized category of medial and lateral medullary infarctions. Clinical findings based on the arterial territory involved were strongly correlated with the signs produced by dysfunction of anatomical structures in the corresponding arterial territory. Tongue deviation was rarely seen in our patients, as reported previously.8 All patients with contralateral tongue paralysis had lesions in the upper medulla oblongata, which suggests involvement of the corticobulbar tract before its decussation.24 However, all patients with tongue paralysis had lesions in the lateral territory. This finding was unexpected because tongue paralysis is known to be a symptom of medial medullary infarction.25,26 The hypoglossal nucleus is typically located in the anteromedial territory near the border between the anteromedial and lateral territories in the dorsal portion of the medulla oblongata.6 Infarctions involving the anteromedial territory are usually located in the ventral portion of the medulla oblongata and they do not extend to the dorsal medulla oblongata. Therefore, the hypoglossal nucleus is seldom affected by anteromedial territory infarctions. In addition, there are variations and overlaps between the arterial territories. These anatomical characteristics and infarction patterns in the medulla oblongata may be responsible for the infrequent occurrence of tongue paralysis in the lateral territory.
Our study was subject to several limitations. First, arterial lesions were determined based on magnetic resonance angiography, in which flow-related artifacts occur in about 60% of cases. Second, the presence of clinical features was based on a retrospective review of medical records. Therefore, their frequencies in each arterial territory may differ somewhat from those determined in a prospective design. Finally, although all patients with tongue paralysis in our study had a lesion in the lateral territory, few of our patients actually had tongue paralysis. Future investigations of this issue may be necessary to confirm our findings.
Acknowledgements
This work was supported by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, the Republic of Korea (A102065).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Sacco RL, Freddo L, Bello JA, Odel JG, Onesti ST, Mohr JP. Wallenberg's lateral medullary syndrome. Clinical-magnetic resonance imaging correlations. Arch Neurol. 1993;50:609–614. doi: 10.1001/archneur.1993.00540060049016. [DOI] [PubMed] [Google Scholar]
- 2.Kim JS, Lee JH, Choi CG. Patterns of lateral medullary infarction: vascular lesion-magnetic resonance imaging correlation of 34 cases. Stroke. 1998;29:645–652. doi: 10.1161/01.str.29.3.645. [DOI] [PubMed] [Google Scholar]
- 3.Kim JS, Choi-Kwon S. Sensory sequelae of medullary infarction: differences between lateral and medial medullary syndrome. Stroke. 1999;30:2697–2703. doi: 10.1161/01.str.30.12.2697. [DOI] [PubMed] [Google Scholar]
- 4.Kwon M, Lee JH, Kim JS. Dysphagia in unilateral medullary infarction: lateral vs medial lesions. Neurology. 2005;65:714–718. doi: 10.1212/01.wnl.0000174441.39903.d8. [DOI] [PubMed] [Google Scholar]
- 5.Kim JS. Pure lateral medullary infarction: clinical-radiological correlation of 130 acute, consecutive patients. Brain. 2003;126:1864–1872. doi: 10.1093/brain/awg169. [DOI] [PubMed] [Google Scholar]
- 6.Duvernoy HM. Human Brain Stem Vessels. 2nd ed. New York: Springer; 1999. [Google Scholar]
- 7.Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of human brain: brainstem and cerebellum. Neurology. 1996;47:1125–1135. doi: 10.1212/wnl.47.5.1125. [DOI] [PubMed] [Google Scholar]
- 8.Kim JS, Han YS. Medial medullary infarction: clinical, imaging, and outcome study in 86 consecutive patients. Stroke. 2009;40:3221–3225. doi: 10.1161/STROKEAHA.109.559864. [DOI] [PubMed] [Google Scholar]
- 9.Lee BI, Nam HS, Heo JH, Kim DI Yonsei Stroke Team. Analysis of 1,000 patients with acute cerebral infarctions. Cerebrovasc Dis. 2001;12:145–151. doi: 10.1159/000047697. [DOI] [PubMed] [Google Scholar]
- 10.Kim YD, Choi HY, Jung YH, Nam CM, Yang JH, Cho HJ, et al. Classic risk factors for atherosclerosis are not major determinants for location of extracranial or intracranial cerebral atherosclerosis. Neuroepidemiology. 2009;32:201–207. doi: 10.1159/000195690. [DOI] [PubMed] [Google Scholar]
- 11.Cho HJ, Choi HY, Kim YD, Nam HS, Han SW, Ha JW, et al. Transoesophageal echocardiography in patients with acute stroke with sinus rhythm and no cardiac disease history. J Neurol Neurosurg Psychiatry. 2010;81:412–415. doi: 10.1136/jnnp.2009.190322. [DOI] [PubMed] [Google Scholar]
- 12.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 13.Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Trial Investigators. Design, progress and challenges of a double-blind trial of warfarin versus aspirin for symptomatic intracranial arterial stenosis. Neuroepidemiology. 2003;22:106–117. doi: 10.1159/000068744. [DOI] [PubMed] [Google Scholar]
- 14.Kim JS, Lee JH, Suh DC, Lee MC. Spectrum of lateral medullary syndrome. Correlation between clinical findings and magnetic resonance imaging in 33 subjects. Stroke. 1994;25:1405–1410. doi: 10.1161/01.str.25.7.1405. [DOI] [PubMed] [Google Scholar]
- 15.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 16.Yonas H, Agamanolis D, Takaoka Y, White RJ. Dissecting intracranial aneurysms. Surg Neurol. 1977;8:407–415. [PubMed] [Google Scholar]
- 17.Yamaura A, Watanabe Y, Saeki N. Dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1990;72:183–188. doi: 10.3171/jns.1990.72.2.0183. [DOI] [PubMed] [Google Scholar]
- 18.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157:364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 19.Seçkin H, Ateş O, Bauer AM, Başkaya MK. Microsurgical anatomy of the posterior spinal artery via a far-lateral transcondylar approach. J Neurosurg Spine. 2009;10:228–233. doi: 10.3171/2008.12.SPINE08289. [DOI] [PubMed] [Google Scholar]
- 20.Shono Y, Koga M, Toyoda K, Matsuoka H, Yokota C, Uehara T, et al. Medial medullary infarction identified by diffusion-weighted magnetic resonance imaging. Cerebrovasc Dis. 2010;30:519–524. doi: 10.1159/000319887. [DOI] [PubMed] [Google Scholar]
- 21.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32:2735–2740. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 22.Han SW, Kim SH, Lee JY, Chu CK, Yang JH, Shin HY, et al. A new subtype classification of ischemic stroke based on treatment and etiologic mechanism. Eur Neurol. 2007;57:96–102. doi: 10.1159/000098059. [DOI] [PubMed] [Google Scholar]
- 23.Bejot Y, Caillier M, Ben Salem D, Couvreur G, Rouaud O, Osseby GV, et al. Ischaemic stroke subtypes and associated risk factors: a French population based study. J Neurol Neurosurg Psychiatry. 2008;79:1344–1348. doi: 10.1136/jnnp.2008.150318. [DOI] [PubMed] [Google Scholar]
- 24.Urban PP, Hopf HC, Connemann B, Hundemer HP, Koehler J. The course of cortico-hypoglossal projections in the human brainstem. Functional testing using transcranial magnetic stimulation. Brain. 1996;119:1031–1038. doi: 10.1093/brain/119.3.1031. [DOI] [PubMed] [Google Scholar]
- 25.Kameda W, Kawanami T, Kurita K, Daimon M, Kayama T, Hosoya T, et al. Lateral and medial medullary infarction: a comparative analysis of 214 patients. Stroke. 2004;35:694–699. doi: 10.1161/01.STR.0000117570.41153.35. [DOI] [PubMed] [Google Scholar]
- 26.Kumral E, Afsar N, Kirbas D, Balkir K, Ozdemirkiran T. Spectrum of medial medullary infarction: clinical and magnetic resonance imaging findings. J Neurol. 2002;249:85–93. [PubMed] [Google Scholar]