Abstract
Background and Purpose
Cardiovascular risk factors are considered to also be risk factors for dementia. Recent studies have shown that the prevalence of cognitive dysfunction is high in patients with cardiac diseases. However, few studies have investigated the influence of cardiac function on cognition and brain structural changes in dementia. The aims of this study were to determine the relationship between cardiac and cognitive function, and to characterize any structural changes in the brain that could be caused by cardiac function in patients with dementia.
Methods
Dementia patients (n=93) were recruited prospectively with checking for the presence of vascular risk factors such as hypertension. Cognitive function was measured by the Mini-Mental State Examination, modified Mini-Mental State test, and Korean version of the Dementia Rating Scale. Brain magnetic resonance imaging was conducted to evaluate the cerebral white-matter changes (WMC), ventricular dilation, and cortical and hippocampal atrophy. Cardiac function was evaluated using two-dimensional echocardiography. We divided the patients into two groups according to the presence (+) or absence (-) of WMC.
Results
In the entire cohort, the size of the left atrium (LA) was positively correlated with the degree of WMC, irrespective of age (p<0.05). The LA was larger in the WMC (+) group (n=42) than in the WMC (-) group. General cognitive function was significantly lower in the WMC (+) group than in the WMC (-) group. Subjects with an enlarged LA tended to exhibit lower cognitive function and more-severe cerebral WMC.
Conclusions
Cardiac dysfunction represented by LA enlargement could be related to cognitive decline and WMC of the brain resulting from impairment of the cerebral hemodynamic process in dementia.
Keywords: dementia, cardiac function, cognitive function, brain structural changes
Introduction
The South Korean population is becoming increasingly aged, with the proportion of those aged at least 65 years having doubled over the past two decades to now account for 11% of the total.1 Along with the growing number of elderly, there has been a concomitant increase in vascular risk factors such as hypertension, diabetes, and hyperlipidemia, which in turn have increased the incidences of cardiovascular and cerebrovascular diseases.2 According to recent studies, these vascular risk factors cause not only cerebrovascular diseases but also degenerative brain diseases such as Alzheimer's disease (AD).3
Several studies have shown that cardiac dysfunction is correlated with cognitive decline, and that serious cardiac dysfunction such congestive heart failure leads to severe cerebral white-matter changes (WMC) in patients with decreased cardiac output (CO).4,5 In particular, diastolic dysfunction of the left ventricle is correlated with lower cognitive function in patients with diverse heart diseases.6
Previous studies have evaluated the relationship between cardiac and cognitive functions only in patients with heart failure or end-stage cardiac diseases. However, the possibility of systemic debility or accompanying complications secondarily affecting cognitive function exists in patients with severe cardiac dysfunction. In addition, the relevance of cardiac function to cognitive function in dementia patients has yet to be evaluated.
This study investigated the relationship between cardiac and cognitive functions in dementia patients, as well as the relevance of cardiac function to cerebral structural changes shown on brain magnetic resonance imaging (MRI) such as WMC, ventricular enlargement (VE), medial temporal atrophy (MTA), and cortical atrophy (CA).
Subjects and Methods
Subjects
Ninety-three patients who underwent brain MRI and detailed cognitive and cardiac function evaluations at the memory clinic of Chungnam National University Hospital from June 2009 to June 2010 were enrolled prospectively. Fifty-four patients were diagnosed with AD using the diagnostic criteria of the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association.7
Sixteen patients were diagnosed with subcortical ischemic vascular dementia (SIVD) using the diagnostic and research criteria for subcortical vascular dementia in clinical trials.8 Twenty-three patients were diagnosed with Parkinson's disease with dementia (PDD) according to the diagnostic criteria of the Movement Disorder Society.9
Hypertension was defined in patients who were currently taking anti hypertensive agents or had a blood pressure (BP) of >140/90 mm Hg recorded on two or more occasions during hospitalization or at an out patient clinic. Diabetes was defined in patients currently taking hypoglycemic agents or conforming to the diabetes criteria for blood-sugar testing and glucose tolerance examination. Hyperlipidemia was defined in patients taking lipid-lowering agents or having a total cholesterol level of >240 mg/dL or a triglyceride level of >200 mg/dL.
Methods
Cognitive evaluation
All patients were evaluated using the modified Mini-Mental State (3MS) test10,11 and Mini-Mental State Examination (MMSE)12 to determine general cognitive performance. The Korean version of the Dementia Rating Scale (K-DRS) test13 was applied to patients who were 80 years of age or older (n=18) in order to compare the results with those of age- and education-matched controls. Any drugs that might influence cerebral function or cognition were not given until the cognitive evaluation was completed. A neuropsychologist who was not aware of patients' clinical states performed the cognitive function tests independently.
Evaluation of structural changes on brain MRI
Brain MRI was performed (1.5-T scanner, General Electric, Milwaukee, WI, USA; Achieva 3.0T X, Philips, Eindhoven, the Netherland) on each patient to determine the cerebral structural changes. The degree of cerebral WMC was evaluated using T2-weighted and fluid attenuated inversion recovery axial images. The degrees of CA and VE were assessed using T1-weighted axial and sagittal images. The severity of MTA was scored from T1-weighted coronal images. The structural changes on brain MRI were rated visually by two neurologists who did not know the clinical states of the patients. The interrater reliability was calculated, and one of the neurologists reevaluated the degree of brain structural changes 1 week after the initial assessment to confirm the intrarater reliability.
Degree of cerebral WMC
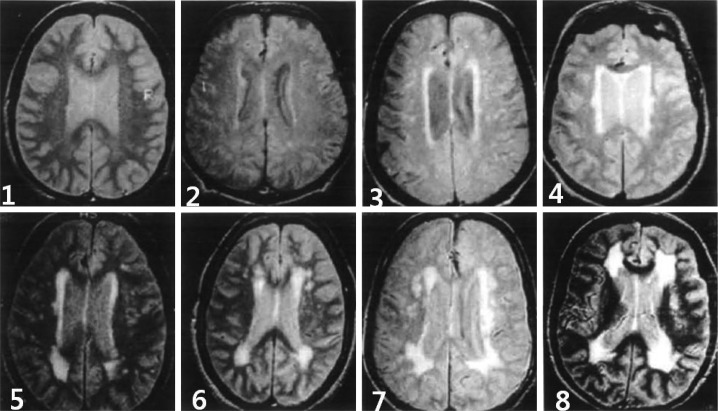
The degree of WMC was evaluated using the Cardiovascular Health Study (CHS) scale,14 and was scored on a scale of 1-8, regardless of whether the WMC were periventicular or deep (Fig. 1). The patients were then divided into two groups according to their CHS WMC scores: 1) the WMC-negative group [WMC (-)], comprising those with a score of 1 or 2; and 2) the WMC-positive group [WMC (+)], comprising those with a score of 3-8.
Fig. 1.
Severity of the cerebral white matter changes, as determined using the Cardiovascular Health Study Scale (score 1-8).
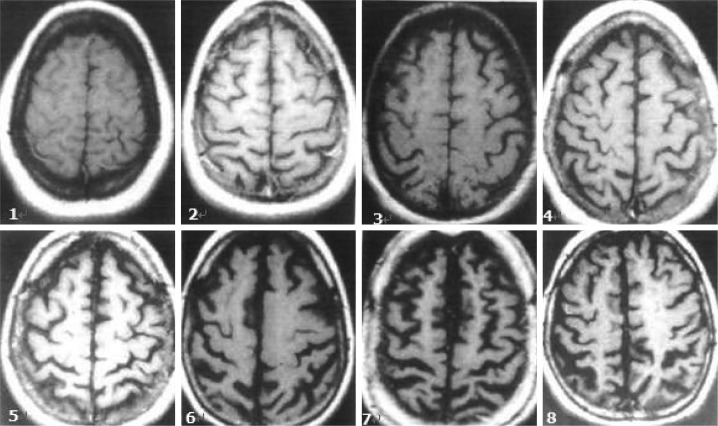
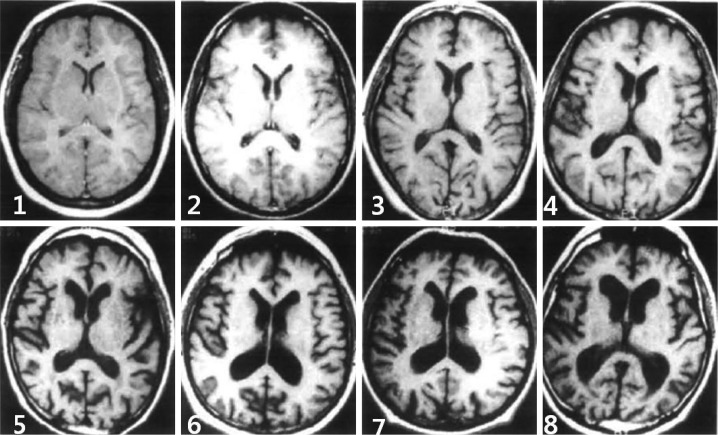
Degree of CA and VE
The degrees of CA and VE were also evaluated using the CHS14 scoring system, each on a scale from 1 to 8, using T1-weighted axial images (Figs. 2 and 3, respectively).
Fig. 2.
Degree of cortical atrophy, as determined using the Cardiovascular Health Study Scale (score 1-8).
Fig. 3.
Degree of ventricular enlargement, as determined using the Cardiovascular Health Study Scale (score 1-8).
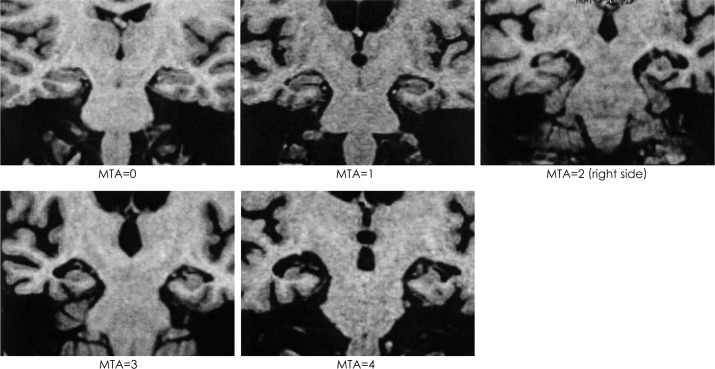
Degree of MTA
The degree of MTA was evaluated on a scale from 0 to 4 using Scheltens' scale (Fig. 4).15
Fig. 4.
Severity of medial temporal lobe atrophy, as determined using Scheltens' Scale (score 0-4).
Evaluation of cardiac function
Two-dimensional echocardiography was performed (Vivid 7, General Electric Vingmed Ultrasound AS, Horten, Norway; I33, Philips, Seattle, WA, USA) to evaluate the following parameters of cardiac function: left atrial diameter, left ventricle end-diastolic volume, end-systolic volume, and ejection fraction. The presence of left ventricular (LV) hypertrophy and valvular disease was also determined. Stroke volume (SV) was calculated as SV=(LV end-diastolic volume)-(LV end-systolic volume), and CO was calculated by multiplying SV by the heart rate (HR) as CO=SV×HR.16
Cardiac function was evaluated independently by a cardiologist who did not know the patients' clinical states. Left atrium (LA) enlargement was regarded as an LA enlargement of >40 mm for men and >38 mm for women.17
Statistical analysis
SPSS-PC software for Windows (version 16.0) was used for the statistical analysis. Analysis of variance and the χ2 test were conducted to compare demographics, cognitive status, cardiac function, and brain structural changes between the groups. The relationships between cardiac function, cognitive status, and brain structural changes were determined by correlation analysis using the Pearson coefficient and multiple regression analysis. The intrarater confidence and reliability of the visual scoring system were determined using Cohen's kappa coefficient. The level of statistical significance was set at p<0.05.
Results
General characteristics of the entire cohort
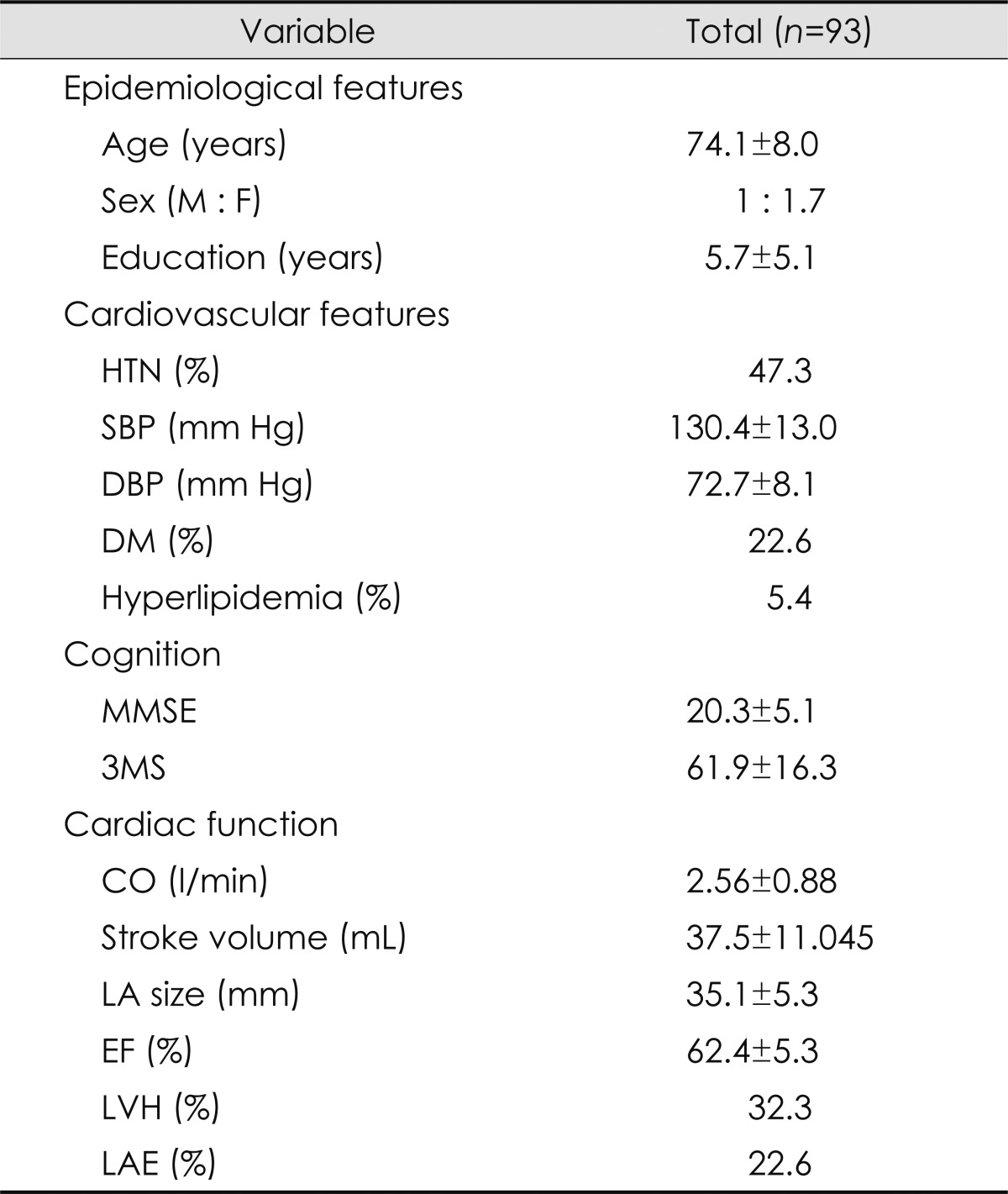
The mean age of the entire cohort was 74.1 years (range, 52-96 years), and the ratio of men to women was 34:59. The average duration of education was 5.7 years (range, 0-19 years). Forty-four patients were hypertensive (47.3%), 21 patients had diabetes mellitus (22.6%), and 5 (5.4%) had hyperlipidemia. The mean MMSE and 3MS scores were 20.3 and 61.9, respectively. None of the 93 patients was diagnosed with heart failure or valvular heart disease, although 3 had atrial fibrillation and 2 had angina pectoris (symptoms that often herald heart failure or heart disease). The mean ejection fraction was 62.4%, the mean size of the LA was 35.1 mm, and the CO was 2.56±0.88 l/min (mean±SD). LV hypertrophy and LA enlargement were found in 32.3% and 22.6% of the entire cohort, respectively (Table 1).
Table 1.
Baseline characteristics of the entire cohort (n=93)
Data are mean±SD values except where stated otherwise.
CO: cardiac output, DBP: diastolic blood pressure, DM: diabetes mellitus, EF: ejection fraction, F: female, HTN: hypertension, LA: left atrium, LAE: left atrial enlargement, LVH: left ventricular hypertrophy, M: male, MMSE: the Mini-Mental State Examination, SBP: systolic blood pressure, SD: standard deviation, 3MS: the modified Mini-Mental State test.
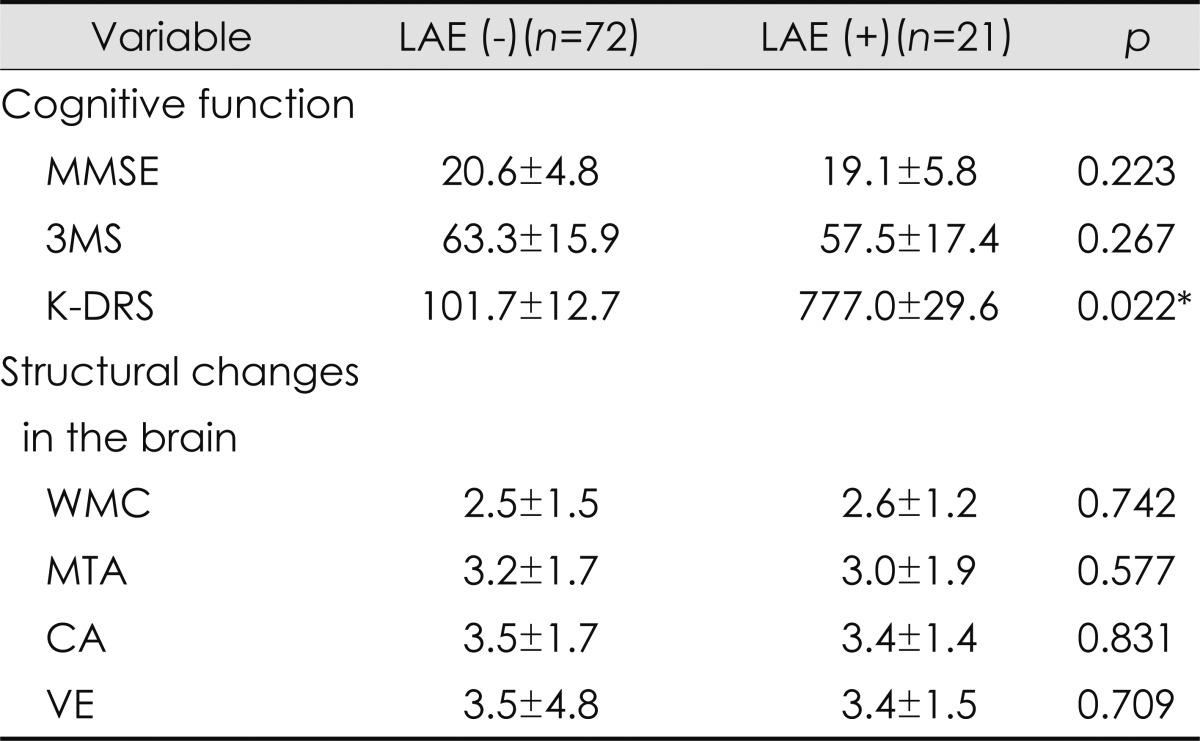
A greater SV was associated with a higher MMSE score, and a higher CO was correlated with higher MMSE, 3MS, and K-DRS scores. The size of the left ventricle was negatively correlated with the K-DRS score in patients who were 80 years of age or older, with age adjustment. The mean K-DRS score was significantly lower in patients with LA enlargement than in patients with a normally sized LA (Table 2).
Table 2.
Comparison of cognitive function and brain structural changes between subjects with LAE [LVE (-)] and without LAE [LAE (+)]
*Significantly different at p<0.05.
CA: cortical atrophy, K-DRS: Korean version of the Dementia Rating Scale, LAE: left atrial enlargement, MMSE: the Mini-Mental State Examination, MTA: medial temporal atrophy, VE: ventricular enlargement, WMC: white matter changes, 3MS: the modified Mini-Mental State test.
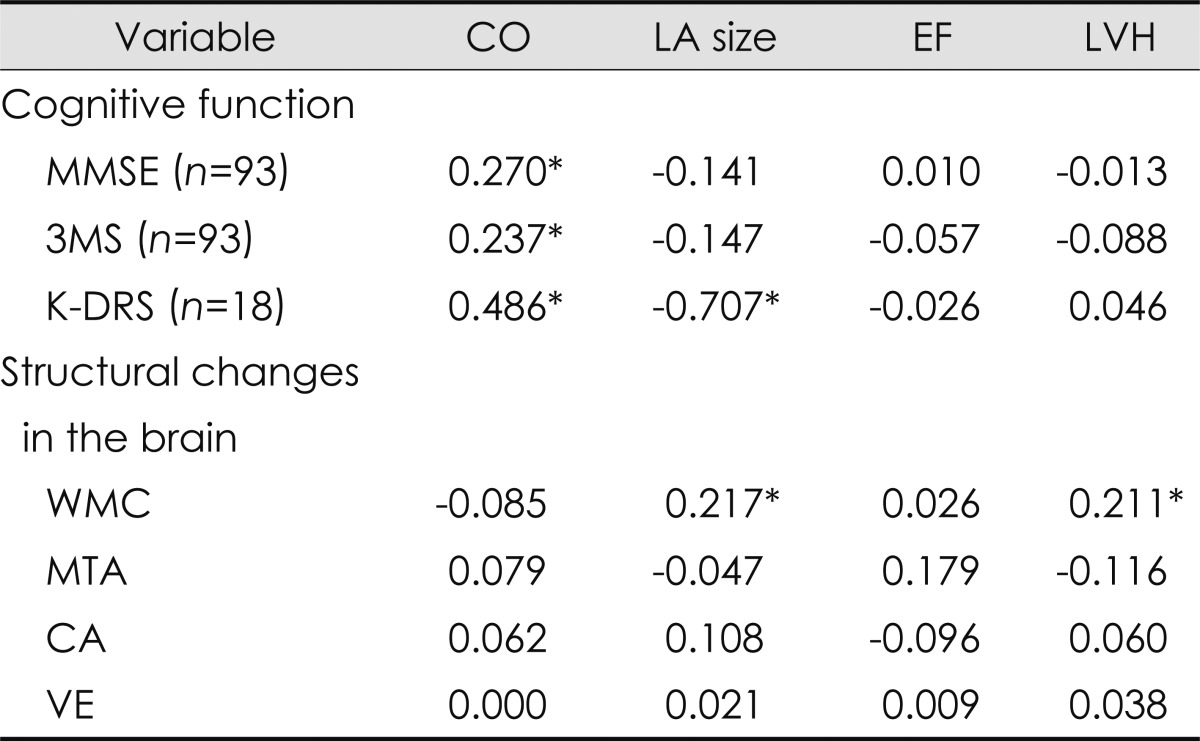
Neither systolic nor diastolic BP was associated with cognitive function or cerebral structural changes. The degree of cerebral WMC was correlated with LV hypertrophy and LA enlargement, but no relationship was found with CA, VE, or MTA (Table 3).
Table 3.
Correlations between cardiac function, cognition, and structural changes in the brain
*Significantly different at p<0.05.
CA: cortical atrophy, CO: cardiac output, EF: ejection fraction, K-DRS: Korean version of the Dementia Rating Scale, LA: left atrium, LVH: left ventricular hypertrophy, MMSE: the Mini-Mental State Examination, MTA: medial temporal atrophy, VE: ventricular enlargement, WMC: white matter changes, 3MS: the modified Mini-Mental State test.
Comparison of clinical characteristics between the WMC (+) and WMC (-) groups
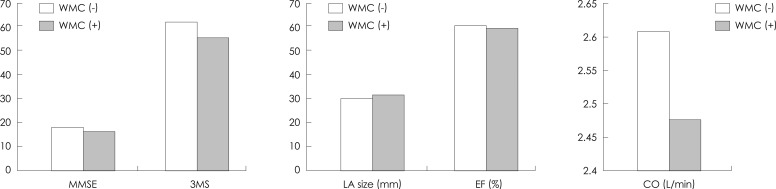
No significant differences were found between the WMC (+) group (n=42) and the WMC (-) group (n=51) with respect to age, gender, education level, diabetes, and hyperlipidemia. In contrast, the number of patients with hypertension was significantly greater in the WMC (+) group (61.9%) than in the WMC (-) group (35.3%; p=0.01). The general cognitive performance was significantly lower in the WMC (+) group than in the WMC (-) group (p=0.021). No differences were found between the two groups for ejection fraction, SV, CO, and LV hypertrophy, while the LA was larger in the WMC (+) group (p=0.014) (Fig. 5).
Fig. 5.
Comparison of cardiac and cognitive functions between WMC-positive (+) and WMC-negative (-) groups. CO: cardiac output, EF: ejection fraction, LA: left atrium, MMSE: the Mini-Mental State Examination, WMC: white matter changes, 3MS: the modified Mini-Mental State test.
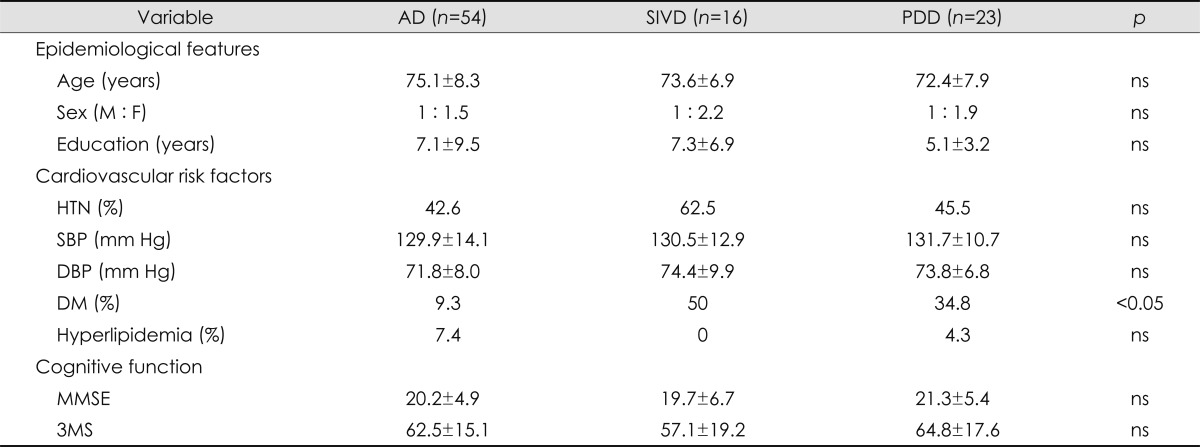
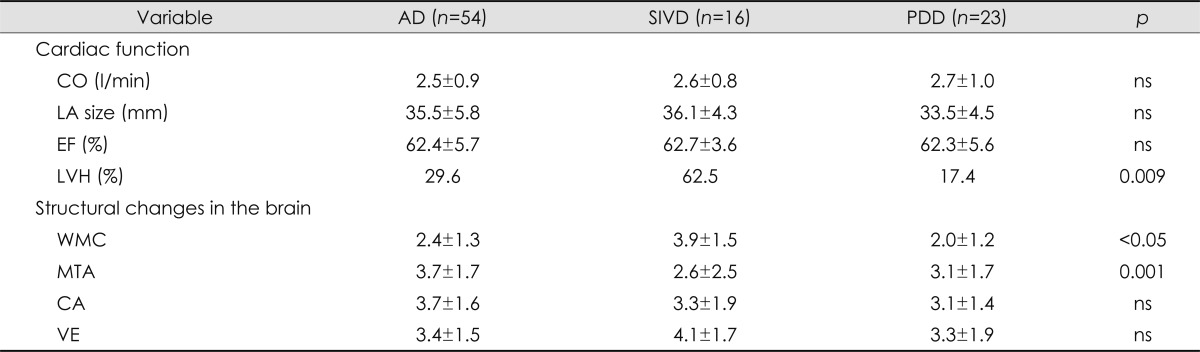
Comparison of clinical characteristics according to dementia subtype
Dementia was categorized into three subgroups: AD (n=54), SIVD (n=16), and PDD (n=23). Age, gender, education level, and MMSE and 3MS scores did not differ significantly among the three groups. Although there were significantly more patients with diabetes in the SIVD group (p=0.01) than in the other two groups, hypertension, systolic BP, diastolic BP, and hyperlipidemia did not differ with the dementia subtype (Table 4). The degree of cerebral WMC was more severe in the SIVD group than in the AD and PDD groups, and the severity of MTA was greatest in the AD group (Table 5).
Table 4.
Comparison of the baseline characteristics among the three dementia subtypes
AD: Alzheimer's disease, DBP: diastolic blood pressure, DM: diabetes mellitus, F: female, HTN: hypertension, M: male, MMSE: the Mini-Mental State Examination, ns: no significant difference, PDD: Parkinson's disease with dementia, SBP: systolic blood pressure, SIVD: subcortical ischemic vascular dementia, 3MS: the modified Mini-Mental State test.
Table 5.
Comparison of the cardiac and structural changes in the brain among the three dementia subtypes
AD: Alzheimer's disease, CA: cortical atrophy, CO: cardiac output, EF: ejection fraction, LA: left atrium, LVH: left ventricular hypertrophy, MTA: medial temporal atrophy, PDD: Parkinson's disease with dementia, SIVD: subcortical ischemic vascular dementia, VE: ventricular enlargement, WMC: white matter changes.
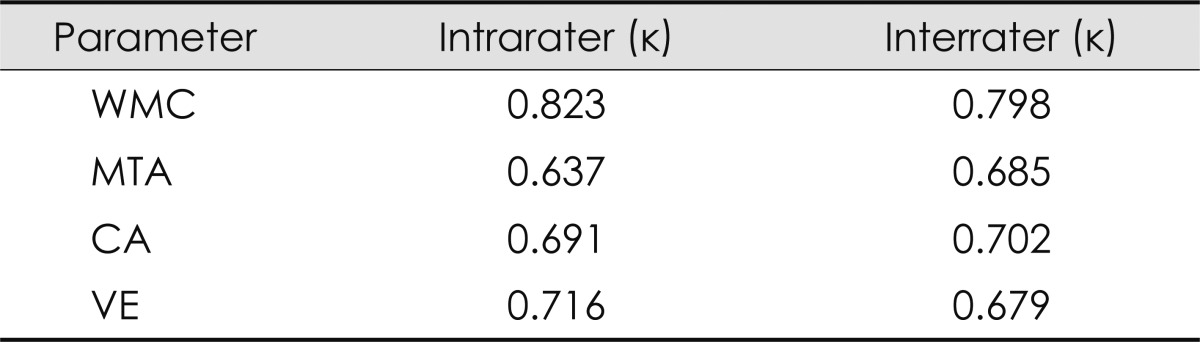
Reliability of visual rating of the structural changes on brain MRI
The reliability of visually rating the structural changes on brain MRI was examined by determining the intrarater and interrater variabilities. Cohen's kappa coefficients for interrater and intrarater reliabilities were 0.68-0.80 and 0.64-0.82, respectively, revealing are latively high consistency (Table 6).
Table 6.
Intrarater and interrater reliabilities for the visual rating parameters
CA: cortical atrophy, MTA: medial temporal atrophy, VE: ventricular enlargement, WMC: white matter changes.
Discussion
There is a growing interest in the relationship between cardiac and cognitive functions, both of which are often impaired in the elderly, who constitute an increasing proportion of the general population. Previous studies have found that LV dysfunction and atrial fibrillation exert negative influences on cognitive function via a reduction in cerebral blood flow.18,19
The LA acts as a contractile pump that delivers 15-30% of LV filling and augments LV stroke volume. Enlargement of the LA is affected by the increased wall tension that results from increased filling pressure. LA enlargement is a marker of both the severity and chronicity of diastolic dysfunction and the magnitude of LA pressure elevation.17
Two-dimensional echocardiography in the parasternal long axis of the LA enables the measurement of its anteroposterior linear dimensions; in this way, LA enlargement can be defined as >40 mm for men and >38 mm for women.17 LA enlargement refers to an increase in the LA size due to the increased pressure associated with mitral valve disease, or fibrosis or calcification of the LA, and it is known to be a predictive factor for cardiovascular diseases such as atrial fibrillation and diastolic cardiomyopathy. The possible mechanisms underlying cerebral dysfunction due to LA enlargement are 1) atrial fibrillation with increased frequency of asymptomatic cerebral infarction, and 2) lowering the CO since the LA serves as a LV reservoir during systole, so that LA enlargement may lead to reduce cerebral perfusion.20-22
In the present study, the ejection fraction and LV hypertrophy had no significant impact on the general cognitive function as measured by MMSE and 3MS scores. In contrast, the performance in all cognitive function tests was worse in patients who had a lower CO. These results support the finding that a lower CO leads to faster cerebral aging and a decline of cognition. Disturbance of cerebral homeostasis in the elderly, who have a lower CO, impedes cerebral perfusion, thereby accelerating the speed of cerebral aging and cognitive decline.23
In the present study, LA enlargement was significantly correlated with cerebral WMC, and cognitive function was worse in the WMC (+) group than in the WMC (-) group. This negative correlation between LA enlargement and cognition was a distinctive result, particularly so in those aged over 80 years, and is consistent with Ott et al.22,24 finding that LA size was related to poor cognitive function in a large-scale study targeting subjects aged at least 65 years.
It has been reported that smaller ejection fractions were associated with lower MMSE scores in patients with heart failure.4,25 In addition, a lower CO was found to be associated with cerebral WMC, and a positive correlation was found between the duration of heart failure and the severity of WMC and MTA.20 These results suggest that cardiac dysfunction, such as reduced CO, strongly influences cognitive function by causing structural changes in the brain.
The WMC were most severe in the SIVD group, which also contained the most cases of LV hypertrophy. LV hypertrophy and cerebral microvascular damage may give rise to disturbance of cerebral autoregulation, so that it causes cerebral WMC in SIVD.
In conclusion, LA enlargement might impact the development of structural changes in the brain, which may compromise cognitive function in the elderly. Therefore, the evaluation of cardiac function, including measurement of the LA size and LV hypertrophy, could play an important role in the prediction of structural changes in the brain and cognitive decline in dementia.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Statistics Korea. 2010. Available from: URL: http://www.kostat.go.kr.
- 2.McCullagh CD, Craig D, McIlroy SP, Passmore AP. Risk factors for dementia. Adv Psychiatr Treat. 2001;7:24–31. [Google Scholar]
- 3.Fillit H, Nash DT, Rundek T, Zuckerman A. Cardiovascular risk factors and dementia. Am J Geriatr Pharmacother. 2008;6:100–118. doi: 10.1016/j.amjopharm.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Zuccalà G, Cattel C, Manes-Gravina E, Di Niro MG, Cocchi A, Bernabei R. Left ventricular dysfunction: a clue to cognitive impairment in older patients with heart failure. J Neurol Neurosurg Psychiatry. 1997;63:509–512. doi: 10.1136/jnnp.63.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogels RL, van der Flier WM, van Harten B, Gouw AA, Scheltens P, Schroeder-Tanka JM, et al. Brain magnetic resonance imaging abnormalities in patients with heart failure. Eur J Heart Fail. 2007;9:1003–1009. doi: 10.1016/j.ejheart.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Suwa M, Ito T. Correlation between cognitive impairment and left ventricular diastolic dysfunction in patients with cardiovascular diseases. Int J Cardiol. 2009;136:351–354. doi: 10.1016/j.ijcard.2008.04.099. [DOI] [PubMed] [Google Scholar]
- 7.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 8.Erkinjuntti T, Inzitari D, Pantoni L, Wallin A, Scheltens P, Rockwood K, et al. Research criteria for subcortical vascular dementia in clinical trials. J Neural Transm Suppl. 2000;59:23–30. doi: 10.1007/978-3-7091-6781-6_4. [DOI] [PubMed] [Google Scholar]
- 9.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 10.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 11.Sohn EH, Lee AY, Park HJ. The validity and reliability of the Korean Modified Mini-Mental State (K-3MS) examination. J Korean Neurol Assoc. 2003;21:346–356. [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Chey J, Na DR, Park SH, Park EH. The validity and reliability of the Korean dementia rating scale. Korean J Clin Psychol. 1998;17:247–258. [Google Scholar]
- 14.Manolio TA, Kronmal RA, Burke GL, Poirier V, O'Leary DH, Gardin JM, et al. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994;25:318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 15.Korf ES, Wahlund LO, Visser PJ, Scheltens P. Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology. 2004;63:94–100. doi: 10.1212/01.wnl.0000133114.92694.93. [DOI] [PubMed] [Google Scholar]
- 16.Edhouse J, Thakur RK, Khalil JM. ABC of clinical electrocardiography. Conditions affecting the left side of the heart. BMJ. 2002;324:1264–1267. doi: 10.1136/bmj.324.7348.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Bursi F, Rocca WA, Killian JM, Weston SA, Knopman DS, Jacobsen SJ, et al. Heart disease and dementia: a population-based study. Am J Epidemiol. 2006;163:135–141. doi: 10.1093/aje/kwj025. [DOI] [PubMed] [Google Scholar]
- 19.Kilander L, Andrén B, Nyman H, Lind L, Boberg M, Lithell H. Atrial fibrillation is an independent determinant of low cognitive function: a cross-sectional study in elderly men. Stroke. 1998;29:1816–1820. doi: 10.1161/01.str.29.9.1816. [DOI] [PubMed] [Google Scholar]
- 20.Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 21.Patel DA, Lavie CJ, Milani RV, Shah S, Gilliland Y. Clinical implications of left atrial enlargement: a review. Ochsner J. 2009;9:191–196. [PMC free article] [PubMed] [Google Scholar]
- 22.Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population-based study. The Rotterdam Study. Stroke. 1997;28:316–321. doi: 10.1161/01.str.28.2.316. [DOI] [PubMed] [Google Scholar]
- 23.Jefferson AL. Cardiac output as a potential risk factor for abnormal brain aging. J Alzheimers Dis. 2010;20:813–821. doi: 10.3233/JAD-2010-100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ott HC, Rhomberg HP, Gosch M. Left atrial size and low cognitive function in very old patients. Z Gerontol Geriatr. 2007;9:130–139. [Google Scholar]
- 25.Almeida OP, Tamai S. Congestive heart failure and cognitive functioning amongst older adults. Arq Neuropsiquiatr. 2001;59:324–329. doi: 10.1590/s0004-282x2001000300003. [DOI] [PubMed] [Google Scholar]