Abstract
Prostate cancer is one of the most common types of cancer and one of the leading causes of cancer death among men in the Western countries. The aim of the present analysis is to assess the cancer burden in order to ensure accurate strategies for chemoprevention and treatment, including the major therapeutic approaches for localized high-risk disease - surgery and radiation therapy - and quality of life issues related to each option.
Keywords: Prostate cancer, Epidemiology, Chemoprevention, Radiotherapy
INTRODUCTION
Although often regarded as an indolent, chronically evolving disease of aging with which, rather than from which, men will die, prostate cancer is indeed one of the most common causes of cancer death among men[1]. Therefore, the perspective to prevent or reduce prostate cancer risk and mortality is a very relevant and debated topic today.
EPIDEMIOLOGY AND EARLY DIAGNOSIS
Excluding superficial skin cancers, prostate cancer is now the most common cancer in humans. Worldwide, over 660 000 new cases are diagnosed each year, accounting for 10% of all new cancers in males. Prostate cancer is the most numerous cancer diagnosed in men with 382 000 new cases (22.2% of the total) in Europe during 2008, followed by lung (291 000, 17%) and colorectal (231 000, 13.5%) cancers, and is the third leading cause of death in men (89 000, 9.3%), preceded by lung (255 000, 26.6%) and colorectal (110 000, 11.5%) cancers[2]. In women, the major cancer was by far breast cancer (421 000, 28.2% of the total), followed by colorectal (204 000, 13.7%) and lung (100 000, 6.7%) cancers. In 2008, in the US, prostate cancer was estimated to have an incidence of 186 300 new cases corresponding to 25% of all cancers in males, followed by lung cancer with 114 700 new cases (15%), with mortality rates of 28 660 deaths per year, corresponding to 10% of all cancer deaths in males, preceded only by lung cancer (90 800, 31%). These data parallel those of women in whom, in 2008, breast cancer was estimated to have an incidence of 182 400 new cases (26% of all female cancers), followed by lung cancer with 100 330 new cases (14%)[1,2], and with mortality rates of 40 400 deaths per year (15% of all female cancer deaths), surpassed only by those of lung cancer (71 000, 26%)[1].
Prostate cancer is predominantly a disease of old age and is rare before the age of 40-50 years. Autopsy studies worldwide have shown that histological disease increases with age and that roughly three-quarters of men older than 80 years will have some evidence of latent disease[3,4]. In parallel, more than 80% of clinically apparent disease occurs in men older than 65 years. In the US, it is estimated that 1 in 55 men between the ages of 40 and 60 years will develop a clinically apparent disease. This incidence rises almost exponentially to 1 in 7 for men between 60 and 80 years[5].
The probability of prostate cancer being diagnosed over 60 years of age is higher than that of breast cancer, reaching a peak of 5.5% in 75-year-old men compared to a probability of breast cancer of being diagnosed in 2.5% in 80-year-old women. The introduction in the 1980s of the PSA test has resulted in a dramatic increase in the incidence of prostate cancer, reaching a peak, in the US, of 240 cases per 100 000 males from about 100 cases in the previous years, settling in the 2000s around a current rate of 160 per 100 000. The boom of births registered in the USA in the 1960s will lead in 2020 to a parallel increase in the number of prostate cancer cases. The estimated number in 2015 will be 280 000 cases of prostate cancers, and 270 000 cases of breast cancer, and will roughly rise to 350 000 and 300 000, respectively, in 2025. Mortality from prostate and breast cancer will be equal in 2030, with a projected estimation of 70 000 deaths (Chan J, unpublished).
In addition to the dramatic increase in incidence of prostate cancer, the widespread use of PSA testing in the US and elsewhere has resulted also in a stage migration at diagnosis, with a significant reduction in the number of patients presenting initially with evidence of advanced disease. The incidence of the number of cases defined as high-risk according the National Comprehensive Cancer Network (NCCN) definition[6] has decreased in the US from 30% in the first half of the 1990s to 16% in the early 2000s[7], yielding an improvement in clinician ability to treat prostate cancer early and, consequently, increase the chances of successful cure.
Actually, the preliminary reports from the 2 largest PSA-based screening trials, the Prostate Lung Colorectal and Ovarian (PLCO) Screening Study in the USA and the European Randomized Study of Screening for Prostate Cancer (ERSPC)[8,9], have made the controversy surrounding PSA screening more confusing. The former study found no improvement in mortality after a median follow-up of 9 years. In contrast, the European study showed a significant improvement in survival, although at a cost of a large number of men needing to be screened, biopsied and treated to result in a relatively small number of lives saved. There were, however, some pitfalls and inconsistencies in the conduction of the studies (high rates of pre-screened men, contamination, etc., in the PLCO study; still short follow-up in ERSPC study) that may limit the significance of their conclusions.
The measurement of PSA levels has attracted some criticism concerning the best time to perform prostate biopsies for histological confirmation of disease. The recommendation to perform biopsies beyond the limit of 4 ng/mL PSA level, adopted in a prevention study of over 9000 cases, has led to a detection of histologically confirmed prostate cancer in about 80% of patients, thus implying a delay in early diagnosis. In the same study, even men with a PSA level < 4 ng were found to have prostate cancer in 15% of cases, 15% of which had aggressive histological features (Gleason score > 6), thus demonstrating the absence of a level of normality and a threshold limit of PSA (above 1 ng/mL) beyond which the presence of prostate cancer with high histological grade can be excluded[10].
Three main procedures are presently recommended to increase the specificity of PSA: (1) Determination of a baseline PSA value within 40 years of age, before the development of benign prostatic hyperplasia. At PSA levels of 0.6 ng/mL or more, the risk of developing prostate cancer during lifetime is 4 times higher and, therefore, a closer follow-up is recommended in subsequent years[11]; (2) Determination of “PSA velocity” (annual increments expressed in ng/mL per year): for PSA < 4 ng/mL, an annual increase of even 0.2 to 0.4 ng should be considered alarming and is associated with an increase in mortality from prostate cancer, for PSA > 4 ng/mL, biopsies are indicated if the PSA increase exceeds 0.75 ng/mL per year[12]; and (3) Determination of free PSA: for levels of total PSA of 4 to 10 ng/mL, a progressive reduction of free PSA below 25% indicates an increased likelihood of disease: up to 28% for values between 10% and 15%, and up to 55% for values below 10%.
The guidelines of the NCCN suggest the determination of PSA at age 40, repeated at 45 and 50 years with PSA level ≤ 0.6 ng/mL, or annual checks of PSA and digital rectal examination with PSA level > 0.6 ng/mL. Biopsy should be considered if PSA level is 2.6 to 4 ng/mL, or “velocity” is > 0.5 ng/mL per year, or free PSA is ≤ 25%. Despite the conflicting results of the two largest screening trials, PLCO and ERSPC[8,9], the adoption of early diagnosis and definitive treatment has led since 1995 to an annual mortality reduction of 3%-4%, while before the introduction of the PSA screening the mortality was increasing by 2% per year. It has been estimated that, if mortality rates had remained unchanged from 1991 to 2004 in the US, death from cancer would have been avoided by 408 400 men[1].
CHEMOPREVENTION AND DIET
Chemoprevention consists of the administration of drugs or other agents to prevent, slow or reverse prostate cancer progression. Because of its high prevalence, slowly progressive nature, and long latency period, prostate cancer is a suitable target for chemoprevention, the aim of which would ideally be the arrest of cancer development during the latency period, and a decrease in the incidence of clinical disease. Several promising chemopreventive approaches and agents are identified and are currently under laboratory and clinical investigation for their potential in reducing the risk of prostate cancer[13].
Dietary manipulations
Epidemiological studies have shown that the incidence of a clinically significant prostate cancer is significantly lower in countries where people eat a predominantly low fat, plant-based diet[14,15]. The reduction of caloric and fat intake in dairy products (red meat, milk, butter, cheese, cream, etc.), the increase in the intake of lycopene, and the maintenance of an adequate supply of vitamin D and sun exposure[16], without an excessive intake of calcium, have been recognized as important constituents of a diet related to a reduction of prostate cancer.
On the other hand, the excessive use of multivitamins (more than 7 times per week) increases the risk of advanced and fatal prostate cancer and must be avoided[17].
Inhibitors of 5α-reductase: These are drugs that inhibit the conversion of testosterone into dihydrotestosterone (DHT) (the most powerful promoter of prostate cancer growth among androgens) by lowering its blood level, and thereby reducing the risk of developing prostate cancer. The Prostate Cancer Prevention Trial, involving over 18 000 men, showed that finasteride, an inhibitor of 5α-reductase (5AR) type 2, is able to reduce the risk of prostate cancer by about 25% compared to the control group. However, in the finasteride group there were a greater number of more aggressive cancers with high histological grade[18]. Furthermore, the major reduction occurred because 15% fewer men underwent a biopsy and in the men who underwent a biopsy the reduction was only 10% which was not statistically significant. This suggests a detection bias, likely as a result of prostate volume reduction in the finasteride group[19,20], which raised doubts about the usefulness of this agent.
Dutasteride: Unlike finasteride, dutasteride is a dual inhibitor of both types 1 and 2 5AR isoenzymes and is able to reduce serum levels of DHT by > 90% compared with 70% seen with finasteride[21]. Recently published results from the large-scale randomized study Reduction by Dutasteride of Prostate Cancer Events, involving nearly 8000 healthy men[22], showed that dutasteride reduced the incidence of prostate cancer detected on biopsy among men who had an increased risk of prostate cancer, and that this reduction was observed mainly among men with low grade tumors (GS ≤ 6). The number of high grade tumors was similar in the dutasteride and placebo group; however, during years 3 and 4 of the study, only 1 tumor with GS of 8 to 10 was found among the 2343 men in the placebo group, whereas 12 of such tumors were detected among the 2447 men in the dutasteride group (P = 0.003).
Selenium/Vitamin E: Some molecular and epidemiological studies, together with some clinical evidence, have suggested that selenium and vitamin E may reduce the risk of prostate cancer[23]. For these reasons, two large-scale randomized double-blind trials, SELECT and The Physicians’ Health Study II, were planned to test the efficacy in preventing prostate cancer of selenium and Vitamin E, alone or in combination, and of selenium with Vitamins E or C, respectively. The first study (SELECT) was closed in 2004 after a recruitment of over 35 000 men. An initial analysis in September 2008 showed no benefit for this prevention and, indeed, noted a non-statistically significant increase in the incidence of type 2 diabetes, so the study was closed prematurely[24].
The Physicians’ Health Study II that began in 1997 and included 14 641 male physicians in the US, initially aged 50 years or older, was closed in 2007 and, after a median follow-up of 8-year, found that neither vitamin E nor C supplementation reduced the risk of prostate or total cancer[25].
SERMs: These drugs are selective estrogen receptor modulators which have the potential to prevent the growth of prostate cancer cells through a modulation of estrogens[26] and a decrease in testosterone levels by a suppression of the hypothalamic-pituitary axis. A double-blind randomized phase II study involving the use of toremifene for 12 mo in over 500 patients with high-grade intraepithelial neoplasia of the prostate showed a reduced risk of cancer by 21.8% compared to patients in the placebo group[27]. A large-scale randomized trial to examine the potential of toremifene to reduce the progression from PIN to prostate cancer is ongoing.
TREATMENT
The natural history of prostate cancer shows that, in many patients, the course of disease is slow and indolent and, therefore, the definitive treatment may be deferred. Randomized clinical trials have shown that a definitive treatment among younger patients with high and intermediate risk disease leads to a survival benefit[28] but may have a detrimental effect on quality of life[29]. In order to avoid the risk of treating clinically insignificant cancers, various institutions have taken into consideration, in patients with limited life expectancy or for those with low-risk localized disease, active surveillance with the aim of administering an adequate therapy only to patients whose cancer will show a trend to local progression during follow-up. Beyond the ongoing debates on the optimal timing and type of therapeutic intervention in low risk cancers, high-risk cancers - which may affect survival - need immediate treatment to permanently cure the disease and to reduce the risk of bone metastases which significantly affect the quality of life. Radical prostatectomy and high-dose radiotherapy, in combination or not with hormone therapy, are both treatment options in these cases. The surgical approach provides the removal of the prostate, seminal vesicles, and pelvic lymph nodes. However, in high-risk prostate cancer surgery it is not always possible to eradicate the whole disease. Due to the infiltration of surgical margins, or involvement of seminal vesicles or pelvic nodes - quite common in high-risk cases - often postoperative radiotherapy, in combination or not with hormone therapy, is mandatory. Complications of radical prostatectomy imply permanent erectile dysfunction in almost all patients, and a 7% to 15% risk of permanent urinary incontinence. Radiation therapy consists of administration of high-dose ionizing radiation to the prostate and seminal vesicles, and elective lower radiation doses to pelvic lymph nodes. With the introduction in the last decade of the modern techniques of three-dimensional conformal or intensity modulated radiotherapy, it is possible to increase local tumor control by delivering higher and more focused radiation doses to the target, and concomitantly decrease the grade ≥ 2 urinary and rectal toxicity to the present 5-year rates of 14%-18% and 2%-14%, respectively[30,31], with a tendency for these to subside later with time[32]. The risk of radiation-induced erectile dysfunction develops slowly, reaching 40%-50% at 5 years in the elderly population usually referred for radiotherapy, with an increased risk when combined with hormonal therapy[33]. Radiation-mediated impotence is multifactorial, and most of the patients evaluated for impotence have arteriogenesis or cavernosal dysfunctions[34].
There are no large-scale randomized controlled trials designed to define the most appropriate treatment between the demolitive or conservative approach. A recent update of a Japanese randomized trial showed no difference in the long-term outcomes between radical prostatectomy and external beam radiotherapy when combined with endocrine therapy[35]. However, the small number of patients enrolled in this study has no power to detect even a large statistical difference between the two treatment modalities. Furthermore, despite numerous retrospective reports showing no difference in survival and PSA-failure, the optimal treatment remains controversial, especially in high-risk prostate cancer. The correct comparison of the results in several retrospective studies or meta-analyses has always been hampered by the two different staging procedures[36] which, in contrast to that usually used for radiotherapy patients, often include an intraoperative ascertainment of the freedom from microscopic lymph node involvement in the surgical series before proceeding to radical prostatectomy[6]. Thus, patients in the radiotherapy series, although staged as having localized disease, could have microscopic lymph node involvement, with a higher risk of distant spread and, therefore, might be under-staged in comparison to those of the surgical series, resulting in an exaggeration of the benefits[37] of radical prostatectomy and making a correct interpretation of the results impossible. Furthermore, localized prostate cancer is a slow progressing disease. Thus, the potential therapeutic benefit could be hidden by deaths from other causes in the elderly population, who are often referred for radiotherapy.
At the Regina Elena Cancer Institute in Rome, Italy, the results of radical prostatectomy and external beam radiotherapy were analyzed and compared in two groups of 122 and 162 patients with a pre-treatment classification of high-risk, clinically localized prostate cancer, consecutively treated between 2003 and 2007 at the Urology and Radiotherapy Departments, respectively. The effectiveness between the two treatments was assessed by comparing the biochemical relapse, which is the most sensitive and specific indicator of disease. The actuarial analysis of the freedom from biochemical failure showed a clear benefit of radiotherapy over prostatectomy, with 3-year rates of 86.8% and 69.8%, respectively (P = 0.001) (Figure 1)[38]. The benefit was even larger (86.8% vs 67.1%) when the radiotherapy series was compared to patients with unfavorable pathological features in the prostatectomy group, who underwent a postoperative radiotherapy ± hormonal therapy (Figure 2)[38]. Radical prostatectomy in high-risk prostate cancer may be beneficial in small tumors with histological Gleason score < 8, PSA < 20 and life expectancy > 10 years, or in patients with bulky prostate and obstructive symptoms.
Figure 1.
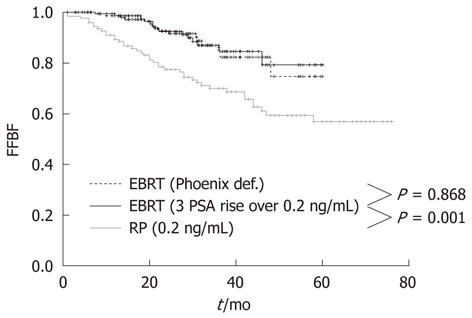
Freedom from Biochemical Failure for patients treated with radical prostatectomy or external beam radiotherapy using Phoenix definition and 3 consecutive PSA increases > 0.2 ng/mL definition. FFBF: Freedom from Biochemical Failure; EBRT: External beam radiotherapy; RP: Radical prostatectomy.
Figure 2.
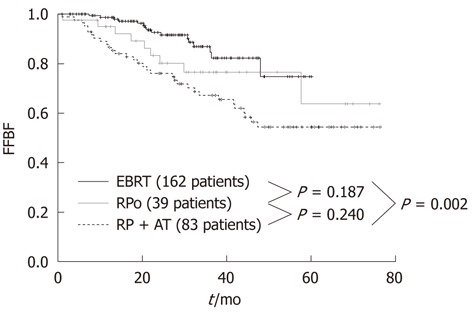
Freedom from Biochemical Failure for patients treated with external beam radiotherapy, radical prostatectomy only and radical prostatectomy plus adjuvant treatment (external beam radiotherapy ± androgen deprivation therapy). FFBF: Freedom from Biochemical Failure; EBRT: External beam radiotherapy; RPo: Radical prostatectomy only; RP: Radical prostatectomy; AT: Adjuvant treatment.
CONCLUSION
Prostate cancer is one of the most significant medical problems of the elderly male population, with a gradual progressive increase in its incidence that, in turn, is likely related to the increase in life expectancy. The high prevalence, long latency, morbidity, and still significant mortality make prostate cancer an important target for prevention with drug therapies which could have the advantage of avoiding “the burden of care” (anxiety, cost and iatrogenic effects of treatments). The increase in early detection through the PSA test, with the consequent reduction in high-risk cancers, and the significant improvement of diagnostic and therapeutic procedures have recently contributed to a slow but progressive decrease in the disease-specific mortality and to a better tolerance of the presently available therapeutic procedures.
Footnotes
Peer reviewer: Cem Onal, MD, Department of Radiation Oncology, Adana Research and Treatment Centre, Baskent University Medical Faculty, 01120 Yuregir, Adana, Turkey
S- Editor Cheng JX L- Editor Logan S E- Editor Zheng XM
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Guileyardo JM, Johnson WD, Welsh RA, Akazaki K, Correa P. Prevalence of latent prostate carcinoma in two U.S. populations. J Natl Cancer Inst. 1980;65:311–316. [PubMed] [Google Scholar]
- 4.Billis A. Latent carcinoma and atypical lesions of prostate. An autopsy study. Urology. 1986;28:324–329. doi: 10.1016/0090-4295(86)90019-1. [DOI] [PubMed] [Google Scholar]
- 5.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8–31, 1. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Prostate cancer, V.I.2007. Available from: http://www.nccn.org/professionals/physician_gls/PDF/prostate.pdf. [Google Scholar]
- 7.Cooperberg MR, Moul JW, Carroll PR. The changing face of prostate cancer. J Clin Oncol. 2005;23:8146–8151. doi: 10.1200/JCO.2005.02.9751. [DOI] [PubMed] [Google Scholar]
- 8.Andriole GL, Crawford ED, Grubb RL, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 10.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 11.Fang J, Metter EJ, Landis P, Chan DW, Morrell CH, Carter HB. Low levels of prostate-specific antigen predict long-term risk of prostate cancer: results from the Baltimore Longitudinal Study of Aging. Urology. 2001;58:411–416. doi: 10.1016/s0090-4295(01)01304-8. [DOI] [PubMed] [Google Scholar]
- 12.Carter HB, Ferrucci L, Kettermann A, Landis P, Wright EJ, Epstein JI, Trock BJ, Metter EJ. Detection of life-threatening prostate cancer with prostate-specific antigen velocity during a window of curability. J Natl Cancer Inst. 2006;98:1521–1527. doi: 10.1093/jnci/djj410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleshner N, Zlotta AR. Prostate cancer prevention: past, present, and future. Cancer. 2007;110:1889–1899. doi: 10.1002/cncr.23009. [DOI] [PubMed] [Google Scholar]
- 14.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33–64, 1. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- 15.Hebert JR, Hurley TG, Olendzki BC, Teas J, Ma Y, Hampl JS. Nutritional and socioeconomic factors in relation to prostate cancer mortality: a cross-national study. J Natl Cancer Inst. 1998;90:1637–1647. doi: 10.1093/jnci/90.21.1637. [DOI] [PubMed] [Google Scholar]
- 16.Hanchette CL, Schwartz GG. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992;70:2861–2869. doi: 10.1002/1097-0142(19921215)70:12<2861::aid-cncr2820701224>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.Lawson KA, Wright ME, Subar A, Mouw T, Hollenbeck A, Schatzkin A, Leitzmann MF. Multivitamin use and risk of prostate cancer in the National Institutes of Health-AARP Diet and Health Study. J Natl Cancer Inst. 2007;99:754–764. doi: 10.1093/jnci/djk177. [DOI] [PubMed] [Google Scholar]
- 18.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 19.Kulkarni GS, Al-Azab R, Lockwood G, Toi A, Evans A, Trachtenberg J, Jewett MA, Finelli A, Fleshner NE. Evidence for a biopsy derived grade artifact among larger prostate glands. J Urol. 2006;175:505–509. doi: 10.1016/S0022-5347(05)00236-3. [DOI] [PubMed] [Google Scholar]
- 20.Cohen YC, Liu KS, Heyden NL, Carides AD, Anderson KM, Daifotis AG, Gann PH. Detection bias due to the effect of finasteride on prostate volume: a modeling approach for analysis of the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2007;99:1366–1374. doi: 10.1093/jnci/djm130. [DOI] [PubMed] [Google Scholar]
- 21.Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab. 2004;89:2179–2184. doi: 10.1210/jc.2003-030330. [DOI] [PubMed] [Google Scholar]
- 22.Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, Pettaway CA, Tammela TL, Teloken C, Tindall DJ, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 23.Clark LC, Combs GF, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 24.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Sesso HD, Buring JE. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steiner MS, Raghow S. Antiestrogens and selective estrogen receptor modulators reduce prostate cancer risk. World J Urol. 2003;21:31–36. doi: 10.1007/s00345-002-0316-x. [DOI] [PubMed] [Google Scholar]
- 27.Price D, Stein B, Sieber P, Tutrone R, Bailen J, Goluboff E, Burzon D, Bostwick D, Steiner M. Toremifene for the prevention of prostate cancer in men with high grade prostatic intraepithelial neoplasia: results of a double-blind, placebo controlled, phase IIB clinical trial. J Urol. 2006;176:965–970; discussion 970-971. doi: 10.1016/j.juro.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Bill-Axelson A, Holmberg L, Ruutu M, Häggman M, Andersson SO, Bratell S, Spångberg A, Busch C, Nordling S, Garmo H, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352:1977–1984. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- 29.Litwin MS, Gore JL, Kwan L, Brandeis JM, Lee SP, Withers HR, Reiter RE. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109:2239–2247. doi: 10.1002/cncr.22676. [DOI] [PubMed] [Google Scholar]
- 30.Arcangeli S, Saracino B, Petrongari MG, Gomellini S, Marzi S, Landoni V, Gallucci M, Sperduti I, Arcangeli G. Analysis of toxicity in patients with high risk prostate cancer treated with intensity-modulated pelvic radiation therapy and simultaneous integrated dose escalation to prostate area. Radiother Oncol. 2007;84:148–155. doi: 10.1016/j.radonc.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Zelefsky MJ, Levin EJ, Hunt M, Yamada Y, Shippy AM, Jackson A, Amols HI. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 32.Karlsdóttir A, Muren LP, Wentzel-Larsen T, Dahl O. Late gastrointestinal morbidity after three-dimensional conformal radiation therapy for prostate cancer fades with time in contrast to genitourinary morbidity. Int J Radiat Oncol Biol Phys. 2008;70:1478–1486. doi: 10.1016/j.ijrobp.2007.08.076. [DOI] [PubMed] [Google Scholar]
- 33.Padula GD, Zelefsky MJ, Venkatraman ES, Fuks Z, Lee HJ, Natale L, Leibel SA. Normalization of serum testosterone levels in patients treated with neoadjuvant hormonal therapy and three-dimensional conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2002;52:439–443. doi: 10.1016/s0360-3016(01)02604-9. [DOI] [PubMed] [Google Scholar]
- 34.Merrick GS, Butler WM, Wallner KE, Galbreath RW, Anderson RL, Kurko BS, Lief JH, Allen ZA. Erectile function after prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2005;62:437–447. doi: 10.1016/j.ijrobp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Akakura K, Suzuki H, Ichikawa T, Fujimoto H, Maeda O, Usami M, Hirano D, Takimoto Y, Kamoto T, Ogawa O, et al. A randomized trial comparing radical prostatectomy plus endocrine therapy versus external beam radiotherapy plus endocrine therapy for locally advanced prostate cancer: results at median follow-up of 102 months. Jpn J Clin Oncol. 2006;36:789–793. doi: 10.1093/jjco/hyl115. [DOI] [PubMed] [Google Scholar]
- 36.Barry MJ, Fleming C, Coley CM, Wasson JH, Fahs MC, Oesterling JE. Should Medicare provide reimbursement for prostate-specific antigen testing for early detection of prostate cancer? Part I: Framing the debate. Urology. 1995;46:2–13. doi: 10.1016/s0090-4295(99)80151-4. [DOI] [PubMed] [Google Scholar]
- 37.Wasson JH, Cushman CC, Bruskewitz RC, Littenberg B, Mulley AG, Wennberg JE. A structured literature review of treatment for localized prostate cancer. Prostate Disease Patient Outcome Research Team. Arch Fam Med. 1993;2:487–493. doi: 10.1001/archfami.2.5.487. [DOI] [PubMed] [Google Scholar]
- 38.Arcangeli G, Strigari L, Arcangeli S, Petrongari MG, Saracino B, Gomellini S, Papalia R, Simone G, De Carli P, Gallucci M. Retrospective comparison of external beam radiotherapy and radical prostatectomy in high-risk, clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2009;75:975–982. doi: 10.1016/j.ijrobp.2008.12.045. [DOI] [PubMed] [Google Scholar]


