Abstract
Prostate cancer (PC) is one of the most frequently diagnosed cancers in men. There are a number of treatment options for PC with a different therapeutic approach between USA and Europe. Radical prostatectomy is one of the most used therapies but focal gland therapy is an emerging approach, especially for localized tumors. In this scenario, high intensity focused ultrasound (HIFU) has been incorporated in certain medical association guidelines. HIFU has been employed for about 10 years especially for localized PC. Results are promising with a 5-year biochemical survival rate ranging from 45% to 84%. Collateral events are rare and HIFU retreatment is not common. Magnetic resonance guided focused ultrasound surgery (MRgFUS) was recently presented as a method for ablation with focused ultrasound under magnetic resonance imaging guidance. It has the advantage of improved targeting and real time temperature monitoring but only a few studies have been conducted with human patients. The aim of this review is to describe the current status of HIFU and MRgFUS in the therapy of PC.
Keywords: Prostate cancer, Magnetic resonance guided focused ultrasound surgery, High intensity focused ultrasound
INTRODUCTION
Prostate cancer (PC) is one of the most frequently diagnosed cancers in the male population in the world[1]. According to the American Cancer Society, PC represents 25% of newly diagnosed cancers every year[1]. In Europe, the mortality rate for PC was 21.1% in 2008[2]. Tumor stage (TNM staging), grading of the tumor (Gleason score) and serum prostate-specific antigen (PSA) levels are the most important prognostic factors for PC. Based on prognostic factors, the risk of PC can be divided in three categories, (high, intermediate and low risk) with different management strategies. Additionally, the patient’s age, concomitant disease, life expectancy and personal preferences may also influence the therapeutic approach[3].
Treatment options for PC demonstrate a large spectrum. The American Urological Association recommends active surveillance, interstitial prostate brachytherapy, external beam radiation therapy (EBRT) and radical prostatectomy (RP) as therapy option for patients with PC[4]. On the other hand, the European Association of Urology (EAU) guidelines recommend RP for intermediate and high-risk populations[5]. Active surveillance is recommended for the low risk population but the patient needs to be informed of risks and other therapy options[5]. A recently published randomized trial that compared retropubic RP to “watchful waiting” showed that RP reduced PC mortality and the risk of metastases[6]. This wide spectrum of treatment option for PC arises from an incomplete comprehension of the natural history of PC biology[7]. Several studies demonstrated that low risk prostatic tumors are correlated with a better prognosis in terms of biochemical free survival and metastatic-free disease[5-7].
Standard treatment for PC has long been “whole-gland” therapy including RP (i.e., complete surgical removal of the prostate) or radiation therapy of the entire prostate (via external beam or brachytherapy). However, widespread PSA-based PC screening has led to a profound stage migration with a large proportion of men being diagnosed with a low-stage, low-grade cancer that has minimal risk of progression. Several men are diagnosed with a PC not destined to become clinically evident during their natural lifespan. There is growing evidence and clinical judgment that patients with low-risk disease may benefit significantly from a minimally invasive focal therapy and avoid complications associated with prostatectomy and other “whole gland” treatments. Analogous to lumpectomy for breast cancer, the goal of focal therapy for PC is to effectively treat the area of the prostate that contains the cancer while minimizing the risk of treatment-related side effects. There is still ongoing debate between the efficiency of focal treatment but at the same time different focal options emerge. Various approaches with different energy sources are proposed to achieve whole gland, subtotal or focus ablation with the guidance of imaging modalities, such as ultrasound (US) or magnetic resonance (MR). Brachytherapy and radiation external beam therapy are the most used as minimally invasive techniques, not only for the therapy of localized PC but also for the palliation of high-grade tumors[4-6]. Laser and cryoablation have been used for focal therapy with promising results[4,5].
Some medical associations recommend high intensity focused ultrasound (HIFU) for treatment of PC but its accuracy is still not clear[5,8]. HIFU is a non-invasive therapy that has been used for localized PC or salvage therapy in the 1990’s. It is a technique that uses focused ultrasound waves to thermally ablate a portion of tissue situated at the focal point. High power ultrasound can be focused on a targeted point to cause a rise in temperature between 70-80 °C. This can result in thermal tissue coagulation necrosis, cavitation and heat shock. Each sonication heats only a small focal target, so multiple sonications, raster scanner, volumetric focus steering or some other beam translating method must be utilized to ablate an entire target area[9]. It is important to note that due to the propagation mechanism of ultrasound, this technique should be performed with caution near bone and gaseous interfaces. Recently, the combination of magnetic resonance guided focused ultrasound surgery (MRgFUS) has been introduced due to a better ability to plan and monitor treatments in real-time[10]. This technique is approved by the Federal and Drugs Administration (FDA) for fibroid ablation and shows great potential in bone metastasis pain palliation. Promising results for treatment of liver, breast and brain tumor and prostate, were also obtained[10]. This article reviews the HIFU experiences and the new developments on MRgFUS for PC.
HIFU OF PROSTATE: TEN YEARS OF EXPERIENCE
The first HIFU prototype was built in 1940 and the general technology existed in an experimental setting for over 50 years. However, only recently has this technology been employed for approved clinical applications. Two HIFU devices are currently available for patient care: Ablatherm (EDAP TMS SA, Vaulx-en-Velin, France) and Sonablate (Focus Surgery Inc., Indianapolis, IN, USA). The most important difference is in their patient positioning. Both devices operate under ultrasound guidance and are approved for commercial distribution in the European Union, Canada, South Korea, Japan and Russia but they have yet to be approved by the Federal Drug Administration in the US.
The role of HIFU for PC management is still controversial but the literature reports that HIFU has been used in a total of 3018 published patients: 93% of these treatments were for primary care PC and only 7% of patients were treated with salvage HIFU[3].
HIFU as primary treatment method in localized PC
Most patients treated with HIFU presented with localized cancer[7]. Usually HIFU is used as a stand-alone procedure with a 5-year disease-free survival rate (biochemical) of 77% for Ablatherm and 45%-84% for Sonablate[3]. Table 1 summarizes the difference in disease free survival rate at various time points as well as the negative biopsies rate at 3 and 15 mo. Best results after HIFU in terms of negative biopsies and low PSA levels were achieved in patients with low-grade PC[3]. Based on The French Association of Urology review, HIFU is the best short-term cancer control in terms of percentage of negative biopsies and decrease of PSA serum levels[11]. However, cautious optimism is recommended, as long-term results have not yet been provided.
Table 1.
Results of high intensity focused ultrasound in terms of survival free disease and negative biopsy rate[11]
| Survival free disease (yr) | Negative biopsies rate (mo) | ||||
| 1 | 2 | 3 | 5 | 7 | 86% (3) |
| 78%-84% | 0%-91% | 20%-86% | 45%-84% | 69% | 80% (5) |
HIFU treatment could be complicated by adverse events involving the bladder function (2%-58%)[3]. Other complications included rectal burn (0%-15%) and rectourethral fistula (0%-3%). The studies performed using an Ablatherm device reported higher complication rates with respect to the Sonablate studies, as shown in Table 2.
Table 2.
Complications in Ablatherm and Sonablate series[11]
| Ablatherm | Sonablate | |
| Bladder neck/urethral stricture stenosis | 2%-17% | 4%-30% |
| Urinary tract infection | 2%-58% | 4%-24% |
| Urinary incontinence | 2%-34% | 1%-2% |
| Urinary retention | 3%-14% | 1%-13% |
| Impotence | 20%-39% | |
| Rectal burn | 0%-15% | - |
| Rectourethral fistula | 0%-3% | - |
HIFU retreatment is rare with a variable rate from 7.7% to 43%[7]; the second treatment always showed an increase in complications such as incontinence and impotence[7].
Salvage HIFU
Five case series reported HIFU with the Ablaterm device as salvage therapy for patients with local recurrence after EBRT with a 5-year survival rate of 17%[12-14]; adverse reactions in these studies are more frequent than in HIFU primary care with a urinary incontinence rate of 50%, bladder outlet obstruction rate of 20% and recto-urethral fistula rate of 3%. In 2005 the National Institute for Health and Clinical Excellence guidelines reported HIFU as the primary treatment or salvage therapy after radiotherapy with the option of carrying out TURP immediately before HIFU[15].
MRGFUS OF PROSTATE: A NOVEL TECHNIQUE
Multi-parametric MR imaging (MRI) utilizing T2-weighted, diffusion weighted MR (DWI) and dynamic contrast enhanced MRI (DCE-MRI) represents the state of the art for detection, localization and staging of PC[16,17]. T2 weighted imaging provides evaluation of morphology. DWI and DCE-MRI provide functional information about the prostate, which helps to improve PC detection as well as characterization of tumor aggressiveness[18]. This advantage makes MRI the more suitable technique for targeting focal cancer lesions in the prostate. In addition, MR thermometry enables the operator to monitor the temperature and amount of estimated tissue damage real time both at the site of ablation as well as in vulnerable areas that have to be protected from ablation. The MRgFUS device is shown in Figure 1.
Figure 1.
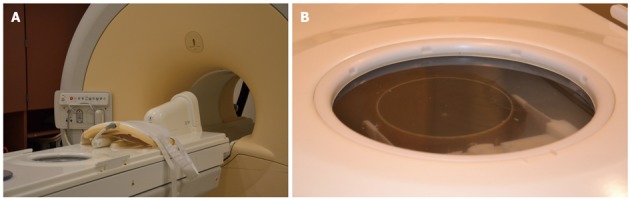
Magnetic resonance guided focused ultrasound surgery suite at the University of Chicago. A: Magnetic resonance guided focused ultrasound surgery (MRgFUS) probe and phase array; B: Focused picture of MRgFUS phased array transducer.
Currently, there is no standard MR protocol for treating PC with MRgFUS across all centers and vendors. Based on the literature available, MRgFUS protocols for treatment of PC include:
Anatomical MRI
It is performed in order to identify and target the lesion for ablation. In MRgFUS of the prostate, T2 weighted sequences are usually preferred because of their high spatial resolution and ability to detect PC[18]. DWI and DCE-MRI sequences can also be used in cases where tumor focus can best be seen on these sequences.
MR thermometry
It is imperative to monitor temperature and thus thermal dose during the ablation procedure. MRI thermometry uses proton resonance frequency shifts to determine the relative temperature[19-21]. This allows monitoring to take place in near real time and improves the safety profile for MRgFUS. In addition, due to immediacy to the peripheral prostate gland, the rectal wall is constantly monitored during treatment in order to avoid major damage, eventually causing perforation.
MR imaging based assessment following the procedure
It is usually performed with contrast-enhanced imaging since the heated area will have vascular damage and less perfusion, which will cause it to present as non-enhancing foci after contrast administration[18]. Hazle et al[22] reported that the area lacking enhancement could underestimate the size of tissue necrosis after treatment as verified by histology. Some authors suggested the use of DWI, and in particular of ADC maps, where the treated tissue presents a lower ADC value with respect to untreated tissue[18].
The most recent studies on MRgFUS of the prostate are given in Table 3 with their study protocol. The currently available FDA and CE (Conformité Européenne, meaning “European Conformity”) approved for uterine fibroids MRgFUS treatment device is GE Insightec ExAblate 2000 and ExAblate 2100. Additionally, Haifu (models JC and JC200) and Philips Sonalleve have CE approval. Moreover, Philips Sonalleve has been accepted for an FDA phase 2 and phase 3 trial: while the usefulness of MRI for guiding focused ultrasound seems to be the most viable method for localized PC therapy, experience is still limited. Initially, one study of human patients that underwent transurethral MRgFUS immediately prior to RP has been published[20]. It demonstrated the feasibility of MRgFUS in humans.
Table 3.
The most recent papers on magnetic resonance guided focus ultrasound surgery prostate treatment
| Population | MR scan | Protocol | |
| Napoli et al[23] | 3 humans patients prior radical prostatectomy | Transrectal probe | - |
| 3 T | |||
| Cheng et al[24] | 18 humans patients with low risk PC | Transrectal probe | - |
| 3 T | |||
| Siddiqui et al[20] | 5 humans patients with localized PC | Transurethral probe | Temperature monitor with PRF sequences |
| 17 dogs | 1.5 T and 3 T | ||
| McDannold et al[25] | 4 dogs | Transrectal probe | Temperature monitor with FSP GR (single slice |
| 3 T | fast spoiled gradient). T1 w post treatment | ||
| Kinsey et al[19] | 3 dogs | Transurethral probe | Monitor of temperature with PRF |
MR: Magnetic resonance; PC: Prostate cancer; FSP GR: Fast spoiled gradient; PRF: Proton resonance frequency.
One of the first experiences in human prostate MRgFUS was presented by Napoli et al[23]: 3 patients with localized PC underwent to MRgFUS with ExAblate system before RP, as shown in Figure 2. The analysis of the specimen confirmed an extensive coagulative necrosis at the site of sonification and the patients didn’t show any particular complications. More recently, Cheng et al[24] presented their multi-centric experience in 18 patients with low risk cancer treated only by MRgFUS approach. The results were promising, with minimum discomfort for the patients and negative post-enhancement MRI that persisted 1 mo after therapy.
Figure 2.
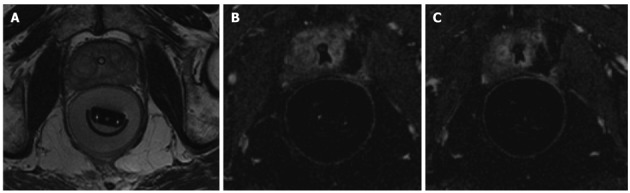
Magnetic resonance guided focused ultrasound surgery of human prostate. A: Pre-sonification scan, catheter is visible in prostate urethra and transrectal probe is present in the rectum, filled by water. Hypointense focus in the left peripheral zone represents patient`s biopsy proven prostate cancer; B, C: Post-contrast T1-weighted images through prostate demonstrates non-enhancing ablation defect in the left peripheral zone.
All the presently available literature on MRgFUS for prostate uses a canine model for research. To date, two different probes (transrectal and transurethral) have been used for MRgFUS of the prostate. These represent two approaches for reaching the prostate less invasively than surgery, but still interacting with as few other critical structures as possible. Regarding the transrectal probe, McDannold et al[25] reported a consistently ablated volume (sharp boundary 4 mm) of prostate tissue after MRgFUS (3 T) using an endorectal coil in dogs. On the other hand, the transurethral probe has been reported with wider variety of applicators: planar, curvilinear and tubular. A planar applicator can be used with faster penetration and large volume of treatment. The advantage of curvilinear applicators is greater control over selective heating. The tubular applicator allows electronic control of an angular heating pattern. Both the planar and curvilinear applicator needed about 10-30 min to ablate larger lesions in the prostate because of a narrow acoustic beam width (4 mm)[19]. The tubular applicator does not need mechanical rotation, unlike the others, so it may reduce potential motion artifacts[18].
CONCLUSION
Focal therapy methods for PC are emerging and among them, focused ultrasound is one of the techniques used to achieve especially localized ablation. HIFU is the technique which has been used most in humans with promising results in term of survival rate. By the books, MRgFUS presented some advantages over regular HIFU for improved lesion targeting and real time temperature monitoring. Experience on MRgFUS of the prostate is still limited, as described in Table 4.
Table 4.
Comparison between high intensity focused ultrasound and magnetic resonance guided focus ultrasound surgery in prostate cancer treatment
| HIFU | MRgFUS | |
| Number of published patients (pt) | 3018 patients[3] | 5 patients[21] |
| 21 patients[24,25] reported in International Meeting and Congress | ||
| Free biological disease survival rate | 1 yr survival rate: 78%-84%[11] | No provide |
| Complication rate | 0%-58%[11] | No provide in human patients |
| Retreatment | 7%-43%[7] | None |
HIFU: High intensity focused ultrasound; MRgFUS: Magnetic resonance guided focused ultrasound surgery.
More studies are needed to demonstrate the oncologic effectiveness of such methods and validate its positive effect on patient outcomes.
Footnotes
Peer reviewer: Feng Wu, MD, PhD, Professor of Surgery, Clinical Consultant, High Intensity Focused Ultrasound Unit, The Churchill Hospital, Headington, Oxford, OX3 7LJ, United Kingdom
S- Editor Cheng JX L- Editor O’Neill M E- Editor Zheng XM
References
- 1.American Cancer Society. Prostate Cancer Facts. In: Cancer Facts and Figures 2009., editor. Atlanta, GA: American Cancer Society; 2009. pp. 19–20. [Google Scholar]
- 2.European Cancer Observatory. Cancer Fact Sheets. Available from: http://eu-cancer.iarc.fr/2-cancer-fact-sheets.html,en.
- 3.Warmuth M, Johansson T, Mad P. Systematic review of the efficacy and safety of high-intensity focussed ultrasound for the primary and salvage treatment of prostate cancer. Eur Urol. 2010;58:803–815. doi: 10.1016/j.eururo.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 4.American Urological Association Education and Research, Inc. Guidelines for the management of clinically localized prostate cancer: 2007 update. Available from: http://www.auanet.org/content/clinical-practice-guidelines/clinical-guidelines/main-reports/proscan07/appendixes.pdf.
- 5.Heidenreich A, Bolla M, Mason MD, Matveev V, Mottet N, Schmid HP, van der Kwast TH, Wiegel T, Zattoni F. Guidelines on Prostate Cancer. January, 2011. Available from: http://www.uroweb.org/guidelines/online-guidelines/ [DOI] [PubMed] [Google Scholar]
- 6.Bill-Axelson A, Holmberg L, Filén F, Ruutu M, Garmo H, Busch C, Nordling S, Häggman M, Andersson SO, Bratell S, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst. 2008;100:1144–1154. doi: 10.1093/jnci/djn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lukka H, Waldron T, Chin J, Mayhew L, Warde P, Winquist E, Rodrigues G, Shayegan B. High-intensity focused ultrasound for prostate cancer: a systematic review. Clin Oncol (R Coll Radiol) 2011;23:117–127. doi: 10.1016/j.clon.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 8. Available from: http://www.uroweb.org/guidelines/online-guidelines/
- 9.Jolesz FA. MRI-guided focused ultrasound surgery. Annu Rev Med. 2009;60:417–430. doi: 10.1146/annurev.med.60.041707.170303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dick EA, Gedroyc WM. ExAblate magnetic resonance-guided focused ultrasound system in multiple body applications. Expert Rev Med Devices. 2010;7:589–597. doi: 10.1586/erd.10.38. [DOI] [PubMed] [Google Scholar]
- 11.Blana A, Rogenhofer S, Ganzer R, Lunz JC, Schostak M, Wieland WF, Walter B. Eight years’ experience with high-intensity focused ultrasonography for treatment of localized prostate cancer. Urology. 2008;72:1329–1333; discussion 1333-1334. doi: 10.1016/j.urology.2008.06.062. [DOI] [PubMed] [Google Scholar]
- 12.Murat FJ, Poissonnier L, Rabilloud M, Belot A, Bouvier R, Rouviere O, Chapelon JY, Gelet A. Mid-term results demonstrate salvage high-intensity focused ultrasound (HIFU) as an effective and acceptably morbid salvage treatment option for locally radiorecurrent prostate cancer. Eur Urol. 2009;55:640–647. doi: 10.1016/j.eururo.2008.04.091. [DOI] [PubMed] [Google Scholar]
- 13.Murat FJ, Poissonnier L, Pricaz E, Rouviere O, Mege F, Chapelon JY, Colombel M, Gelet A. Prognostic factors for salvage HIFU success after external beam radiation failure (EBRT) failure. Eur Urol Suppl. 2008;7:119. [Google Scholar]
- 14.Rebillard X, Soulié M, Chartier-Kastler E, Davin JL, Mignard JP, Moreau JL, Coulange C. High-intensity focused ultrasound in prostate cancer; a systematic literature review of the French Association of Urology. BJU Int. 2008;101:1205–1213. doi: 10.1111/j.1464-410X.2008.07504.x. [DOI] [PubMed] [Google Scholar]
- 15.National Institute for Health and Clinical Excellence (NICE) IPG118 High-intensity focused ultrasound for prostate cancer: guidance. Available from: http://guidance.nice.org.uk/IPG118/Guidance/pdf.
- 16.Bonekamp D, Jacobs MA, El-Khouli R, Stoianovici D, Macura KJ. Advancements in MR imaging of the prostate: from diagnosis to interventions. Radiographics. 2011;31:677–703. doi: 10.1148/rg.313105139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoeks CM, Barentsz JO, Hambrock T, Yakar D, Somford DM, Heijmink SW, Scheenen TW, Vos PC, Huisman H, van Oort IM, et al. Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology. 2011;261:46–66. doi: 10.1148/radiol.11091822. [DOI] [PubMed] [Google Scholar]
- 18.Pauly KB, Diederich CJ, Rieke V, Bouley D, Chen J, Nau WH, Ross AB, Kinsey AM, Sommer G. Magnetic resonance-guided high-intensity ultrasound ablation of the prostate. Top Magn Reson Imaging. 2006;17:195–207. doi: 10.1097/RMR.0b013e31803774dd. [DOI] [PubMed] [Google Scholar]
- 19.Kinsey AM, Diederich CJ, Rieke V, Nau WH, Pauly KB, Bouley D, Sommer G. Transurethral ultrasound applicators with dynamic multi-sector control for prostate thermal therapy: in vivo evaluation under MR guidance. Med Phys. 2008;35:2081–2093. doi: 10.1118/1.2900131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddiqui K, Chopra R, Vedula S, Sugar L, Haider M, Boyes A, Musquera M, Bronskill M, Klotz L. MRI-guided transurethral ultrasound therapy of the prostate gland using real-time thermal mapping: initial studies. Urology. 2010;76:1506–1511. doi: 10.1016/j.urology.2010.04.046. [DOI] [PubMed] [Google Scholar]
- 21.Chopra R, Tang K, Burtnyk M, Boyes A, Sugar L, Appu S, Klotz L, Bronskill M. Analysis of the spatial and temporal accuracy of heating in the prostate gland using transurethral ultrasound therapy and active MR temperature feedback. Phys Med Biol. 2009;54:2615–2633. doi: 10.1088/0031-9155/54/9/002. [DOI] [PubMed] [Google Scholar]
- 22.Hazle JD, Diederich CJ, Kangasniemi M, Price RE, Olsson LE, Stafford RJ. MRI-guided thermal therapy of transplanted tumors in the canine prostate using a directional transurethral ultrasound applicator. J Magn Reson Imaging. 2002;15:409–417. doi: 10.1002/jmri.10076. [DOI] [PubMed] [Google Scholar]
- 23.Napoli A, Pediconi F, Anzidei M, Catalano C, Passariello R. Noninvasive Treatment of Prostate and Breast Cancer: Initial Clinical Experience Using High Intensity Focused Ultrasound Therapy with 3T Magnetic Resonance Guidance. Interventional Oncology Series: Molecular Targeting and Emerging Clinical Foci in Interventional Oncology. Code VO41-16.RSNA; 2010. [Google Scholar]
- 24.Cheng CWS, Kwek JW, Thng CH, Lau W, Ho H, Khoo JBK. Initial experience with MRg FUS focal therapy for low risk prostate cancer; European Congress of Radiology; Mar 3-7; Vienna, Austria. 2011. [Google Scholar]
- 25.McDannold N, Ziso H, Assif B, Hananel A, Vykhodtseva N, Gretton P, Pilatou M, Haker S, Tempany CM. Proc SPIE Photonics. 2009. MRI-guided Focused Ultrasound (MRgFUS) System for Thermal Ablation of Prostate Cancer: Pre-clinical Evaluation in Canines. Available from: http://www.spl.harvard.edu/publications/item/view/1726. [Google Scholar]


