Abstract
Background
Intravaginal cleansing may predispose women to adverse health outcomes and may interfere with the effectiveness and safety of female-initiated methods for preventing sexually transmitted infections (STIs). In a 4-week randomized study of 192 Malagasy sex workers, we evaluated associations between self-reported intravaginal cleansing and randomization assignment: diaphragm with viscous candidate microbicide gel (Acidform™, TOPCAD, Chicago, IL, licensed to Instead, Coppell, TX), diaphragm with placebo hydroxyethylcellulose gel (HEC, ReProtect LLC, Baltimore, MD), Acidform alone, or HEC alone.
Methods
Women were counseled to avoid intravaginal cleansing and were blinded to gel assignment. We evaluated changes in self-reported intravaginal cleansing across the study and assessed the effects of treatment assignment and covariates on frequent (more than once daily) intravaginal cleansing. Significant predictors in domain-specific models were evaluated in an all-domain multiple regression model.
Results
The proportion of women reporting intravaginal cleansing decreased from baseline (97%) to week 1 (82%) (p < 0.001). Self-reported frequent intravaginal cleansing decreased from baseline (87% to 56%) during the same time period (p < 0.001). In adjusted analyses, the Acidform-diaphragm group had 60% lower odds of frequent intravaginal cleansing during the study (odds ratio [OR] 0.4, 95% confidence interval [CI] 0.2-0.8) compared to the control group (HEC only). HEC-diaphragm and Acidform only users did not differ from controls. Living on the coast of Madagascar, not cohabiting, frequent intravaginal cleansing at enrollment, and high coital frequency predicted frequent intravaginal cleansing during follow-up.
Conclusions
Gel characteristics and the diaphragm's presence likely influenced women's cleansing. Viscous gel delivered by a cervical barrier (such as a diaphragm) may minimize the likelihood of frequent intravaginal cleansing.
Introduction
Intravaginal cleansing includes wiping the vagina with fingers or other objects or substances. It also includes douching, which is the pressurized application of water or another liquid into the vagina. Common motivations for cleansing include personal hygiene (e.g., cleaning after menstruation or sex), desire to meet the expectations of sexual partners, pregnancy prevention, and prevention or self-treatment of vaginal discharge or sexually transmitted infections (STI).1,2 Women who cleanse intravaginally may disrupt their vaginal environment in ways that predispose them to adverse health outcomes. Douching, for example, has been linked to pelvic inflammatory disease (PID), ectopic pregnancy, preterm birth, low birth weight, bacterial vaginosis (BV), candidiasis, and STIs.3–6 In addition to being a condition of concern in its own right, BV is a risk factor for preterm birth and low birth weight.3 BV may also place women at increased risk for HIV7 and herpes simplex virus type 2 (HSV-2) infection.8 It is important to note, however, that data about the effect of intravaginal cleansing on women's health are inconsistent and difficult to interpret.2,4 Indeed, some studies(e.g.,9) have suggested that particular forms of cleansing may be beneficial.
Until recently, studies of cervical barriers and microbicides for HIV/STI prevention have yielded discouraging results.10,11 The Methods for Improving Reproductive Health in Africa (MIRA) trial, a large-scale randomized controlled trial (RCT) of the diaphragm with lubricant gel for HIV prevention, did not demonstrate effectiveness against HIV12 or cervical STIs13 in intent-to-treat analyses. It has been hypothesized, however, that differential condom use between treatment arms and less than optimal adherence to diaphragm use may have biased results toward the null. Notably, per-protocol analyses of data from the MIRA study suggested an effect against Neisseria gonorrhoeae.13 Although the results of the MIRA study dampened enthusiasm for cervical barriers as methods of HIV/STI prevention, it and other studies demonstrated that the diaphragm may be acceptable in sub-Saharan Africa.14,15 In light of the debate about the meaning of the MIRA trial's results, additional trials of cervical barriers as methods for STI prevention may still be warranted. As for microbicides, early studies of non-oxynol-9 generated concerns about increased susceptibility to HIV infection.11 Two RCTs of the candidate microbicide cellulose sulfate gel were halted because of safety concerns in one of the studies.16,17 Another candidate microbicide, BufferGel, failed to show an effect against HIV in a large RCT, whereas PRO 2000 gel showed signs of effectiveness against HIV in the same trial.18 In 2010, CAPRISA 004 study investigators released groundbreaking results demonstrating the effectiveness of tenofovir gel against HIV acquisition.19
Intravaginal cleansing may interfere with the safety and effectiveness of female-initiated methods, such as microbicides2,20 and cervical barriers. Diaphragms and other cervical barriers may become dislodged as a result of cleansing, potentially allowing exposure of the cervix to semen and sexually transmitted pathogens during intercourse. Additionally, women who cleanse intravaginally may be less consistent in their use of cervical barriers compared to women who do not cleanse intravaginally.21 If a microbicide is even partially removed through vaginal cleansing, this may diminish its effect. Furthermore, substances inserted into the vagina for cleansing may interact with microbicides in detrimental ways.2
There has been substantial discussion of the importance of women's intravaginal practices in the context of microbicide use,2,22 and some studies have evaluated the relationship between intravaginal practices and the use of other female-initiated methods for STI prevention, such as the diaphragm.(e.g., 23) Nevertheless, there remains a need for information about the effect of intravaginal practices on the safety and efficacy of female-initiated methods for STI prevention and, conversely, the effect of these methods on intravaginal practices. Using data from a 4-week randomized, prospective study among female sex workers in Madagascar (a setting with a high prevalence of intravaginal cleansing24), we examined the effects of four treatment assignments, including a diaphragm with a candidate microbicide gel, on intravaginal cleansing.
Materials and Methods
Study design
From September 2005 to November 2005, we conducted a randomized pilot study in health clinics in four cities in Madagascar (Antananarivo, Antsiranana, Mahajanga, and Toamasina) to assess the feasibility and acceptability of conducting a large-scale RCT of the diaphragm with candidate microbicide for the prevention of N. gonorrhoeae and Chlamydia trachomatis infection.25 Enrollment criteria included being aged 15–55 years, not being pregnant and not intending to become pregnant in the next 2 months, reporting ≥4 sex partners in the past month, reporting <100% condom use in the past 2 weeks, not reporting an allergy to latex, and having no physical abnormalities precluding diaphragm use.
Each participant was randomly assigned to one of the following: (1) candidate microbicide gel (Acidform™, TOPCAD, Chicago, IL, licensed to Instead, Coppell, TX) applied once daily in the dome of the latex All-flex® Arcing Spring Diaphragm (Ortho-McNeil Pharmaceutical, Inc., Titusville, NJ), (2) inert placebo hydroxyethylcellulose gel (HEC) (ReProtect LLC, Baltimore, MD) applied once daily in the dome of the latex All-flex Arcing Spring Diaphragm, (3) Acidform gel applied intravaginally before every act of sex, and (4) HEC gel applied intravaginally before every act of sex (the control group). Acidform is a viscous candidate microbicide gel with acid-buffering and bioadhesive properties.26 Block randomization ensured an equal number of participants in each treatment group at each study site. Sequenced opaque envelopes containing treatment assignments were provided to the four sites. As each eligible participant was consented and enrolled, a study clinician at the site opened the next envelope in the sequence, revealing the participant's treatment assignment. The study was partially masked; neither participants nor study staff knew which gel each participant received.
Face-to-face structured interviews and pelvic examinations were conducted at enrollment and at 4 weekly follow-up visits. During pelvic examinations, participants were instructed not to cleanse their internal genitalia while enrolled in the study, and they were counseled accordingly. Participants were provided with sufficient supplies of condoms and counseled to use condoms for every act of sex. To minimize social desirability bias, we conducted face-to-face interviews before pelvic examinations and counseling, we trained interviewers to ask about intravaginal cleansing and other sensitive behaviors in a nonjudgmental fashion, and we provided counseling of similar duration and intensity to women in all study groups.
Procedures were approved by the ethics committee of the Ministry of Health in Antananarivo, Madagascar, and institutional review boards at the University of North Carolina at Chapel Hill, North Carolina, and the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia.
Measures
Although the main outcomes of this study were acceptability and adherence to use of study products during sex, we planned a priori to conduct an analysis of intravaginal cleansing during follow-up. Throughout follow-up, women were asked to report on their typical frequency of intravaginal cleansing in the past week during the structured face-to-face interview. Women who chose the response “not at all” were considered to have refrained from intravaginal cleansing. Our outcome variable for this analysis, frequent intravaginal cleansing, was defined as choosing the response “more than once daily.” We focused on frequent cleansing because there were sufficient numbers of women reporting this for a robust multivariable regression analysis; lack of variability in the data precluded a robust comparison of cleansers to noncleansers.
Our primary predictor variable was treatment assignment as randomized. Other variables were grouped into three domains: (1) study site and demographic factors, (2) sexual/reproductive factors, and (3) factors related to product use and interpersonal power/control. Demographic factors included age (quartile), cohabitation/marriage status (three categories), years of education (above/below the median), income from a source other than sex work (yes/no), ever received more money for not using a condom (yes/no), crowding in the home (three categories based on sharing of living quarters), and possession of household items considered indicative of higher socioeconomic status (SES) (electricity, television, refrigerator, cell phone, tap water, and flush toilet). Sexual/reproductive factors included coital frequency in the past week (tertile), sex with a main partner in the past week (yes/no), number of sex partners in a typical week (tertile), percentage of partners who are new in a typical week (quartile, with middle two quartiles combined), self-reported consistent condom use in the past week (100% vs. <100%), hormonal contraception at last sex act (yes/no), gravidity (quartile), having ever been told by a clinician that she had an STI (yes/no), clinician-assessed vaginal discharge at previous study visit, self-reported frequency of intravaginal cleansing at enrollment (quartile), and knowledge that douching cannot prevent pregnancy or STI (yes/no). Variables related to product use and interpersonal power/control included self-reported adherence to study product use during sex in the past week (100% vs. <100%), perceived control over condom use with clients (three categories representing increasing control), having ever refused a client because he would not use a condom (yes/no), any client having ever been violent because of a condom request (yes/no), and perceived importance of preventing main partners from learning of study products (three categories representing increasing importance). Variables assessed during follow-up (coital frequency, sex with a main partner, condom use, vaginal discharge, adherence to study product use, and perceived importance of main partners not learning of study products) were treated as time varying.
Statistical analyses
To assess bivariable associations, we entered each predictor variable into an unadjusted logistic regression model of frequent intravaginal cleansing. We used generalized estimating equations (GEE) to account for repeated measurements on the same participant. We then entered predictor variables into domain-specific logistic regression models (one for each domain). We conducted manual backward elimination on each domain-specific model until all remaining variables were significant at alpha of 0.20. Significant predictors in domain-specific models were evaluated in an all-domain multiple regression model. We again conducted manual backward elimination, this time at alpha of 0.05. Using domain-specific models to identify candidate variables for final models can be helpful when one wishes to limit the number of variables under consideration at any one time. This approach has been applied in various contexts.(e.g., 27)
We adjusted domain-specific models and all subsequent models for study visit (a proxy for change over time), treatment assignment, and site. Based on a priori theoretical considerations (e.g., the role that coital frequency could play in determining if women cleanse intravaginally), we also adjusted for coital frequency and adherence to study product use during sex. We tested all variables in our final main effects model for first-order interactions (at alpha = 0.05) between each predictor variable and the following independent variables: treatment assignment, site, and coital frequency.
Finally, to address the possibility that our findings about the effect of treatment assignment were driven by differential adherence, we conducted a sensitivity analysis in which we restricted our final main effects model to weeks of follow-up for which women reported consistent (100%) use of study products during sex. Because the regression model for our sensitivity analysis would not converge when we used GEE, we did not adjust for repeated measurements in that model. In all other regression models, however, we used GEE to account for repeated measurements on the same participants, and we specified an unstructured working correlation matrix. All analyses were conducted in SAS (SAS Institute, version 9.1.3, Cary, NC).
Results
Among 314 women, 192 (61%) met eligibility criteria for the pilot study and were enrolled. Women were most commonly found ineligible because they reported consistent condom use (37%), had <4 sexual partners (25%), were pregnant or planned to become pregnant (15%), or had an allergy to latex (11%). The analytic sample for the present analysis consisted of participants who provided data on intravaginal cleansing and use of their assigned study products. These 189 participants contributed 752 weeks of follow-up data (4 weeks of data were missing).
Baseline characteristics and differences between groups
Participant characteristics at enrollment are shown in Table 1. Relatively few (12%) were cohabiting with a husband or other partner. Less than half (41%) said they had a main sex partner (husband or boyfriend). About half (48%) said they always or almost always used condoms with clients. Median values for coital frequency and total number of partners in a typical week were 10 (interquartile range [IQR] 8.5) and 7 (IQR 5), respectively.
Table 1.
Participant Characteristics at Enrollment
| |
Treatment assignment |
|
|||
|---|---|---|---|---|---|
| Characteristic | Diaphragm with Acidform™ n (%) | Diaphragm with HEC n (%) | Acidform only n (%) | HEC only n (%) | pa |
| Age, years | |||||
| 36+ | 14 (31.1) | 11 (22.9) | 18 (37.5) | 14 (29.2) | 0.003 |
| 29–35 | 8 (17.8) | 7 (14.6) | 9 (18.8) | 9 (18.8) | – |
| 24–28 | 8 (17.8) | 15 (31.3) | 8 (16.7) | 14 (29.2) | – |
| <24 | 15 (33.3) | 15 (31.3) | 13 (27.1) | 11 (22.9) | – |
| Marital status | |||||
| Cohabitation/married | 6 (13.3) | 5 (10.4) | 5 (10.4) | 6 (12.5) | 0.04 |
| Divorced/separated/widowed | 21 (46.7) | 19 (39.6) | 18 (37.5) | 24 (50) | – |
| Never married | 18 (40) | 24 (50) | 25 (52.1) | 18 (37.5) | – |
| Education, years | |||||
| ≥5.5 | 24 (53.3) | 23 (47.9) | 25 (52.1) | 23 (47.9) | 0.71 |
| <5.5 | 21 (46.7) | 25 (52.1) | 23 (47.9) | 25 (52.1) | – |
| Household items | |||||
| No electricity | 20 (44.4) | 18 (37.5) | 19 (39.6) | 21 (43.8) | <0.001 |
| Electricity only | 3 (6.7) | 11 (22.9) | 10 (20.8) | 6 (12.5) | – |
| Electricity plus other itemb | 22 (48.9) | 19 (39.6) | 19 (39.6) | 21 (43.8) | – |
| Sex acts, typical weekc | |||||
| 15+ | 15 (33.3) | 11 (22.9) | 12 (25.5) | 9 (18.8) | <0.001 |
| 10–14 | 12 (26.7) | 12 (25.0) | 12 (25.5) | 13 (27.1) | – |
| 6–9 | 15 (33.3) | 17 (35.4) | 12 (25.5) | 13 (27.1) | – |
| <6 | 3 (6.7) | 8 (16.7) | 11 (23.4) | 13 (27.1) | – |
| Have main partner | |||||
| Yes | 18 (40) | 18 (37.5) | 17 (35.4) | 25 (52.1) | 0.004 |
| No | 27 (60) | 30 (62.5) | 31 (64.6) | 23 (47.9) | |
| Sex partners, typical weekd | |||||
| 10+ | 21 (46.7) | 15 (31.3) | 16 (33.3) | 16 (33.3) | 0.01 |
| 5–9 | 16 (35.6) | 21 (43.8) | 23 (47.9) | 20 (41.7) | – |
| ≤4 | 8 (17.8) | 12 (25.0) | 9 (18.8) | 12 (25.0) | – |
| Typical condom use with clients | |||||
| Always/almost always | 20 (44.4) | 19 (39.6) | 26 (54.2) | 25 (52.1) | 0.02 |
| Never/rarely/sometimes | 25 (55.6) | 29 (60.4) | 22 (45.8) | 23 (47.9) | – |
| Hormonal contraception at last sex | |||||
| Yes | 12 (26.7) | 10 (20.8) | 12 (25) | 10 (20.8) | 0.55 |
| No | 33 (73.3) | 38 (79.2) | 36 (75) | 38 (79.2) | – |
| Gravidity | |||||
| 4+ | 16 (35.6) | 15 (31.3) | 18 (37.5) | 17 (35.4) | 0.11 |
| 3 | 10 (22.2) | 6 (12.5) | 9 (18.8) | 11 (22.9) | – |
| 2 | 9 (20.0) | 12 (25.0) | 11 (22.9) | 9 (18.8) | – |
| 0–1 | 10 (22.2) | 15 (31.3) | 10 (20.8) | 11 (22.9) | – |
| Ever told by clinician that she had STI | |||||
| Yes | 16 (36.4) | 18 (37.5) | 22 (46.8) | 21 (44.7) | 0.14 |
| No | 28 (63.6) | 30 (62.5) | 25 (53.2) | 26 (55.3) | – |
| Intravaginal cleansing frequency, past week | |||||
| 28+ times | 11 (24.4) | 17 (35.4) | 17 (35.4) | 15 (31.3) | <0.001 |
| 21–27 times | 13 (28.9) | 11 (22.9) | 10 (20.8) | 16 (33.3) | – |
| 14–20 times | 14 (31.1) | 14 (29.2) | 9 (18.8) | 7 (14.6) | – |
| <14 times | 7 (15.6) | 6 (12.5) | 12 (25.0) | 10 (20.8) | – |
Due to rounding and missing data, not all categories sum to 100%.
p value for Cochran-Mantel-Haenszel test of general association.
Television, refrigerator, cell phone, tap water, or flush toilet.
Median 10; interquartile range 8.5.
Median 7; interquartile range 5.
HEC, hydroxyethylcellulose gel; STI, sexually transmitted infection.
Compared to other women, HEC only participants were most often divorced, separated, or widowed. They were also the most likely to have a main partner. Acidform-diaphragm participants most often had electricity plus other household items indicative of higher SES. They had higher sexual frequency and more partners than women in other groups. HEC-diaphragm participants tended to be younger. Women in the HEC only and Acidform only groups reported higher levels of condom use. At baseline, HEC-diaphragm and Acidform only participants were most often in the highest category of intravaginal cleansing.
Intravaginal cleansing at enrollment
At enrollment, the median number of acts of intravaginal cleansing in the past week was 21 (IQR 14). Of women who reported intravaginal cleansing in the past week (97%), nearly all (99%) reported the use of water. Soap (5%), disinfectant (2%), detergent (2%), permanganate (2%), alum (1%), and lemon (<1%) were less commonly used. Among women who reported inserting an object during cleansing (98% of those who cleansed), fingers (98%) and cloth (98%) were the objects most commonly used.
Changes in intravaginal cleansing during follow-up
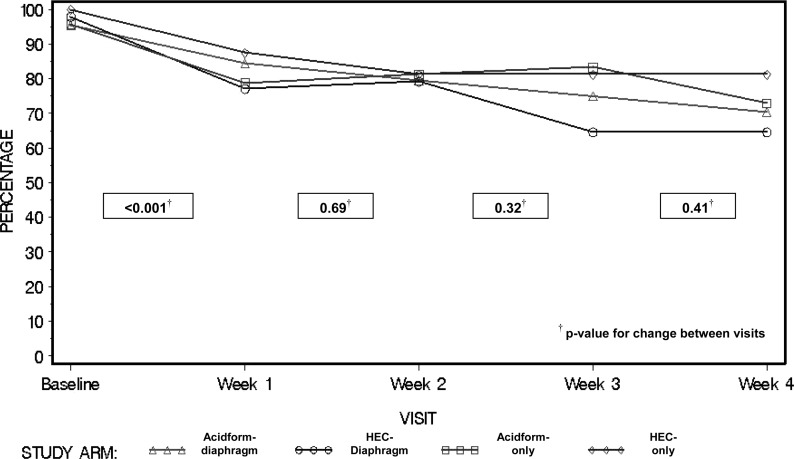
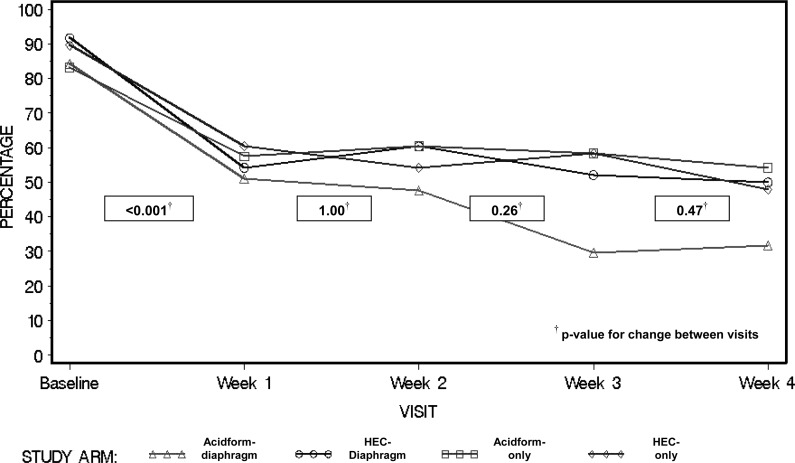
The percentage of women reporting any intravaginal cleansing dropped from 97% at enrollment to 82% at week 1 (p < 0.001) (Fig. 1). It continued to drop in the Acidform-diaphragm and HEC-diaphragm groups but not in other groups. At week 3, significantly fewer women in the two diaphragm groups reported intravaginal cleansing (compared to women who did not receive a diaphragm) (70% vs. 82%, p = 0.04). This difference did not achieve statistical significance at week 4 (67% vs. 77%, p = 0.14). Self-reported frequent (>once daily) intravaginal cleansing dropped from 87% at enrollment to 56% at week 1 (p < 0.001). The percentage reporting frequent cleansing then leveled off for three of the four treatment groups but continued to drop within the Acidform-diaphragm group (Fig. 2). Compared to others, Acidform-diaphragm participants less often reported frequent cleansing at week 3 (30% vs. 56%, p = 0.03) and week 4 (32% vs. 51%, p = 0.002).
FIG. 1.
Percentage of participants reporting any intravaginal cleansing at baseline and throughout follow-up. HEC, hydroxyethylcellulose gel.
FIG. 2.
Percentage of participants reporting frequent (>1 time daily) intravaginal cleansing at baseline and throughout follow-up.
Predictors of intravaginal cleansing during follow-up
Several variables achieved statistical significance in unadjusted models. Study site, the presence of electricity in the home (in the absence of other household items indicative of higher SES), coital frequency, number of partners in a typical week, percentage of partners who are new in a typical week, hormonal contraception use at last sex act, self-reported frequency of intravaginal cleansing at baseline, belief that douching can prevent pregnancy or STI, and violence from a client after a condom request were all positively associated with frequent intravaginal cleansing during the study. Educational attainment, cohabitation, crowding in the home, receipt of more money for sex without a condom, perceived control over condom use with clients, and refusal of sex without a condom were inversely associated with frequent intravaginal cleansing.
Although not significant in unadjusted analyses, treatment assignment was statistically significant in the final all-domain model. Assignment to the Acidform-diaphragm group was associated with 60% lower odds of reporting frequent intravaginal cleansing during follow-up (odds ratio [OR] 0.4, 95% confidence interval [CI] 0.2-0.8) compared to the HEC only group (Table 2). For HEC-diaphragm and Acidform only participants, the odds of frequent cleansing did not differ significantly from those of HEC only participants.
Table 2.
Bivariable and Multivariable Associations for Predictors of Frequent Intravaginal Cleansing During Follow-Up
| Characteristic | Visits at which women reported frequent cleansing n (%) | Bivariable OR (95% CI) | Multivariable OR (95% CI) |
|---|---|---|---|
| Treatment assignment | |||
| Diaphragm with Acidform™ | 71 (40.1) | 0.56 (0.30-1.05) | 0.37 (0.16-0.84) |
| Diaphragm with HEC | 104 (54.2) | 0.93 (0.49-1.79) | 0.97 (0.48-1.95) |
| Acidform only | 110 (57.6) | 1.08 (0.56-2.08) | 1.14 (0.60-2.17) |
| HEC only (referent) | 106 (55.2) | 1 | 1 |
| Site | |||
| Mahajanga | 160 (85.1) | 42.54 (19.30-93.77) | 37.3 (16.35-85.12) |
| Toamasina | 145 (75.5) | 22.63 (11.46-44.68) | 17.84 (8.02-39.69) |
| Antsiranana | 64 (35.6) | 4.10 (2.18-7.70) | 3.12 (1.50-6.45) |
| Antananarivo (referent) | 22 (11.5) | 1 | 1 |
| Cohabitation/marriage | |||
| Never married, noncohabiting | 210 (62.3) | 7.50 (3.34-16.84) | 3.41 (1.16-9.97) |
| Ever married, noncohabiting | 166 (50.8) | 4.93 (2.19-11.08) | 2.44 (0.88-6.76) |
| Cohabiting (referent) | 15 (17.1) | 1 | 1 |
| Coital frequency, past week | |||
| 18+ | 161 (63.6) | 1.69 (1.20-2.39) | 1.80 (1.10-2.96) |
| 11–17 | 132 (50.6) | 1.21 (0.88-1.66) | 1.35 (0.84-2.18) |
| <11 (referent) | 98 (41.2) | 1 | 1 |
| Intravaginal cleansing at enrollment, past week | |||
| 28+times | 146 (61.9) | 2.56 (1.27-5.17) | 2.52 (1.17-5.41) |
| 21–27 times | 105 (52.5) | 1.74 (0.85-3.56) | 1.93 (0.92-4.07) |
| 14–20 times | 82 (46.6) | 1.38 (0.66-2.88) | 1.73 (0.72-4.15) |
| <14 times (referent) | 58 (41.4) | 1 | 1 |
Generalized estimating equations (GEE) were used to account for repeated measurements on the same participant. Multivariable ORs and CIs reflect adjustment for all variables presented here plus study visit and adherence to study product use during sex.
CI, confidence interval; OR, odds ratio.
Other statistically significant variables in the final all-domain model included study site, marital/cohabitation status, coital frequency, and self-reported frequency of intravaginal cleansing at baseline. Living in Mahajanga was associated with nearly 40 times the odds of cleansing frequently compared with the reference category of living in Antananarivo. Residence in Toamasina and Antsiranana was associated with 18 times and 3 times the odds of frequent cleansing, respectively. Notably, the CIs for the estimated ORs for residence in Mahajanga and Toamasina were wide, indicating imprecision. Compared with women currently living with a partner, ever-married noncohabiting women had more than twice the odds of reporting frequent cleansing; never-married noncohabiting women had more than three times the odds of reporting frequent cleansing. Women in the highest category of coital frequency had nearly twice the odds of reporting frequent cleansing compared with those in the lowest category. Those in the highest category of intravaginal cleansing at baseline had 2.5 times the odds of reporting frequent cleansing during the study compared with those in the lowest category.
Neither consistent condom use nor adherence to study product use in the past week emerged as a predictor of frequent intravaginal cleansing. All tests for interaction were nonsignificant.
The sensitivity analysis relied on 321 weeks of follow-up data. Assignment to the Acidform-diaphragm group was significantly associated with 80% lower odds of reporting frequent intravaginal cleansing compared to the HEC only group (OR 0.2, 95% CI 0.1-0.7).
Discussion
In this randomized prospective study, we evaluated the effect of receipt of four product combinations on frequent intravaginal cleansing: the diaphragm with candidate microbicide gel (Acidform), the diaphragm with placebo gel (HEC), Acidform alone, and HEC alone. We found that women whose gel was viscous and delivered by a diaphragm (i.e., women in the Acidform-diaphragm group) were most successful in avoiding frequent intravaginal cleansing during the study. Other predictors of frequent cleansing included living in coastal areas of Madagascar (Mahajanga, Toamasina, and Antsiranana), reporting frequent intravaginal cleansing at enrollment, and reporting high coital frequency during the study; cohabiting with a male partner was inversely related to frequent cleansing.
Little has been published about intravaginal practices in Madagascar. A mixed-method study conducted among sex workers in Antananarivo, Toamasina, and Mahajanga revealed that participants generally cleansed intravaginally with their forefinger and water whenever they bathed and between clients.24 A qualitative study conducted at a public clinic in Antananarivo28 found that women considered intravaginal cleansing necessary to remove impurities before and after sex. Additionally, women believed that their male partners would be displeased if they failed to cleanse before sex. In the present study, anecdotal reports from the field revealed that some women found the added moisture from study gels uncomfortable and that some were concerned that men would confuse the gels with ejaculate from previous partners.
We consider it likely that the presence of a diaphragm and the physical characteristics of the gels influenced women's choices about intravaginal cleansing. Gels may have been sensed as moisture or wetness, and vaginal cleansing may have been motivated by the volume of gel that was present; that is, as more gel accumulated in the vagina, women may have experienced a greater compulsion to cleanse despite having been instructed not to do so. In the two diaphragm-gel groups, there would have been comparatively less gel in the vagina, and we would therefore expect less cleansing. Nevertheless, we observed lower levels of cleansing in the Acidform-diaphragm group but not in the HEC-diaphragm group. HEC is less viscous than Acidform, and it may have been more likely to leak around the edges of the diaphragm. Thus, it is possible that women in the HEC-diaphragm group were exposed to more gel on the exterior (noncervix) side of the diaphragm and more often sensed an unacceptable degree of moisture or wetness compared to women in the Acidform-diaphragm group. In summary, women in the Acidform-diaphragm group may have been least likely to reach the point where an unacceptable amount of gel had accumulated, and this may explain why Acidform-diaphragm participants had the lowest odds of frequent intravaginal cleansing during the study.
We considered alternative explanations for our finding that receipt of a diaphragm with Acidform was inversely associated with frequent intravaginal cleansing. These included differential adherence, differential condom use, and differing expectations of product efficacy. As we reported previously,25 the Acidform-diaphragm group reported somewhat higher levels of study product use during sex (compared to the control group of HEC alone). Nevertheless, the results of our sensitivity analysis do not support differential adherence as an explanation for our findings. The sensitivity analysis replicated the final model of intravaginal cleansing but included only weeks of follow-up during which women reported 100% adherence to study regimens. Results were similar to those of the main analysis: when self-reported adherence was optimal, the inverse association between receipt of diaphragm with Acidform and frequent cleansing remained.
The literature suggests an inverse association between condom use and intravaginal cleansing.21,29 Although all groups received equivalent counseling about condom use, we previously reported that self-reported condom use was lowest in the Acidform-diaphragm group (62% of acts vs. the overall average of 66%).25 This would lead us to expect comparatively more cleansing in the Acidform-diaphragm group. Thus, differential condom use is an unlikely explanation for our findings.
If study participants believed that intravaginal cleansing protects women from STI or pregnancy and if expectations of product efficacy varied by study group, this could have caused differences between groups in intravaginal cleansing. It is unlikely that the type of gel received led to differing expectations of efficacy. Participants were blinded to whether they received active or placebo gel. Although some women presumably noticed that their gel was thicker or thinner than the alternative, we have no reason to believe that women knew whether they had received active or placebo gel. Women in diaphragm arms may have felt more protected than women who received a gel alone. Nevertheless, this increased sense of protection would have led to lower levels of cleansing in both diaphragm groups, not just the Acidform-diaphragm group.
As for other predictors of frequent cleansing, coastal and inland regions of Madagascar have different cultural norms surrounding intravaginal cleansing; intravaginal cleansing is more strongly encouraged on the coast. Additionally, coastal areas are less temperate, and the heat and resulting perspiration may increase women's desires to cleanse. Women who cleansed frequently before enrolling in the study may have found it particularly difficult to avoid frequent cleansing during the study. Our finding that women with the highest coital frequency cleansed most frequently is consistent with other research in this population, which found that many women cleanse intravaginally between clients.24 Cohabiting with a husband or other sexual partner may afford less privacy for intravaginal cleansing compared to not sharing living space with a partner.
Limitations of this study include small sample size, relatively short follow-up, reliance on self-reported data, and apparent imbalance on baseline characteristics. Despite the limited statistical power that our small sample size afforded, we identified a significant effect of receiving the diaphragm with Acidform on frequent cleansing. Although we could not evaluate the long-term effect of treatment assignment on cleansing, 4 weeks may have been sufficient for participants to become accustomed to their study products and adapt their intravaginal cleansing habits accordingly. Reliance on self-reported data, which can be subject to recall and social desirability biases, is another limitation. Social desirability is a plausible explanation for the large overall drops in self-reported intravaginal cleansing from enrollment to the first follow-up visit. However, we would not expect differences between groups in either recall or the social desirability of reporting intravaginal cleansing during the study, and, thus, we do not consider our main findings about predictors of frequent cleansing to have been biased by social desirability. Although some may consider baseline differences among treatment groups to be a limitation of the study, we would argue that it is unrealistic to expect balance every time random selection is implemented30 and that we have likely achieved better overall balance than we would have with nonrandom selection.
Strengths of this study include the longitudinal design and, despite some apparent imbalances among treatment groups, randomization of study participants. The longitudinal design allowed us to account for temporality (e.g., by adjusting regression models for change over time). By randomizing participants, we reduced the likelihood of substantial confounding by women's measured or unmeasured baseline characteristics.
Conclusions
Concerns have been raised about the potential for harmful effects of intravaginal practices on the safety and effectiveness of vaginal microbicides and other methods that are used intravaginally, such as the diaphragm. Should there be an adverse effect of frequent intravaginal cleansing on microbicide effectiveness or safety, the mode of microbicide delivery may influence the likelihood of frequent cleansing and, therefore, influence the likelihood of harm. Identifying modes of delivery that make abstaining from cleansing more acceptable may lead to better outcomes for women. Our results suggest that in a population in which intravaginal cleansing is prevalent and at least partially motivated by the desire to regulate the amount of material in the vagina, women may be more successful in avoiding frequent intravaginal cleansing if a gel (e.g., a microbicide) is viscous and delivered by a cervical barrier.
More research is needed on the effects of female-initiated methods of STI prevention on intravaginal practices. Additionally, more research is needed on the impact of intravaginal practices on the safety and efficacy of these methods. Counseling women not to engage in intravaginal cleansing is a common strategy for preventing cleansing in research studies of new methods. However, the degree to which counseling and educational messages can modify women's vaginal cleansing habits is uncertain20–22 and requires further study.31 Research studies addressing the safety and effectiveness of methods that are used intravaginally should include subanalyses among women who, despite counseling, cleanse intravaginally. Without a better understanding of the safety and efficacy of methods that are used intravaginally, we may find ourselves unable to recommend these methods to women who cleanse but, nevertheless, are at risk of unintended pregnancy and STIs.
Acknowledgments
This study was funded by the U.S. Centers for Disease Control and Prevention through an interagency agreement with the U.S. Agency for International Development and CONRAD. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Disclosure Statement
No competing financial interests exist.
References
- 1.Cottrell BH. Vaginal douching. J Obstet Gynecol Neonatal Nurs. 2003;32:12–18. doi: 10.1177/0884217502239796. [DOI] [PubMed] [Google Scholar]
- 2.Hilber AM. Chersich MF. van de Wijgert JH, et al. Vaginal practices, microbicides and HIV: What do we need to know? Sex Transm Infect. 2007;83:505–508. doi: 10.1136/sti.2007.028597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martino JL. Vermund SH. Vaginal douching: Evidence for risks or benefits to women's health. Epidemiol Rev. 2002;24:109–124. doi: 10.1093/epirev/mxf004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myer L. Kuhn L. Stein ZA, et al. Intravaginal practices, bacterial vaginosis, and women's susceptibility to HIV infection: Epidemiological evidence and biological mechanisms. Lancet Infect Dis. 2005;5:786–794. doi: 10.1016/S1473-3099(05)70298-X. [DOI] [PubMed] [Google Scholar]
- 5.Simpson T. Merchant J. Grimley DM, et al. Vaginal douching among adolescent and young women: More challenges than progress. J Pediatr Adolesc Gynecol. 2004;17:249–255. doi: 10.1016/j.jpag.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Ness RB. Hillier SL. Richter HE, et al. Douching in relation to bacterial vaginosis, lactobacilli, and facultative bacteria in the vagina. Obstet Gynecol. 2002;100:765. doi: 10.1016/s0029-7844(02)02184-1. [DOI] [PubMed] [Google Scholar]
- 7.St John E. Mares D. Spear GT. Bacterial vaginosis and host immunity. Curr HIV/AIDS Rep. 2007;4:22–28. doi: 10.1007/s11904-007-0004-y. [DOI] [PubMed] [Google Scholar]
- 8.Cherpes TL. Meyn LA. Krohn MA, et al. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319–325. doi: 10.1086/375819. [DOI] [PubMed] [Google Scholar]
- 9.Gresenguet G. Kreiss JK. Chapko MK, et al. HIV infection and vaginal douching in central Africa. AIDS. 1997;11:101–106. doi: 10.1097/00002030-199701000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Padian NS. McCoy SI. Balkus JE, et al. Weighing the gold in the gold standard: Challenges in HIV prevention research. AIDS. 2010;24:621–635. doi: 10.1097/QAD.0b013e328337798a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wetmore CM. Manhart LE. Wasserheit JN. Randomized controlled trials of interventions to prevent sexually transmitted infections: Learning from the past to plan for the future. Epidemiol Rev. 2010;32:121–136. doi: 10.1093/epirev/mxq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padian NS. van der Straten A. Ramjee G, et al. Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: A randomised controlled trial. Lancet. 2007;370:251–261. doi: 10.1016/S0140-6736(07)60950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramjee G. van der Straten A. Chipato T, et al. The diaphragm and lubricant gel for prevention of cervical sexually transmitted infections: Results of a randomized controlled trial. PLoS One. 2008;3:e3488. doi: 10.1371/journal.pone.0003488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montgomery ET. Cheng H. van der Straten A, et al. Acceptability and use of the diaphragm and Replens lubricant gel for HIV prevention in Southern Africa. AIDS Behav. 2010;14:629–638. doi: 10.1007/s10461-009-9609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bukusi EA. Gallo MF. Sharma A, et al. Adherence to diaphragm use for infection prevention: A prospective study of female sex workers in Kenya. Infect Dis Obstet Gynecol. 2009;2009:420196. doi: 10.1155/2009/420196. Epub March 7, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Damme L. Govinden R. Mirembe FM, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359:463–472. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 17.Halpern V. Ogunsola F. Obunge O, et al. Effectiveness of cellulose sulfate vaginal gel for the prevention of HIV infection: Results of a phase III trial in Nigeria. PLoS One. 2008;3:e3784. doi: 10.1371/journal.pone.0003784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karim SA. Coletti A. Richardson BA Safety and effectiveness of vaginal microbicides BufferGel and PRO 2000 gel for the prevention of HIV infection in women. Presented at the 16th Conference on Retroviruses and Opportunistic Infections (CROI); Montreal, Canada. Feb 8–11;2009 . [Google Scholar]
- 19.Abdool Karim Q. Abdool Karim SS for CAPRISA 004 Trial Group. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koblin BA. Mayer K. Mwatha A, et al. Douching practices among women at high risk of HIV infection in the United States: Implications for microbicide testing and use. Sex Transm Dis. 2002;29:406–410. doi: 10.1097/00007435-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 21.van der Straten A. Cheng H. Chidanyika A, et al. Vaginal practices and associations with barrier methods and gel use among sub-Saharan African women enrolled in an HIV prevention trial. AIDS Behav. 2010;14:590–599. doi: 10.1007/s10461-010-9690-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braunstein S. van de Wijgert J. Preferences and practices related to vaginal lubrication: Implications for microbicide acceptability and clinical testing. J Womens Health. 2005;14:424–433. doi: 10.1089/jwh.2005.14.424. [DOI] [PubMed] [Google Scholar]
- 23.Gallo MF. Sharma A. Bukusi EA, et al. Intravaginal practices among female sex workers in Kibera, Kenya. Sex Transm Infect. 2010;86:318–322. doi: 10.1136/sti.2009.040345. [DOI] [PubMed] [Google Scholar]
- 24.Behets FM. Van Damme K. Turner AN, et al. Evidence-based planning of a randomized controlled trial on diaphragm use for prevention of sexually transmitted infections. Sex Transm Dis. 2008;35:238–242. doi: 10.1097/OLQ.0b013e31815abaa2. [DOI] [PubMed] [Google Scholar]
- 25.Behets FM. Turner AN for Mad STI Prevention Group. Vaginal microbicide and diaphragm use for sexually transmitted infection prevention: A randomized acceptability and feasibility study among high-risk women in Madagascar. Sex Transm Dis. 2008;35:818–826. doi: 10.1097/OLQ.0b013e318175d8ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garg S. Anderson RA. Chany CJ, et al. Properties of a new acid-buffering bioadhesive vaginal formulation (Acidform) Contraception. 2001;64:67–75. doi: 10.1016/s0010-7824(01)00217-7. [DOI] [PubMed] [Google Scholar]
- 27.Al Habashneh R. Guthmiller JM. Levy S, et al. Factors related to utilization of dental services during pregnancy. J Clin Periodontol. 2005;32:815–821. doi: 10.1111/j.1600-051X.2005.00739.x. [DOI] [PubMed] [Google Scholar]
- 28.Legardy-Williams J. Bell AJ. Jamieson DJ, et al. Attitudes and beliefs about vaginal cleansing among women, men and healthcare providers in Antananarivo, Madagascar; Presented at the Microbicides 2006 Conference; Cape Town, South Africa. Apr 23–26;2006 . [Google Scholar]
- 29.Scorgie F. Kunene B. Smit JA, et al. In search of sexual pleasure and fidelity: Vaginal practices in KwaZulu-Natal, South Africa. Cult Health Sex. 2009;11:267–283. doi: 10.1080/13691050802395915. [DOI] [PubMed] [Google Scholar]
- 30.Senn S. Testing for baseline balance in clinical trials. Stat Med. 1994;13:1715–1726. doi: 10.1002/sim.4780131703. [DOI] [PubMed] [Google Scholar]
- 31.McClelland RS. Ndinya-Achola JO. Baeten JM. Re: distinguishing the temporal association between women's intravaginal practices and risk of human immunodeficiency virus infection: A prospective study of South African women [Letter] Am J Epidemiol. 2007;165:474–475. doi: 10.1093/aje/kwk101. [DOI] [PubMed] [Google Scholar]