Abstract
Since their initial discovery over a century ago, our knowledge of the functions of myoglobin and the mitochondrion has gradually evolved. The mitochondrion, once thought to be solely responsible for energy production, is now known to be an integral redox and apoptotic signal tranducer within the cell. Likewise, myoglobin, traditionally thought of only as an oxygen store, has emerged as a physiological catalyst that can modulate reactive oxygen species levels, facilitate oxygen diffusion and scavenge or generate nitric oxide (NO) depending on oxygen tensions within the cell. By virtue of its unique ability to regulate O2 and NO levels within the cell, myoglobin can modulate mitochondrial function in energy-demanding tissues such as the beating heart and exercising muscle. In this review, we present the conventional functions of myoglobin and mitochondria, and describe how these roles have been reassessed and advanced, particularly in the context of NO and nitrite signaling. We present the mechanisms by which mitochondria and myoglobin regulate one another within the cell through their interactions with NO and oxygen and discuss the implications of these interactions in terms of health and disease.
Keywords: cytochrome c oxidase, nitrite, nitrite reductase, facilitated diffusion, hypoxia
INTRODUCTION
The first true spectroscopic description of myoglobin was in 1897 by Mörner, who suggested the term “myochrome” for this muscle pigment [1] Shortly thereafter, Carl Benda (in 1898) coined the term “mitochondria” to describe the threadlike granules that had been observed by many scientists in cells for decades prior [2]. Though myoglobin and mitochondria were discovered in such close chronology, these distinct cellular structures were studied predominantly in isolation from one another for several decades. However, in the late-1900s, with the discovery that mitochondria mediate oxidative phosphorylation and that myoglobin binds diatomic oxygen, it was recognized that myoglobin and mitochondria potentially interact and form a functional metabolome for efficient oxygen utilization in the heart and muscle. In the last 30 years, our understanding of the traditional function of both the mitochondrion and myoglobin has greatly evolved and new physiological roles have been uncovered for both these cellular components. It is now recognized that mitochondria are not only energy producers but also vital mediators of redox and apoptotic signaling within the cell. Likewise, myoglobin is now known not only to be an oxygen storage protein, but has also been described to be a nitric oxide (NO) scavenger and most recently, a hypoxic nitrite reductase [3; 4]. The expansion of the roles of mitochondria and myoglobin has led to the re-evaluation of the physiological interactions that exist between these two sub-cellular entities. In this review we examine the traditional functional roles of mitochondria and myoglobin and focus on the role of myoglobin in regulating mitochondrial function, particularly in the context of NO and nitrite signaling.
The mitochondrial respiratory chain maintains cellular homeostasis
While mitochondria were recognized by their structure as early as 1857, their function remained elusive until much later. It was only in the early 1900s through the combined work of Warburg and Keilin that oxygen utilization in the cell was linked to the reduction of mitochondrial cytochromes. This sparked a line of discovery that led to the isolation of ATP, the description of oxidative phosphorylation within the mitochondrion, and ultimately culminated in Peter Mitchell’s description of the chemiosmotic theory in 1961[5]. Collectively, these findings describe the fundamental function of the mitochondrion by which complexes I and II, embedded within the inner mitochondrial membrane, derive electrons from NADH and FADH2 and transfer these electrons down the respiratory chain and on to reduce oxygen, which binds to complex IV (cytochrome c oxidase). In response to electron transfer, protons are pumped by the complexes from the matrix to the intermembrane space, creating an electrochemical gradient (ΔμH+)[6]. This gradient drives the re-entry of protons into the matrix through the ATP synthase (Complex V), which is linked to the conversion of ADP to ATP. In this way, the proton gradient drives the synthesis of ATP by ATP synthase and couples oxygen consumption to ATP production. It is now known that this energy producing function of the mitochondrion is essential to the viability of most cells in the body.
In addition to ATP production, mitochondria play a role in maintaining cellular redox environment and contributing to reactive oxygen species (ROS) signaling. This is due to the fact that respiration is not fully coupled to ATP production, and it is estimated that about 2% of cellular mitochondrial oxygen consumption generates superoxide rather than ATP under basal conditions [7]. Indeed, electrons can prematurely leak at complexes I and III, combining with oxygen to form superoxide, which is then converted to hydrogen peroxide (H2O2) by manganese superoxide dismutase in the matrix, and copper/zinc superoxide dismutase in the intermembrane space and cytosol [8]. Although in pathology, high levels of mitochondrial ROS can be deleterious, low physiological levels have been shown to play a significant role in signaling mechanisms that regulate cell growth and proliferation, apoptosis and inflammatory responses [9; 10; 11] [9; 10; 12; 13]. ROS act as regulatory mediators in a number of signaling cascades such as the insulin receptor kinase activity [9], protein kinase C [14], the activation of MAPK cascades [15], and the activation of transcription factors AP-1 and NF-kB [15]. ROS signaling has also been shown to play a significant role in protection of the heart from ischemia/reperfusion [9]. Transient generation of ROS during brief preconditioning ischemia triggers of protection by activating protein kinases such as PKC, p38 and/or JAK/STAT [16; 17;18].
The energetic capacity of the respiratory chain is also linked to cellular viability as the release of the respiratory protein cytochrome c is a critical step in the initiation of the apoptotic pathway. This function also relates to superoxide generated by the respiratory chain, as ROS may oxidize the anionic phospholipid cardiolipin, which anchors cytochrome c to the inner membrane through electrostatic, hydrophobic and hydrogen bonds. Peroxidation of cardiolipin inhibits binding to cytochrome c, which then remains freely soluble in the intermembrane space, and is likely to be released into the cytosol, initiating the apoptotic cascade [19]. Beyond ATP generation, redox signaling and regulation of apoptosis at the level of the respiratory chain, mitochondrial matrix enzymes play a crucial role in heme and steroid biosynthesis [20], iron transport [21], and cell cycle regulation [22].
Regulation of mitochondrial function
Mitochondrial respiratory chain function, particularly oxidative phosphorylation, is regulated at several levels. Mitochondrial number is altered rapidly in the cell to adapt to changing metabolic demand. This process is governed by the PPARγ coactivator 1α (PGC1α), which is found abundantly in tissues rich in mitochondria, and regulates the transcription of both nuclear and mitochondrial DNA necessary to synthesize new mitochondrial proteins [23]. Physiologically, NO is a potent activator of PGC1α and mediates mitochondrial biogenesis instigated by caloric restriction and chronic hypoxia. In addition to the expression of new mitochondrial proteins, existing mitochondria within a cell interact with one another, leading to the dynamic formation and breakage of mitochondrial networks by fission and fusion events respectively. These mitochondria dynamics are regulated by a number of proteins, the most prominent of which are dynamin-related protein 1 (Drp1) and the mitofusins. While Drp1 mediates mitochondrial fission, an event that makes the mitochondrion more susceptible to degradation, phosphorylation of this protein by stimuli such as calcium or NO inhibits fission, rendering the mitochondria more resilient to apoptotic mechanisms [24]. Conversely, expression of mitofusin 1 and 2 modulate fusion and upregulation of these proteins results in the formation of long mitochondrial networks within the cell that have been observed to increase mitochondrial membrane potential and increase respiratory chain activity leading to a number of physiological consequences including modulation of the cell cycle [25], adaptation to oxidative stress [26] and protection against insults such as ischemia/reperfusion injury [27].
Perhaps the most characterized regulatory mechanism of mitochondrial ETC function is the post-translational modification of the respiratory chain complexes. The mitochondrial complexes have been shown to be a target of phosphorylation, acetylation and glutathionylation [28]. For example, phosphorylation of complex IV protects the protein against ATP-induced inhibition of activity. Glutathionylation of subunits of mitochondrial complex I [29] and complex IV [30], decreases the activity of these enzymes and has been postulated to protect them from oxidative damage. Several complexes within the ETC are known to be inhibited by acetylation and the biological implications of this modification has been an active area of research after the discovery of the specific mitochondrial deacetylases, sirtuins 3, 4 and 5, which are regulated by changes in metabolism [31].
The most well characterized interaction of NO with the mitochondrion is the inhibition of cytochrome c oxidase (complex IV) by nanomolar concentrations of NO. NO directly binds to the binuclear center (CuB/heme a3) of the enzyme, resulting in a reversible nitrosylation of the hemea3 and an inhibition of oxygen binding to the enzyme [32]. This competition between NO and oxygen at cytochrome c oxidase results in an inhibition of respiration that is more potent as oxygen tension is decreased [33; 34]. This NO-dependent inhibition of respiration has been suggested to regulate tissue oxygen gradients [35], mediate HIF-1a stabilization [36], and underlie the phenomenon of cardiac hibernation during ischemia/reperfusion [37] and will be discussed more extensively in the context of myoglobin signaling below.
Higher concentrations of NO, leading to the formation of metabolites, such as peroxynitrite, nitrogen dioxide (NO2•) or dinitrogen trioxide (N2O3), have been shown to S-nitrosate critical thiols on complex I, inhibiting its activity [38]. While a persistent S-nitrosation of complex I could lead to detrimental inhibition of oxidative phosphorylation, this modification has been shown to be protective in situations such as reperfusion after organ ischemia, in which gradual entry of electrons into the respiratory chain prevents excessive ROS generation and oxidative damage of mitochondrial proteins and lipids [39].
Regulation of oxidative phosphorylation by oxygen
The requirement of oxygen as a substrate for cytochrome c oxidase suggests that mitochondrial respiration and hence oxidative phosphorylation should be oxygen sensitive. However, much debate surrounds this issue. While respiration is known to be independent of oxygen concentration over a wide range of oxygen tensions (>15μM), it is still unclear whether mitochondria become oxygen limited physiologically in vivo, particularly in more hypoxic, metabolically active tissues such as the heart and skeletal muscle. The KM for oxygen of purified cytochrome c oxidase has been reported to be between 0.3-1 μM depending on the source of the enzyme [40; 41]. However, a larger oxygen range (0.05 – 5.0 μM) has been reported for the half-maximal saturation of respiration rate (P50) in isolated mitochondria or cells, which is likely due to oxygen gradients within the cell. The matter of oxygen limitation is further complicated by the fact that the P50 of respiration varies by cell type as well as in different physiological conditions. For example, changes in respiratory state have been shown to alter P50 of respiration in vitro [42]. More recently, Schumacker and colleagues have shown that exposure of cytochrome c oxidase to brief periods of hypoxia increase the KM of the enzyme[43]. Moreover, the apparent KM of the enzyme is also affected by modulators such as NO[44]. Due to the high affinity of cytochrome c oxidase for NO versus O2 and the competition between the two molecules, nanomolar levels of NO can significantly raise the apparent KM of the enzyme to oxygen concentrations as high as 5μM [45].
Despite the uncertainty of an absolute oxygen concentration at which it occurs, it is clear that mitochondria become oxygen limited only at relatively low oxygen tensions (<3 μM). Studies investigating mitochondrial respiratory rates during physiological conditions suggest that despite low cytoplasmic levels of oxygen (~3μM) in myocytes, mitochondria are not oxygen limited. However, respiratory capacity can rapidly change by 20-fold in response to increased demand, suggesting that in exercising skeletal muscle or the beating heart, where oxygen supply is constant, metabolic function can quickly exceed oxygen supply and become oxygen limited [46]. It is under such conditions that the role of myoglobin (P50 = 3.1μM), an oxygen store and facilitator of diffusion, becomes necessary.
Myoglobin: discovery, structure and localization
The initial identification of myoglobin was made by Mörner, who distinguished myoglobin from hemoglobin by their differences in absorption spectra and named the protein “myochrome”. The term “myoglobin” was coined by Gunther and colleagues in 1921, who confirmed that myoglobin was indeed distinct from hemoglobin in the blood. Using high resolution X-ray crystallography, John Kendrew was the first to resolve the structure of myoglobin in 1959, making it the first protein described at the atomic level. The protein itself is a single polypeptide chain consisting of approximately 153 amino acids that form eight α-helices folded around a hydrophobic core containing the heme prosthetic group [47]. The heme prosthetic group consists of a protoporphyrin ring bound to a central iron atom by four nitrogen atoms and the proximal histidine (His 93) at the fifth coordination site. Small molecule ligands, such as oxygen, NO and carbon monoxide (CO), bind myoglobin at its sixth coordination site. Also at this site lies the distal histidine (His 64), which is not directly bound to the iron but perfectly poised for hydrogen bonding to modulate the affinity of ligands for myoglobin [48]. The specific and conserved folding of the protein is such that the outer surface of myoglobin is predominated by hydrophilic residues, allowing myoglobin molecules in solution to move past one another with little frictional impediment. This property is integral to the functional role of myoglobin as a facilitator of oxygen diffusion within cells [49; 50].
Myoglobin is present in the heart and the skeletal muscle of virtually all mammals. In the heart, the basal myoglobin concentration is approximately 200 to 300 μmol kg−1 wet weight, while in the skeletal muscle levels are much higher reaching approximately 400–500 μmol kg−1 wet weight [50]. The concentration of myoglobin in these tissues is closely associated with the work performed by the tissue and also correlates with cytochrome c oxidase expression as well as capillary density [51; 52]. Myoglobin expression is regulated in these tissues and has been shown to increase in people living at high altitude. Surprisingly, to date, no increase in myoglobin content has been proven with exercise training or chronic hypoxia alone, suggesting a more complicated regulation of myoglobin gene expression. Interestingly, NO increases gene and protein expression of myoglobin [53].
Recent studies suggest that myoglobin expression is not limited to endurance muscle. Physiologically, small concentrations of myoglobin are expressed in smooth muscle cells[54]. Additionally, significant levels of myoglobin have been measured in tumor cells even of non-muscle origin [55]. Studies indicate that this myoglobin expression may enhance tumor oxygenation as well as inactivate ROS within the tumor, leading to growth and invasion [56]. However, these studies are preliminary and the role of the protein in tumorogenesis is still under investigation.
Myoglobin function
Initial work investigating the function of myoglobin established that oxygen reversibly binds ferrous myoglobin at the sixth coordination position of its heme iron and shows a hyperbolic oxygen dissociation curve. The P50 of myoglobin is 3.1μM, demonstrating the high affinity of myoglobin for oxygen [50]. In both cardiac and striated muscle, the cytosolic pool of myoglobin is maintained between 35 and 50% saturated with oxygen. Based on the high affinity of myoglobin for oxygen and its presence in metabolically active endurance muscles, Millikan laid the foundations for identifying the physiological role of myoglobin. In 1939, he hypothesized three potential functions for myoglobin, suggesting that the protein worked as 1) an oxygen store, 2) an oxygen transporter, or 3) “an intracellular catalyst” [57]. While Millikan concluded in his seminal work that myoglobin acted mainly as “a short term oxygen store, tiding muscle over from one contraction to the next”, evidence for all three putative functions has emerged over the last 70 years and will be reviewed below.
Oxygen storage
The most accepted function of myoglobin is that of a temporary store of oxygen. Studies of working skeletal muscle show that upon the onset of muscle contraction, the cytosolic pool of myoglobin reaches a new deoxygenated steady state within a matter of seconds (20-40s depending on workload) suggesting that oxygen is released for mitochondrial consumption during contraction [58]. At high altitude, myoglobin concentrations increase to augment the reservoir of oxygen in the heart and muscle [59] . Similarly, in marine mammals, myoglobin concentrations approach 10-fold higher levels than in terrestrial mammals and directly affects the capacity of these animals to dive to deeper depths of the sea. In these animals, myoglobin supplies oxygen during periods when ventilation is absent. Thus, the ability to remain under water directly correlates to the expression level of myoglobin in aquatic mammals [60].
Facilitated Diffusion
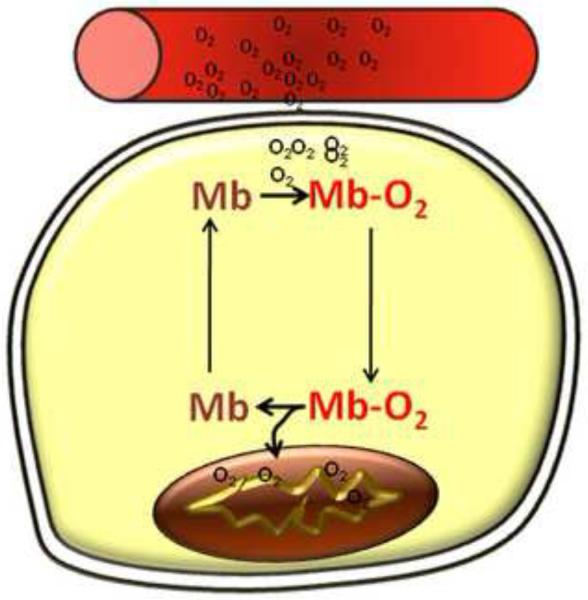
The contribution of myoglobin to mediating facilitated diffusion in vivo is perhaps the most controversial function of the protein. The first evidence of myoglobin-mediated facilitated diffusion was reported by Wittenberg in 1959 in which he proposed that oxygen diffusion was enhanced within the cell, from the sarcolemma to the mitochondrion, by the ability of myoglobin to rapidly and reversibly bind oxygen and translationally diffuse within the cell [61]. Subsequent studies demonstrated the high affinity of myoglobin to bind oxygen could compete with the ability of oxygen to dissolve in solution [62]. Thus, myoglobin is able to capture oxygen as it crosses phases from the capillary to cell and transport it within the cell, offloading the oxygen as it tries to come to equilibrium with the deoxygenated myoglobin pool within the cell. This results in the oxygen gradient from the capillary to cell becomes steeper, leading to increased oxygen flux within the cell. This idea is further supported by data showing that a gradient of oxygenated myoglobin exists in the isolated cardiomyocyte in which maximal oxygenation occurs at the cell membrane and decreases significantly towards the interior of the cell where respiring mitochondria are located [63] (Figure 1).
Figure 1. Myoglobin facilitates oxygen diffusion.
Capillaries deliver oxygen to the myocyte, where myoglobin binds oxygen at the sarcolemmal membrane. Myoglobin diffuses through the cell and releases oxygen at the mitochondrion, where oxygen concentration is low.
Despite these studies demonstrating facilitated diffusion, the role of myoglobin as a regulator of mitochondrial oxygen supply has remained controversial. In 1982, Cole et al. reported that under steady-states of oxygen supply, myoglobin increased the flow of oxygen to myocytes, but had no effect on oxygen consumption and ATP production rate in rat skeletal muscle mitochondria casting a doubt on myoglobin’s effect on mitochondrial respiration [64]. Additionally, the first myoglobin knockout mouse line generated suggested that animals without myoglobin were fertile, exhibiting no significant differences from wild type mice at rest, during endurance exercise, or in response to hypoxia [65]. However, subsequent studies by Godecke et al. on an independent myoglobin knockout line revealed that the deletion of myoglobin led to compensatory mechanisms, resulting in a steepened oxygen gradient between the capillary and mitochondria. These compensations include increased capillary density, augmented coronary flow and elevated hematocrit [66]. Additionally, studies in other systems showed a role for myoglobin in regulating respiration. For example, hypoxic pigeon muscle fibers were shown to increase their rate of oxidative phosphorylation significantly in the presence of myoglobin [67] and in working skeletal muscle from dogs, the maintenance of the rate of oxygen consumption was dependent on the presence of myoglobin [68]. Pharmacological means to inhibit myoglobin’s actions in cells also showed that myoglobin-oxygen binding regulates oxidative phosphorylation [67; 69; 70; 71]. While these experiments have collectively led to the general acceptance of the concept that myoglobin facilitates diffusion within the cell, controversy still exists surrounding the matter of whether mitochondrial function is completely dependent on this function. General consensus holds that under hypoxic conditions in cardiomyocytes or prolonged physiological exercise in skeletal muscle, myoglobin is integral for oxygen supply to drive oxidative phosphorylation [50; 64; 72].
Myoglobin as an intracellular catalyst
It is now clear that myoglobin functions as a physiological catalyst in many respects. In vitro studies have demonstrated that the ferrous (2+) and ferric (Fe3+) forms of myoglobin can directly interact with ROS, particularly H2O2, leading to the oxidation of myoglobin to its ferryl (Fe4+) form. The ferryl myoglobin formed is a strong oxidant that on its own can oxidize proteins and lipids. Additionally, ferryl heme within myoglobin can interact with a protein radical, forming a cross-linked species capable of generating more H2O2as a well as initiating lipid peroxidation (see [73] for a review of this chemistry). In vivo studies investigating the physiological role of this chemistry demonstrate both protective and deleterious effects. For example, hearts from myoglobin knockout mice have been shown to be more susceptible to oxidative stress immediately after ischemia/reperfusion due to the lack of ROS scavenging by myoglobin [74]. However, later in reperfusion, the formation of ferrylMb (Fe4+) has been shown to contribute to reperfusion-induced oxidative damage of the heart [75]. Beyond reperfusion injury, Mb-induced ROS production has been implicated in the pathogenesis of a number of conditions including rhabdomyolysis-associated renal failure, atherosclerosis, and crush injuries [76; 77]. Interestingly, mitochondrial dysfunction, particularly augmented mitochondrial ROS generation, plays a role in the progression of many of these diseases (including ischemia/reperfusion, rhabdomyolysis and atherosclerosis) and thus it is interesting to speculate on the existence of a detrimental pathway by which mitochondrial H2O2oxidizes myoglobin, leading to the generation of ferrylMb, thus perpetuating oxidative chemistry.
In addition to ROS, myoglobin is known to catalyze reactions involving reactive nitrogen species. The next sections will review the role of myoglobin as an NO dioxygenase as well as a nitrite reductase and discuss the implications of these roles on mitochondrial function.
Myoglobin regulates mitochondrial function through its NO dioxygenase activity
The ability of NO to react rapidly with heme centers has long been recognized and is one of its most important chemical characteristics. In the case of hemoglobin, the implications for hemoglobin-based NO scavenging were recognized early on [78; 79]. However, the physiological role of the reaction of myoglobin with NO took longer to emerge. The reaction of oxygenated myoglobin with NO is rapid (k= 5 ×107 M−1 s−1) and results in the two-electron oxidation of NO to nitrate, converting ferrous myoglobin to metmyoglobin (Reaction 1):
Mb (Fe2+)O2 + NO → Mb (Fe3+) + NO3− (Reaction 1)
A physiological role for this reaction was first proposed by Brunori and based on the fact that NO reversibly binds and inhibits cytochrome c oxidase and hence mitochondrial respiration [80]. Brunori hypothesized that in working muscle or heart, oxygenated myoglobin functions as a NO scavenger to prevent NO-mediated inhibition of mitochondrial respiration and hence contributes to higher rates of oxidative phosphorylation [80]. It is estimated from cell culture models that respiratory rate is inhibited 15-30% under basal conditions by endogenous NO [81]. Given that the concentration of NO necessary to half maximally inhibit respiration (at 30μM O2) is less than 100nM [82], the high concentration of myoglobin in the heart and muscle (0.2-0.4mmol/kg wet weight) should be sufficient to scavenge NO and significantly impact respiratory rates in these tissues which contain far less cytochrome c oxidase (30-50μmol/kg wet weight).
This theory was first substantiated by Flogel and colleagues who used the myoglobin knockout mouse model to demonstrate myoglobin regulated NO signaling in the heart [83]. In these studies, perfusion of isolated wildtype hearts with exogenous NO showed a dose dependent decrease in oxygenated myoglobin concentrations with a concomitant increase in metmyoglobin formation, demonstrating that oxymyoglobin scavenges NO, generating metmyoglobin [83]. Further, when endogenous NO production was stimulated in wildtype and myoglobin knockout hearts, myoglobin knockouts were more susceptible to physiological modulation by NO. For example, NO-dependent depression of cardiac contractility was significantly more pronounced in myoglobin knockout hearts than in wildtype and this was associated with a greater decrease in energetics (measured by an increase in myocardial ADP and a decrease in phosphocreatinine levels)[83]. Further studies in vivo demonstrated that myoglobin knockout mice displayed decreased resting oxygen consumption and exercise endurance [84]. Collectively, these studies provided evidence that myoglobin regulates physiological NO signaling in vivo and was consistent with myoglobin-mediated attenuation of NO-dependent cytochrome c oxidase inhibition.
While focus has been placed on the role of myoglobin-dependent NO scavenging on regulation of respiration, it is important to consider the effect of NO on mitochondrial function beyond oxidative phosphorylation. NO is a modulator of not only respiration, but also of ROS generation and apoptotic signaling [85; 86]. Through its interaction with cytochrome c oxidase, NO can effectively increase superoxide generation by the respiratory chain [87]. Thus, it is possible that myoglobin-dependent attenuation of respiratory inhibition also decreases mitochondrial ROS generation, which may attenuate oxidative stress and modulate redox signaling in the heart and skeletal muscle. The mechanism of regulation of mitochondrial cytochrome c release, an initiating step of apoptosis, by NO is not as clearly defined. However, it is known that low concentrations of NO prevent cytochrome c release, while high concentrations stimulate its release [88; 89]. Long term studies examining apoptotic rate in myoglobin knockout mice may provide some insight into mechanisms by which NO regulates apoptosis or into compensatory adaptive mechanisms to prevent premature apoptosis.
Myoglobin regulates mitochondrial function through its nitrite reductase activity
The role of myoglobin, particularly in regulating mitochondrial function, has again been re-assessed with the finding that nitrite is an endocrine reserve of bioactive NO. In both tissues and blood, nitrite (1-10μM found basally in heart and muscle) can be reduced to bioactive NO through its reaction with a number of deoxygenated heme containing nitrite reductase proteins[90]. Deoxygenated myoglobin has been found to be a particularly potent nitrite reductase which, in the presence of a proton, reduces nitrite to NO and is oxidized to metmyoglobin in the process (Reaction 2):
NO2− + deoxyMb (Fe2+) + H+ → NO + metMb (Fe3+) + OH− (Reaction 2)
This second order reaction (k = 12 M−1s−1 at pH 7.4 and 37°C) generates bioavailable NO, which can then mediate signaling [3]. This is interesting given that this reaction shifts myoglobin’s role from an NO scavenger to a NO generator.
We have shown in isolated mitochondria and intact cardiomyocytes that NO production by this reaction can inhibit mitochondrial respiration at oxygen concentrations below the P50 of myoglobin [3]. Interestingly, this is consistent with studies performed prior to the discovery of myoglobin’s nitrite reductase activity in which nitrite was utilized as a pharmacological method to oxidize and inactivate myoglobin to decipher myoglobin’s role in oxygen diffusion to the mitochondrion [69]. In these classical studies, Doeller and Wittenberg demonstrated that nitrite had no effect on the respiration of isolated mitochondria, but in the presence of myoglobin in intact cardiomyocytes, nitrite inhibited respiratory rate [69]. While they attributed this effect to inhibition of myoglobin dependent oxygen diffusion to the mitochondria, it is interesting to speculate that this effect may have been due solely to the nitrite reductase activity of myoglobin.
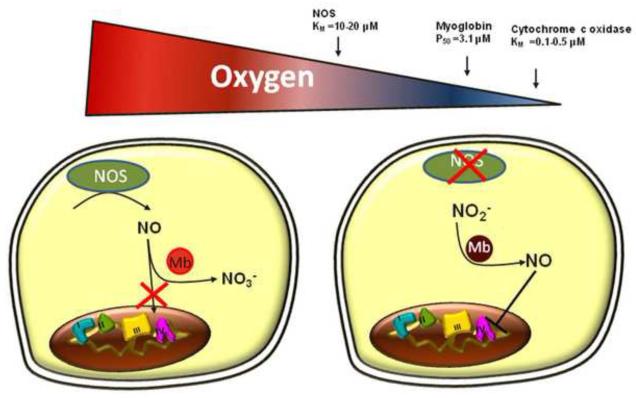
The physiological role of nitrite-myoglobin-mediated inhibition of respiration is likely most relevant in hypoxic conditions. While inhibition of respiration (particularly when oxygen supply is high) can be detrimental, inhibition of respiration could serve as a mechanism to conserve the existing oxygen and extend oxygen gradients in the cell during hypoxia. As mentioned above, the KM for oxygen at cytochrome c oxidase (0.1-0.5μM) is significantly lower than the P50 of myoglobin (3.1μM). Thus, when oxygen supply begins to decrease, myoglobin becomes desaturated and shifts to nitrite reduction to generate NO before respiration rate becomes oxygen limited. It is important to note that oxygen is also a substrate for nitric oxide synthase (KM for oxygen ~10-20μM)[91]. Hence in hypoxic conditions, reduction of nitrite potentially represents the predominant mechanism of NO generation (Figure 2).
Figure 2. Myoglobin is a normoxic NO dioxygenase and hypoxic nitrite reductase.
In normoxia, NOS enzymatically generates NO which is inactivated by oxygenated myoglobin, preventing respiratory inhibition (left side). As oxygen levels begin to decrease, and endogenous sources of NO are inhibited (nitric oxide synthase KM for oxygen ~10-20μM), myoglobin becomes deoxygenated (P50= 3.1 μM) and shifts to nitrite reduction to generate NO and inhibit respiration, thereby conserving existing oxygen.
Given that the nitrite reductase reaction of myoglobin is dependent on the presence of a proton and deoxygenation of myoglobin, it is particularly relevant in conditions of ischemia. Indeed, nitrite has been shown to be protective in a number of models of ischemia/reperfusion [92; 93; 94; 95; 96; 97]. In the heart, nitrite dependent protection after ischemia is dependent on the presence of myoglobin as nitrite treatment does not decrease ischemia-induced infarction in myoglobin knockout mice [37]. Consistent with myoglobin-dependent generation of NO regulating respiration in this model, ischemic hearts of wildtype mice show a decreased energetic status in the presence of myoglobin, while this effect was not present in myoglobin knockout hearts [4]. Further, nitrite was able to inhibit mitochondrial respiration in isolated cardiomyocytes from wildtype but not myoglobin knockout mice [4; 37]. In the context of ischemia, this protective effect is consistent with a phenomenon known as “short term hibernation” in which oxygen consumption is downregulated in response to a decreased oxygen supply. This dampens the rapid decrease in energy stores during ischemia and eventually contributes to the restoration of metabolic balance in the heart [98].
The role of myoglobin as a nitrite reductase in regulating mitochondrial function beyond cytochrome c oxidase inhibition, particularly in the context of ischemia/reperfusion, is an area of ongoing investigation. We have previously shown that nitrite-dependent S-nitrosation of thiols on mitochondrial complex I inhibits mitochondrial ROS generation at reperfusion, and this phenomenon has been associated with the nitrite-dependent protection after ischemia/reperfusion [39; 86]. It is unclear whether myoglobin catalyzes the nitrite-dependent S-nitrosation of complex I. However, novel reductive anhydrase chemistry has recently been proposed for hemoglobin by which hemoglobin catalyzes a multi-step reaction resulting in the dehydration of one molecule of water from two nitrite molecules, generating dinitrogen trioxide N2O3[99]. This species can then directly nitrosate thiols or homolyze to NO and NO2• While this chemistry has not explicitly been defined for myoglobin, it is possible that similar chemistry be at play in the heart and skeletal muscle, leading to nitrosative regulation of the mitochondrion.
Myoglobin: NO Dioxygenase versus Nitrite reductase
When examining the role of myoglobin in NO and nitrite signaling, it is important to consider that both the heart and muscle operate at oxygen tensions around the P50 of myoglobin such that under steady state conditions, the myoglobin pool remains approximately half saturated with oxygen. Thus, it stands to reason that in physiological conditions, both the nitrite reductase and NO dioxygenase reactions occur simultaneously and myoglobin plays a dual role of NO generator and scavenger and effectively regulates the concentration of bioavailable NO in the myocyte. However, both these roles of myoglobin are modulated by oxygen tension, such that augmentation of oxygen supply increases dioxygenase activity resulting in decreased NO bioavailability and oxygen consumption will be upregulated in response. Likewise, a decrease in oxygen supply increases the pool of deoxygenated myoglobin leading to greater rates of nitrite reduction and NO-dependent respiratory inhibition. Through this intricate system, the functions of myoglobin, NO and oxygen are critically linked to regulate the concentrations of one another as well as maintain optimal mitochondrial function.
CONCLUSIONS
The last century brought major advancements in delineating the basic structure and function of both the mitochondrion and myoglobin. Our understanding of the functional role of these two sub-cellular structures continues to evolve, especially with the recognition of the role of NO in physiology. It is now well accepted that mitochondria and myoglobin are intimately linked both in the level of their expression as well as in their functional regulation of one another and that oxygen and NO provide a crucial link between the two structures. The majority of research has thus far focused on the role of myoglobin in regulating mitochondrial oxygen consumption. However, current and future studies will focus on the reactions of myoglobin with NO/nitrite and determine how these interactions regulate all aspects of mitochondrial function in health and disease.
Highlights.
Myoglobin regulates mitochondrial function through modulation of cellular O2 and NO levels
Myoglobin facilitates oxygen diffusion, storage and catalysis
Myoglobin serves as a NO dioxygenase or nitrite reductase depending on pO2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Mörner KAH. Beobachtungen über den Muskelfarbstoff. Nord. Med. Ark. 1897;30:1–8. [Google Scholar]
- [2].Rheinberger HJ. From microsomes to ribosomes: “strategies” of “representation. J Hist Biol. 1995;28:49–89. doi: 10.1007/BF01061246. [DOI] [PubMed] [Google Scholar]
- [3].Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100:654–61. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- [4].Rassaf T, Flogel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circ Res. 2007;100:1749–54. doi: 10.1161/CIRCRESAHA.107.152488. [DOI] [PubMed] [Google Scholar]
- [5].Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–8. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- [6].Rousset S, Alves-Guerra MC, Mozo J, Miroux B, Cassard-Doulcier AM, Bouillaud F, Ricquier D. The biology of mitochondrial uncoupling proteins. Diabetes. 2004;53(Suppl 1):S130–5. doi: 10.2337/diabetes.53.2007.s130. [DOI] [PubMed] [Google Scholar]
- [7].Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–30. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- [8].Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- [9].Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- [10].Hool LC. Reactive oxygen species in cardiac signalling: from mitochondria to plasma membrane ion channels. Clin Exp Pharmacol Physiol. 2006;33:146–51. doi: 10.1111/j.1440-1681.2006.04341.x. [DOI] [PubMed] [Google Scholar]
- [11].Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995;18:775–94. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- [12].Felty Q, Xiong WC, Sun D, Sarkar S, Singh KP, Parkash J, Roy D. Estrogen-induced mitochondrial reactive oxygen species as signal-transducing messengers. Biochemistry. 2005;44:6900–9. doi: 10.1021/bi047629p. [DOI] [PubMed] [Google Scholar]
- [13].Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–28. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- [14].Novalija E, Kevin LG, Camara AK, Bosnjak ZJ, Kampine JP, Stowe DF. Reactive oxygen species precede the epsilon isoform of protein kinase C in the anesthetic preconditioning signaling cascade. Anesthesiology. 2003;99:421–8. doi: 10.1097/00000542-200308000-00024. [DOI] [PubMed] [Google Scholar]
- [15].Genestra M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal. 2007;19:1807–19. doi: 10.1016/j.cellsig.2007.04.009. [DOI] [PubMed] [Google Scholar]
- [16].Penna C, Mancardi D, Rastaldo R, Pagliaro P. Cardioprotection: a radical view Free radicals in pre and postconditioning. Biochim Biophys Acta. 2009;1787:781–93. doi: 10.1016/j.bbabio.2009.02.008. [DOI] [PubMed] [Google Scholar]
- [17].Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol. 1997;29:207–16. doi: 10.1006/jmcc.1996.0265. [DOI] [PubMed] [Google Scholar]
- [18].Costa AD, Pierre SV, Cohen MV, Downey JM, Garlid KD. cGMP signalling in pre- and post-conditioning: the role of mitochondria. Cardiovasc Res. 2008;77:344–52. doi: 10.1093/cvr/cvm050. [DOI] [PubMed] [Google Scholar]
- [19].Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- [20].Nilsson R, Schultz IJ, Pierce EL, Soltis KA, Naranuntarat A, Ward DM, Baughman JM, Paradkar PN, Kingsley PD, Culotta VC, Kaplan J, Palis J, Paw BH, Mootha VK. Discovery of genes essential for heme biosynthesis through large-scale gene expression analysis. Cell Metab. 2009;10:119–30. doi: 10.1016/j.cmet.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Richardson DR, Lane DJ, Becker EM, Huang ML, Whitnall M, Suryo Rahmanto Y, Sheftel AD, Ponka P. Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc Natl Acad Sci U S A. 2010;107:10775–82. doi: 10.1073/pnas.0912925107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Arciuch VG, Elguero ME, Poderoso JJ, Carreras MC. Mitochondrial Regulation of Cell Cycle and Proliferation. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2011.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–68. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- [24].Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–44. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].de Brito OM, Scorrano L. Mitofusin 2: a mitochondria-shaping protein with signaling roles beyond fusion. Antioxid Redox Signal. 2008;10:621–33. doi: 10.1089/ars.2007.1934. [DOI] [PubMed] [Google Scholar]
- [26].Jahani-Asl A, Cheung EC, Neuspiel M, MacLaurin JG, Fortin A, Park DS, McBride HM, Slack RS. Mitofusin 2 protects cerebellar granule neurons against injury-induced cell death. J Biol Chem. 2007;282:23788–98. doi: 10.1074/jbc.M703812200. [DOI] [PubMed] [Google Scholar]
- [27].Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–22. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- [28].Foster DB, Van Eyk JE, Marban E, O’Rourke B. Redox signaling and protein phosphorylation in mitochondria: progress and prospects. J Bioenerg Biomembr. 2009;41:159–68. doi: 10.1007/s10863-009-9217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Taylor ER, Hurrell F, Shannon RJ, Lin TK, Hirst J, Murphy MP. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J Biol Chem. 2003;278:19603–10. doi: 10.1074/jbc.M209359200. [DOI] [PubMed] [Google Scholar]
- [30].Fratelli M, Demol H, Puype M, Casagrande S, Villa P, Eberini I, Vandekerckhove J, Gianazza E, Ghezzi P. Identification of proteins undergoing glutathionylation in oxidatively stressed hepatocytes and hepatoma cells. Proteomics. 2003;3:1154–61. doi: 10.1002/pmic.200300436. [DOI] [PubMed] [Google Scholar]
- [31].Huang JY, Hirschey MD, Shimazu T, Ho L, Verdin E. Mitochondrial sirtuins. Biochim Biophys Acta. 2010;1804:1645–51. doi: 10.1016/j.bbapap.2009.12.021. [DOI] [PubMed] [Google Scholar]
- [32].Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50–4. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- [33].Brookes PS, Kraus DW, Shiva S, Doeller JE, Barone MC, Patel RP, Lancaster JR, Jr., Darley-Usmar V. Control of mitochondrial respiration by NO*, effects of low oxygen and respiratory state. J Biol Chem. 2003;278:31603–9. doi: 10.1074/jbc.M211784200. [DOI] [PubMed] [Google Scholar]
- [34].Shiva S, Brookes PS, Patel RP, Anderson PG, Darley-Usmar VM. Nitric oxide partitioning into mitochondrial membranes and the control of respiration at cytochrome c oxidase. Proc Natl Acad Sci U S A. 2001;98:7212–7. doi: 10.1073/pnas.131128898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu X, Miller MJ, Joshi MS, Thomas DD, Lancaster JR., Jr. Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc Natl Acad Sci U S A. 1998;95:2175–9. doi: 10.1073/pnas.95.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science. 2003;302:1975–8. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- [37].Hendgen-Cotta UB, Merx MW, Shiva S, Schmitz J, Becher S, Klare JP, Steinhoff HJ, Goedecke A, Schrader J, Gladwin MT, Kelm M, Rassaf T. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2008;105:10256–61. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A. 1998;95:7631–6. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Burwell LS, Nadtochiy SM, Brookes PS. Cardioprotection by metabolic shut-down and gradual wake-up. J Mol Cell Cardiol. 2009;46:804–10. doi: 10.1016/j.yjmcc.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chance B. Reaction of oxygen with the respiratory chain in cells and tissues. J Gen Physiol. 1965;49(Suppl):163–95. doi: 10.1085/jgp.49.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gnaiger E, Steinlechner-Maran R, Mendez G, Eberl T, Margreiter R. Control of mitochondrial and cellular respiration by oxygen. J Bioenerg Biomembr. 1995;27:583–96. doi: 10.1007/BF02111656. [DOI] [PubMed] [Google Scholar]
- [42].Gnaiger E, Lassnig B, Kuznetsov A, Rieger G, Margreiter R. Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J Exp Biol. 1998;201:1129–39. doi: 10.1242/jeb.201.8.1129. [DOI] [PubMed] [Google Scholar]
- [43].Budinger GR, Chandel N, Shao ZH, Li CQ, Melmed A, Becker LB, Schumacker PT. Cellular energy utilization and supply during hypoxia in embryonic cardiac myocytes. Am J Physiol. 1996;270:L44–53. doi: 10.1152/ajplung.1996.270.1.L44. [DOI] [PubMed] [Google Scholar]
- [44].Giuffre A, Sarti P, D’Itri E, Buse G, Soulimane T, Brunori M. On the mechanism of inhibition of cytochrome c oxidase by nitric oxide. J Biol Chem. 1996;271:33404–8. doi: 10.1074/jbc.271.52.33404. [DOI] [PubMed] [Google Scholar]
- [45].Torres J, Cooper CE, Sharpe M, Wilson MT. Reactivity of nitric oxide with cytochrome c oxidase: interactions with the binuclear centre and mechanism of inhibition. J Bioenerg Biomembr. 1998;30:63–9. doi: 10.1023/a:1020559528124. [DOI] [PubMed] [Google Scholar]
- [46].Boushel R, Gnaiger E, Calbet JA, Gonzalez-Alonso J, Wright-Paradis C, Sondergaard H, Ara I, Helge JW, Saltin B. Muscle mitochondrial capacity exceeds maximal oxygen delivery in humans. Mitochondrion. 2011;11:303–7. doi: 10.1016/j.mito.2010.12.006. [DOI] [PubMed] [Google Scholar]
- [47].Kendrew JC. The three-dimensional structure of a protein molecule. Sci Am. 1961;205:96–110. doi: 10.1038/scientificamerican1261-96. [DOI] [PubMed] [Google Scholar]
- [48].Springer BA, Egeberg KD, Sligar SG, Rohlfs RJ, Mathews AJ, Olson JS. Discrimination between oxygen and carbon monoxide and inhibition of autooxidation by myoglobin. Site-directed mutagenesis of the distal histidine. J Biol Chem. 1989;264:3057–60. [PubMed] [Google Scholar]
- [49].Wittenberg JB. Myoglobin-facilitated oxygen diffusion: role of myoglobin in oxygen entry into muscle. Physiol Rev. 1970;50:559–636. doi: 10.1152/physrev.1970.50.4.559. [DOI] [PubMed] [Google Scholar]
- [50].Wittenberg JB, Wittenberg BA. Myoglobin function reassessed. J Exp Biol. 2003;206:2011–20. doi: 10.1242/jeb.00243. [DOI] [PubMed] [Google Scholar]
- [51].Lawrie RA. The relation of energy-rich phosphate in muscle to myoglobin and to cytochrome-oxidase activity. Biochem J. 1953;55:305–9. doi: 10.1042/bj0550305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Reis DJ, Wooten GF. The relationship of blood flow to myoglobin, capillary density, and twitch characteristics in red and white skeletal muscle in cat. J Physiol. 1970;210:121–35. doi: 10.1113/jphysiol.1970.sp009199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kanatous SB, Mammen PP. Regulation of myoglobin expression. J Exp Biol. 2010;213:2741–7. doi: 10.1242/jeb.041442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Qiu Y, Sutton L, Riggs AF. Identification of myoglobin in human smooth muscle. J Biol Chem. 1998;273:23426–32. doi: 10.1074/jbc.273.36.23426. [DOI] [PubMed] [Google Scholar]
- [55].Flonta SE, Arena S, Pisacane A, Michieli P, Bardelli A. Expression and functional regulation of myoglobin in epithelial cancers. Am J Pathol. 2009;175:201–6. doi: 10.2353/ajpath.2009.081124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Michieli P. Hypoxia, angiogenesis and cancer therapy: to breathe or not to breathe? Cell Cycle. 2009;8:3291–6. doi: 10.4161/cc.8.20.9741. [DOI] [PubMed] [Google Scholar]
- [57].Millikan G. Muscle hemoglobin. Physiological Reviews. 1939;19:503–523. [Google Scholar]
- [58].Mole PA, Chung Y, Tran TK, Sailasuta N, Hurd R, Jue T. Myoglobin desaturation with exercise intensity in human gastrocnemius muscle. Am J Physiol. 1999;277:R173–80. doi: 10.1152/ajpregu.1999.277.1.R173. [DOI] [PubMed] [Google Scholar]
- [59].Reynafarje B. Myoglobin content and enzymatic activity of muscle and altitude adaptation. J Appl Physiol. 1962;17:301–5. doi: 10.1152/jappl.1962.17.2.301. [DOI] [PubMed] [Google Scholar]
- [60].Snyder GK. Respiratory adaptations in diving mammals. Respir Physiol. 1983;54:269–94. doi: 10.1016/0034-5687(83)90072-5. [DOI] [PubMed] [Google Scholar]
- [61].Lin PC, Kreutzer U, Jue T. Myoglobin translational diffusion in rat myocardium and its implication on intracellular oxygen transport. J Physiol. 2007;578:595–603. doi: 10.1113/jphysiol.2006.116061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sidell BD. Intracellular oxygen diffusion: the roles of myoglobin and lipid at cold body temperature. J Exp Biol. 1998;201:11, 19–28. doi: 10.1242/jeb.201.8.1119. [DOI] [PubMed] [Google Scholar]
- [63].Takahashi E, Endoh H, Doi K. Visualization of myoglobin-facilitated mitochondrial O(2) delivery in a single isolated cardiomyocyte. Biophys J. 2000;78:3252–9. doi: 10.1016/S0006-3495(00)76861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Cole RP, Sukanek PC, Wittenberg JB, Wittenberg BA. Mitochondrial function in the presence of myoglobin. J Appl Physiol. 1982;53:1116–24. doi: 10.1152/jappl.1982.53.5.1116. [DOI] [PubMed] [Google Scholar]
- [65].Garry DJ, Ordway GA, Lorenz JN, Radford NB, Chin ER, Grange RW, Bassel-Duby R, Williams RS. Mice without myoglobin. Nature. 1998;395:905–8. doi: 10.1038/27681. [DOI] [PubMed] [Google Scholar]
- [66].Godecke A, Flogel U, Zanger K, Ding Z, Hirchenhain J, Decking UK, Schrader J. Disruption of myoglobin in mice induces multiple compensatory mechanisms. Proc Natl Acad Sci U S A. 1999;96:10495–500. doi: 10.1073/pnas.96.18.10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wittenberg BA, Wittenberg JB, Caldwell PR. Role of myoglobin in the oxygen supply to red skeletal muscle. J Biol Chem. 1975;250:9038–43. [PubMed] [Google Scholar]
- [68].Cole RP. Skeletal muscle function in hypoxia: effect of alteration of intracellular myoglobin. Respir Physiol. 1983;53:1–14. doi: 10.1016/0034-5687(83)90012-9. [DOI] [PubMed] [Google Scholar]
- [69].Doeller JE, Wittenberg BA. Myoglobin function and energy metabolism of isolated cardiac myocytes: effect of sodium nitrite. Am J Physiol. 1991;261:H53–62. doi: 10.1152/ajpheart.1991.261.1.H53. [DOI] [PubMed] [Google Scholar]
- [70].Wittenberg BA, Wittenberg JB. Myoglobin-mediated oxygen delivery to mitochondria of isolated cardiac myocytes. Proc Natl Acad Sci U S A. 1987;84:7503–7. doi: 10.1073/pnas.84.21.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wittenberg BA, Wittenberg JB. Effects of carbon monoxide on isolated heart muscle cells. Res Rep Health Eff Inst. 1993:1–12. discussion 13-21. [PubMed] [Google Scholar]
- [72].Cole RP. Myoglobin function in exercising skeletal muscle. Science. 1982;216:523–5. doi: 10.1126/science.7071598. [DOI] [PubMed] [Google Scholar]
- [73].Alayash AI, Patel RP, Cashon RE. Redox reactions of hemoglobin and myoglobin: biological and toxicological implications. Antioxid Redox Signal. 2001;3:313–27. doi: 10.1089/152308601300185250. [DOI] [PubMed] [Google Scholar]
- [74].Flogel U, Godecke A, Klotz LO, Schrader J. Role of myoglobin in the antioxidant defense of the heart. FASEB J. 2004;18:1156–8. doi: 10.1096/fj.03-1382fje. [DOI] [PubMed] [Google Scholar]
- [75].Jourd’heuil D, Mills L, Miles AM, Grisham MB. Effect of nitric oxide on hemoprotein-catalyzed oxidative reactions. Nitric Oxide. 1998;2:37–44. doi: 10.1006/niox.1998.0167. [DOI] [PubMed] [Google Scholar]
- [76].Plotnikov EY, Chupyrkina AA, Pevzner IB, Isaev NK, Zorov DB. Myoglobin causes oxidative stress, increase of NO production and dysfunction of kidney’s mitochondria. Biochim Biophys Acta. 2009;1792:796–803. doi: 10.1016/j.bbadis.2009.06.005. [DOI] [PubMed] [Google Scholar]
- [77].Vuletich JL, Osawa Y, Aviram M. Enhanced lipid oxidation by oxidatively modified myoglobin: role of protein-bound heme. Biochem Biophys Res Commun. 2000;269:647–51. doi: 10.1006/bbrc.2000.2349. [DOI] [PubMed] [Google Scholar]
- [78].Lancaster JR., Jr. Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc Natl Acad Sci U S A. 1994;91:8137–41. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kim-Shapiro DB, Schechter AN, Gladwin MT. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler Thromb Vasc Biol. 2006;26:697–705. doi: 10.1161/01.ATV.0000204350.44226.9a. [DOI] [PubMed] [Google Scholar]
- [80].Brunori M. Nitric oxide, cytochrome-c oxidase and myoglobin. Trends Biochem Sci. 2001;26:21–3. doi: 10.1016/s0968-0004(00)01698-4. [DOI] [PubMed] [Google Scholar]
- [81].Brown GC, Bolanos JP, Heales SJ, Clark JB. Nitric oxide produced by activated astrocytes rapidly and reversibly inhibits cellular respiration. Neurosci Lett. 1995;193:201–4. doi: 10.1016/0304-3940(95)11703-y. [DOI] [PubMed] [Google Scholar]
- [82].Borutaite V, Brown GC. Rapid reduction of nitric oxide by mitochondria, and reversible inhibition of mitochondrial respiration by nitric oxide. Biochem J. 1996;315( Pt 1):295–9. doi: 10.1042/bj3150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Flogel U, Merx MW, Godecke A, Decking UK, Schrader J. Myoglobin: A scavenger of bioactive NO. Proc Natl Acad Sci U S A. 2001;98:735–40. doi: 10.1073/pnas.011460298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Merx MW, Godecke A, Flogel U, Schrader J. Oxygen supply and nitric oxide scavenging by myoglobin contribute to exercise endurance and cardiac function. FASEB J. 2005;19:1015–7. doi: 10.1096/fj.04-2886fje. [DOI] [PubMed] [Google Scholar]
- [85].Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol. 2002;3:214–20. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- [86].Brookes PS, Levonen AL, Shiva S, Sarti P, Darley-Usmar VM. Mitochondria: regulators of signal transduction by reactive oxygen and nitrogen species. Free Radic Biol Med. 2002;33:755–64. doi: 10.1016/s0891-5849(02)00901-2. [DOI] [PubMed] [Google Scholar]
- [87].Poderoso JJ, Carreras MC, Lisdero C, Riobo N, Schopfer F, Boveris A. Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys. 1996;328:85–92. doi: 10.1006/abbi.1996.0146. [DOI] [PubMed] [Google Scholar]
- [88].Ghafourifar P, Schenk U, Klein SD, Richter C. Mitochondrial nitric-oxide synthase stimulation causes cytochrome c release from isolated mitochondria. Evidence for intramitochondrial peroxynitrite formation. J Biol Chem. 1999;274:31185–8. doi: 10.1074/jbc.274.44.31185. [DOI] [PubMed] [Google Scholar]
- [89].Brune B. Nitric oxide: NO apoptosis or turning it ON? Cell Death Differ. 2003;10:864–9. doi: 10.1038/sj.cdd.4401261. [DOI] [PubMed] [Google Scholar]
- [90].Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–67. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- [91].Stuehr DJ, Wei CC, Santolini J, Wang Z, Aoyagi M, Getzoff ED. Radical reactions of nitric oxide synthases. Biochem Soc Symp. 2004:39–49. doi: 10.1042/bss0710039. [DOI] [PubMed] [Google Scholar]
- [92].Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101:13683–8. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–40. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Jung KH, Chu K, Ko SY, Lee ST, Sinn DI, Park DK, Kim JM, Song EC, Kim M, Roh JK. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke. 2006;37:2744–50. doi: 10.1161/01.STR.0000245116.40163.1c. [DOI] [PubMed] [Google Scholar]
- [95].Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kumar D, Branch BG, Pattillo CB, Hood J, Thoma S, Simpson S, Illum S, Arora N, Chidlow JH, Jr., Langston W, Teng X, Lefer DJ, Patel RP, Kevil CG. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc Natl Acad Sci U S A. 2008;105:7540–5. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Dezfulian C, Shiva S, Alekseyenko A, Pendyal A, Beiser DG, Munasinghe JP, Anderson SA, Chesley CF, Vanden Hoek TL, Gladwin MT. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation. 2009;120:897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Hearse DJ. Myocardial hibernation. A form of endogenous protection? Eur Heart J. 1997;18(Suppl A):A2–7. doi: 10.1093/eurheartj/18.suppl_a.2. [DOI] [PubMed] [Google Scholar]
- [99].Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, Jiang A, He X, Azarov I, Seibert R, Mehta A, Patel R, King SB, Hogg N, Ghosh A, Gladwin MT, Kim-Shapiro DB. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat Chem Biol. 2007;3:785–94. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]