Abstract
Fifteen years (1984–1998) of records from a Veterinary Teaching Hospital were analyzed to determine whether antimicrobial drug resistance in coagulase-positive Staphylococcus spp. (S. aureus, S. intermedius) isolated from clinical infections in dogs has increased, and whether there has been a change in the species of bacteria isolated from urinary tract infections in dogs. In coagulase-positive Staphylococcus spp., a complex pattern showing both increases and decreases of resistance to different classes of antimicrobial drugs was observed, reflecting the changing use of different antimicrobial drug classes in the hospital over a similar period (1990–1999). In canine urinary tract infections identified from 1984 to 1998, an increase in the incidence of multiresistant Enterococcus spp. was apparent, with marginal increases also in incidence in Enterobacter spp. and in Pseudomonas aeruginosa, both of which, like Enterococcus spp., are innately antimicrobial-resistant bacteria. A survey of directors of veterinary teaching hospitals in Canada and the United States identified only 3 hospitals that had any policy on use of “last resort” antimicrobial drugs (amikacin, imipenem, vancomycin). Evidence is briefly reviewed that owners may be at risk when dogs are treated with antimicrobial drugs, as well as evidence that some resistant bacteria may be acquired by dogs as a result of antimicrobial drug use in agriculture. Based in part on gaps in our knowledge, recommendations are made on prudent use of antimicrobial drugs in companion animals, as well as on the need to develop science-based infection control programs in veterinary hospitals.
Introduction
The crisis of antimicrobial drug resistance in human medicine has brought into question every aspect of use of these drugs in animals. While there is considerable, though often fragmented, data on antimicrobial drug resistance in bacteria of food animal origin (1,2) and increasing, but also fragmented, data on quantities of antimicrobial drug use in food animals, there is little useful data on antimicrobial drug use and resistance in companion animals. Is antimicrobial resistance a problem in companion animal, specifically canine, medicine?
Intuitively, the problem of antimicrobial resistance in bacterial pathogens isolated from dogs should be less severe than in bacterial pathogens isolated from humans; dogs are less likely to be exposed to antimicrobial drugs for other than short, sporadic periods, because they are less commonly hospitalized, they are not kept in “old dog's homes,” chronically ill dogs may be euthanized, and immunocompromised animals are not commonly treated with very broad-spectrum and potent antimicrobial drugs, such as imipenem.
By contrast to the situation in food animal medicine, the effect of antimicrobial drug use in companion animals on the acquisition of their resistant bacteria by humans, or the acquisition by human pathogens of resistance genes derived from resistant bacteria coming from companion animals, has not been explored in any significant way. Are owners at risk when their dogs and cats are treated with antimicrobial drugs?
This paper addresses, primarily with reference to dogs, the following questions: 1) Is antimicrobial drug resistance in bacterial pathogens isolated from dogs increasing? Is it a problem? 2) Because of antimicrobial resistance, has there been a change over time in the identity of bacteria isolated from infections in dogs? 3) What is “prudent use” of antimicrobial drugs in dogs? Are there any specific guidelines for companion animals other than the generic guidelines promulgated by national veterinary organizations? 4) Are owners at risk when their companion animals are treated with antimicrobial drugs? 5) To what extent can resistance problems occurring in bacteria isolated from companion animals be attributed to medical or agricultural use of antimicrobial drugs? 6) Based on these analyses, what recommendations can be made for the future? Excellent overviews of aspects of this topic are available elsewhere (3,4).
The overall objective of this preliminary investigation and discussion is to stimulate dialogue and to focus research on the subject of antimicrobial resistance and antimicrobial drug use in companion animals, rather than to provide definitive answers.
Materials and methods
Data for antimicrobial susceptibility for 495 coagulase-positive Staphylococcus spp. (S. aureus, S. intermedius) isolated from clinical infections in dogs presenting to or hospitalized in the Veterinary Teaching Hospital, Ontario Veterinary College (VTH-OVC), from 1984 to 1998, were retrieved from electronic laboratory records. During the first half of this time period, antimicrobial susceptibility testing was performed by disk diffusion susceptibility testing using standard methods and interpretation as susceptible, intermediate, or resistant, based on published criteria developed for medical pathogens. During the last half of this time period, antimicrobial susceptibility testing was performed using a commercially prepared, customized “break point” broth susceptibility system, and using similar interpretive criteria to those used in the first half. For the purposes of analysis, intermediate susceptibility was regarded as susceptible. Identification of the bacteria for which susceptibility testing was done was by standard methods in use in the laboratory.
Data for all bacterial pathogens isolated from canine urinary tract infections (UTI) in the VTH-OVC from 1984 to 1998 were retrieved from electronic records. These included both nonhospitalized and hospitalized dogs, but no attempt was made to distinguish these 2 groups or determine whether they were referral or primary care patients. Identification of the bacteria was by standard methods in use in the laboratory. However, Enterococcus spp. were only identified generically starting in 1997; before this, they were described simply as either nonhemolytic or alpha-hemolytic streptococci. Enterococcus spp. are naturally resistant to clindamycin, as well as to penicillin G and cephalothin, giving them a characteristic antimicrobial resistance profile. Based on this, in the retrospective analysis of the relative frequency of different bacteria isolated from canine UTI, all clindamycin-resistant “Streptococcus spp.” were classified as Enterococcus spp. Data from both the resistance and UTI studies were managed and regression lines plotted using a spreadsheet program (Microsoft Excel; Microsoft Canada, Mississauga, Ontario). Pearson correlation coefficients were calculated using a statistical analysis system (SAS for Windows, v.8; SAS Institute, Cary, North Carolina, USA).
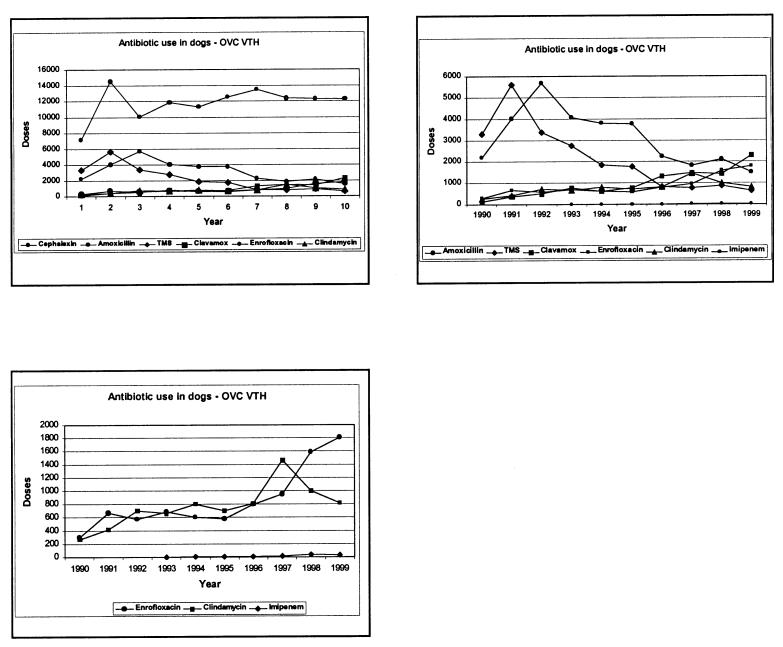
The records of the VTH pharmacy were searched for quantities of antimicrobial drugs (amoxicillin, amoxicillin-clavulanic acid, cephalexin, clindamycin, enrofloxacin, imipenem, trimethoprim-sulphamethoxazole) dispensed for dogs over the period 1990–1999 (records for 1984–1989 were unavailable). These were converted into an estimated “daily dog dose” for each year by dividing the total number of grams of the antimicrobial dispensed by the pharmacy (for canine patients only) by the number of grams of antimicrobial that would be given to a 20 kg dog in 1 d during treatment using recommended dosages. Where a recommended dose range was given, the round number at the midpoint of the range was used; for example, the recommended dosage of amoxicillin for canine patients is 10–20 mg/kg body weight (BW), q12h, so 15 mg/kg BW was used to calculate a daily dose of 0.6 g for a 20 kg dog.
Two informal surveys were performed. The first was a survey of 15 university veterinary clinical microbiology laboratories in 7 different countries (Australia, Belgium, France, Sweden, Switzerland, United Kingdom, United States of America) that were thought likely to have compiled annual data of antimicrobial susceptibilities of bacterial pathogens isolated from dogs and cats. The survey asked each laboratory whether it had antimicrobial resistance data from canine bacterial pathogens recorded over time, whether it was willing to share this data, and what method used to determine antimicrobial susceptibility. The second survey was of the directors of the VTHs of each of the 31 veterinary schools in the United States and Canada, as well as the Angell Memorial Hospital in Boston, Massachusetts, USA. It asked each whether the hospital had a formal policy about the selection of antimicrobial drugs and, in particular, about the use of imipenem, vancomycin, and amikacin, and if it would provide a copy of any written policy.
Results
Changes in antimicrobial resistance and drug use over time
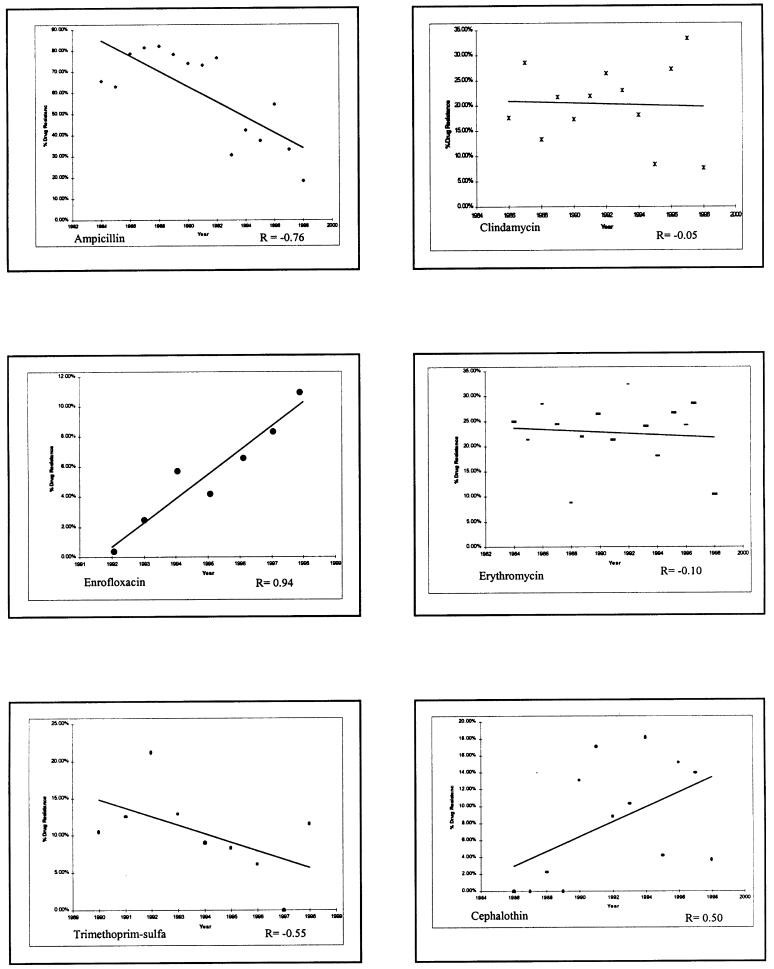
Over the period 1984 to 1998 at the OVC, there was a significant decline in the proportion of S. aureus and S. intermedius isolates resistant to penicillin G (r = -0.57, P = 0.03; data not shown) and ampicillin (r = -0.75, P = 0.001), and a marginal but not significant decline in resistance to trimethoprim-sulfonamides (r = -0.55, P = 0.13) (Figure 1). This trend of decline mirrors an apparent decline in the hospital usage of these drugs (Figure 2). By contrast, the proportion of S. aureus and S. intermedius isolates resistant to clindamycin and erythromycin remained stable and has not changed significantly (Figure 1), corresponding to stable usage of clindamycin over time (Figure 2). Resistance to cephalothin appears to have increased slightly (r = 0.50, P = 0.08), reflecting wide usage of this drug, whereas resistance to enrofloxacin in S. intermedius has shown a marked increase (r = 0.94, P = 0.001) (Figure 1), reflecting the increasing usage of this drug at the OVC in recent years (Figure 2). Resistance to gentamicin has also increased (r = 0.57, P = 0.02; data not shown).
Figure 1. Change in percentage of resistance over time (1984–1998) to selected antimicrobial drugs in coagulase-positive Staphylococcus spp. at the Veterinary Teaching Hospital, Ontario Veterinary College. Note that the vertical scale is different for most drugs tested.
Figure 2. Change in usage over time (1990–1999) of common antimicrobial drugs administered to dogs at the Veterinary Teaching Hospital, Ontario Veterinary College, adjusted to daily dog doses.
Changes in bacterial species isolated from urinary tract infections over time
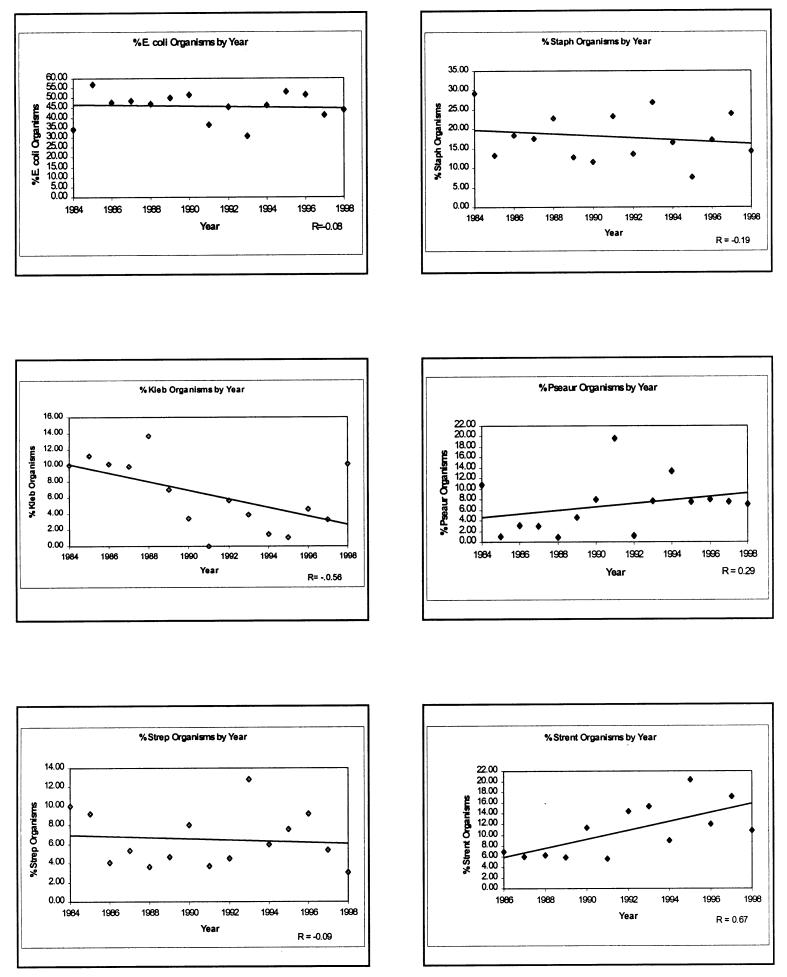
Total numbers of isolates from UTI that were identified at the OVC from 1984 to 1998 were, in order of frequency: Escherichia coli (657 isolates), S. aureus or S. intermedius (262), Enterococcus spp. (113), Pseudomonas aeruginosa (98), Streptococcus spp. (94), Klebsiella spp. (87), Proteus spp. (85), and Enterobacter spp. (36). A mean of 96 of these isolates were obtained per year (range 67–132). At the OVC, Enterococcus spp. have been identified to genus only in the last 3 y. If the reclassification of all pre-1997, clindamycin-resistant “streptococci” in canine UTI as Enterococcus spp. is correct, there has been a significant increase (r = 0.67, P = 0.01) in the proportion of enterococcal UTIs over the last 15 y (Figure 3). By contrast, E. coli, coagulase-positive Staphylococcus spp., and streptococci (including S. canis) have remained a stable proportion of the causes of UTIs; P. aeruginosa and Enterobacter spp. (both inherently resistant bacterial species) have increased slightly; while Klebsiella spp. and Proteus spp. (r = -0.39, P = 0.15; data not shown) have declined as a proportion of the causes of canine UTI (Figure 3). Apart from Enterococcus spp., only Klebsiella spp. have shown a significant change in prevalence as a cause of UTI over time (r = -0.56, P = 0.03).
Figure 3. Proportions of the most common bacteria isolated over time (1984–1998) from canine urinary tract infections at the Veterinary Teaching Hospital, Ontario Veterinary College.
The use of guidelines
In the survey of directors of VTHs in the United States and Canada, only 3 of the 21 who replied described having any policy for the “top shelf” drugs, amikacin, imipenem, and vancomycin. Several others said they did not use these drugs, only used them very exceptionally, or had a quality control system based on “rounds,” which effectively controlled their use. Others commented that veterinarians do not like restrictions on their rights to prescribe.
Discussion
1. Is antimicrobial drug resistance in companion animals increasing? Is it a problem?
Our data on resistance show a marked annual variation in resistance to antimicrobial drugs, which is probably the result of a small sample size derived from various referent populations, although it may also reflect changing fashions in the use of antimicrobial drugs (2,3,4), both at the VTH-OVC and in private practice. The data presented here can only be considered as a rough reflection of the relationship between use and resistance.
It is difficult to obtain a reliable global view of whether antimicrobial resistance in companion animals is increasing. The pattern of changing resistance that we observed is more complex than the conclusion reached in one study of resistance in canine S. intermedius that “resistance to commonly used antibiotics appears to have increased dramatically over the last decade” (5). Our data suggest, rather, a pattern that appears to reflect drug usage. Despite a possible wealth of data in filing cabinets in veterinary clinical microbiology laboratories around the world, there have been virtually no systematic investigations of changes in antimicrobial drug resistance in bacteria isolated from companion animals over time, using standard methodologies for assessing resistance. There are only a few notable exceptions to this devastating criticism (6,7,8,9). Given the impact of small sample sizes and other sampling issues on the apparent prevalence of resistance, some trends are noticeable in the short term. For example, there has been an increase in resistance of companion animal bacteria to fluoroquinolones (10,11), a development that appears temporally associated with the rapid introduction of these drugs into companion animal practice. In a recent U.S. study, only 75% of E. coli from canine infections in 1998 were susceptible to enrofloxacin compared with > 95% more than 6 y earlier at the same institution (6,11). At the OVC, resistance of S. aureus and S. intermedius to fluoroquinolones has risen from 0% to 12% in 8 y (Figure 1). Our data showing a trend to an apparent increase in resistance to cephalothin is suspect, since resistance to first-generation cephalosporins is unusual. It suggests that there may have been errors in test procedures or their interpretation.
Reports of resistance coming from diagnostic laboratories may be those of “worst case” scenarios, since they often represent treatment failures rather than treatment successes, which tend not to reach the laboratory. Antimicrobial drug-treated animals will more likely yield resistant bacteria than will untreated animals (12,13,14). In addition, variation in resistance patterns between multiple isolates of coagulase-positive staphylococci from the same dog suggests that single swab samples or the usual recommendation to perform susceptibility tests on a limited number of colonies may be of limited value in optimally determining susceptibility patterns (8,15).
Internationally, there are considerable differences in the criteria used for susceptibility testing. In our informal survey of veterinary clinical microbiology laboratories around the world, these ranged from the bizarre “all tests are done without standardization on discs and are scored by eye by the same team for 10 y,” to the Australian “calibrated dichotomous sensitivity test,” to the use of the Veterinary Antimicrobial Susceptibility Test criteria of the United States-based National Committee for Clinical Laboratory Standards, which, although an excellent and widely accepted standard, unfortunately limits itself to U.S.-specific veterinary- or farmer-licenced antimicrobial drugs, mostly for food animals (16). In addition to differences in susceptibility test methods and interpretive criteria between laboratories, our survey showed that laboratories may change procedures over time, often do not analyze their data, change computer software that stores data with the result that earlier data are inaccessible, or lose computerized records. It seems evident that even the best run clinical microbiology laboratories have problems with the universality, reliability, and, in many cases, the availability of the data required to determine antimicrobial resistance and trends over time for companion animals.
The congruence of changing resistance with changing drug use is an important concept, although the relation is not necessarily direct. Resistance is not inevitable. For example, S. canis and P. multocida have remained largely highly susceptible to antimicrobial drugs over the last 50 y, for unknown reasons. Such an apparently debilitating lack of adaptability in these bacterial species contrasts with that in E. coli and many other bacteria, whose greater ability to evolve is shown in the often rapid emergence of resistance. Once resistance emerges, the continued selection pressure of antimicrobial drugs will maintain resistant bacteria in populations. In the absence of such selection pressure, resistance will tend to decline (17), since there is a physiological cost to bacteria to maintain unused resistance genes. For example, Naidoo and Lloyd (18) showed the rapid rate with which S. intermedius lost resistance plasmids in the laboratory. However, the genetic ecology of antimicrobial resistance is highly complex (19). The physiological cost to bacteria of possessing resistance genes can be overcome, for example, by down-regulating their expression in the absence of antimicrobial drug exposure, by clustering them on multiple-resistance plasmids, or by arranging them as gene cassettes on integrons in the order in which they are needed, the integrons themselves being maintained within plasmids or transposons (20,21). Thus, even if the selection pressure exerted by antimicrobial drug use is removed, once resistance has emerged, its prevalence will not necessarily decline.
Although, in general, it appears that widespread antimicrobial drug use has led to the on-going development and refinement of mechanisms for spreading resistance, resistant bacteria isolated from companion animals are conspicuous by their absence as key organisms that have been studied to understand resistance gene organization. In other words, no sufficiently dramatic resistance event in bacteria isolated from dogs and cats has attracted anyone's attention. There are, however, few workers in this field, so that even common forms of important resistance have not been reported. For example, methicillin resistance in S. aureus (or S. intermedius) of canine origin may be more common than published reports indicate (22,23), perhaps because only a few workers have deliberately addressed this issue.
In summary, rather than simply becoming increasingly resistant to antimicrobial drugs, over 15 y, S. aureus and S. intermedius in dogs have increased in resistance to some antimicrobial drugs but decreased in resistance to others, changes that appear to reflect the changing patterns of use of individual drugs over a similar period. Antimicrobial resistance is not at a crisis stage in canine medicine, but there are warning signs. More information is needed on antimicrobial resistance and its molecular basis in canine medicine, as well as on antimicrobial drug usage by veterinarians in companion animal practice.
2. Has there been a change over time in the identity of bacteria isolated from infections in animals because of antibiotic resistance?
If antibiotic resistance is a problem in companion animal practice, one might expect to observe a change in the identity of bacteria in different infections over time. There are hints that this has happened. For example, a study of the prevalence and susceptibility of obligate anaerobes isolated from a variety of companion animals, including dogs and cats, associated developing resistance with changes in the prevalence of different anaerobic species over time, although it was acknowledged that changing techniques might have influenced this observation (24). This trend appears to have continued (25).
After making an assumption about the identity of isolates classified earlier as “Streptococcus spp.,” our survey of bacterial species in UTIs identified an apparent increase in infection with Enterococcus spp. over the 15 y examined. While not conclusive, these findings can be interpreted as evidence of an increase in enterococcal UTIs in dogs. This would reflect the inherent resistance of this organism to common antibiotics, so it would be expected to emerge among the type of canine patient seen in the VTH-OVC, which include animals with urinary tract catheters. This is consistent with what has happened in secondary and tertiary care human hospitals (26,27). More studies of the changing patterns of bacterial infections in companion animals are required, however, with fewer assumptions about bacterial identities.
Nosocomial infections with multiresistant bacteria, such as Acinetobacter baumannii (28), Enterobacter spp. (29), Klebsiella spp. (30), and Salmonella enterica serovars (31,32), have been recognized in hospitalized dogs, particularly in intensive care units, for many years. Such infections are probably both under-recognized and under-reported. The increasing availability of modern, simple, DNA-based fingerprinting systems, such as randomly amplified polymorphic DNA, should make it much easier to more fully document such occurrences in the future.
In summary, in the last 15 y, there has been an apparent increase in the proportion of Enterococcus spp. in UTIs in dogs, possibly reflecting the multiresistant character of the bacteria and, perhaps also, the changing nature of patients or procedures in the VTH-OVC. Other innately resistant bacteria may have marginally increased proportionately.
3. What is “prudent use” of antimicrobial drugs in companion animals? Are there any specific guidelines for companion animals other than the generic guidelines promulgated by national veterinary organizations?
The essence of prudent use is to ensure that antimicrobial drugs are used only where necessary; for as short a time as possible, consistent with clinical efficacy; with optimal dosage and administration using the narrowest spectrum antimicrobial; guided, where possible, by laboratory findings or in consultation with a veterinarian; and in a manner that does not cause toxicity to the treated animal and that minimizes the development and spread of resistant bacteria, or of their resistance genes.
Several English-speaking national veterinary associations, including the Canadian Veterinary Medical Association, have produced general guidelines for prudent or judicious antimicrobial drug use in the last 2 y. The effort required to capture these elements in generic guidelines has been considerable. In addition to these generic national association guidelines (available by searching national association Web sites), individual species groups have developed or are developing species-specific guidelines. For example, the American Animal Hospital Association is developing guidelines under the auspices of the American Veterinary Medical Association Steering Committee on the Judicious Use of Antimicrobials. The British Small Animal Veterinary Association has infection-specific guidelines in its Manual on Infectious Diseases (2000).
Should some antimicrobial drugs (amikacin, imipenem, and vancomycin) that are essential in human medicine for the treatment of multiresistant bacteria or of serious, mixed bacterial infections in tertiary care institutions be unavailable to veterinarians? The rumors of the use of imipenem to treat cat-bite abscesses may just be hospital rumors, but international agreement about the circumstances under which “top-shelf” drugs can be used would be helpful. We need to recognize that the use of any broad-spectrum antimicrobial drug to treat a bacterial infection affects all the bacteria in the body, not just the target organism, and is liable to select for considerably enhanced colonization of the intestine and other sites by innately multiresistant bacteria, such as Acinetobacter spp. and Enterococcus spp.
Will prudent use guidelines affect how veterinarians prescribe antimicrobial drugs, and which drugs they prescribe, and will their use reduce resistance? How will we know?
Perhaps with the exception of those in some Scandinavian countries, we won't know. This is because, unlike those Scandinavian countries, we currently lack data on quantities of antimicrobial drugs used in companion animal practice and there are no baselines of resistance data against which to judge any effects. Until such data are available, we will have to go on faith that heightened concerns about resistance will encourage veterinarians to evaluate and improve their decision-making practices for prescribing antimicrobial drugs, reduce their overall use of the drugs, and, thereby, reduce selection pressure for resistance. Antimicrobial drug prescription by Canadian physicians declined by 14% between 1995 and 2000 (33), probably, at least in part, as a result of efforts to enhance prudent use. On-going monitoring of antimicrobial susceptibility of S. pneumoniae has shown a significant reduction of penicillin G and trimethoprim-sulphamethoxazole resistance in Canada in the last 2 y, corresponding to a decline in the use of these 2 drugs (34).
In summary, the lack of reliable data on resistance in canine pathogens means that we will not know whether more prudent use, including reduction, of antimicrobial drugs in canine medicine will lead to reduced resistance. Veterinarians may have to accept on faith that more prudent use is worthwhile, based on the fact that recent reductions in medical prescriptions of antimicrobial drugs in Canada have been followed by a significant reduction in resistance in a selected pathogen (34).
4. Are owners at risk when companion animals are treated with antimicrobial drugs?
This is an area that has received little, if any, systematic attention, so the answer must be a mix of “we don't know,” “possibly however” and “of course.” Bacteria in animals are often and, in some cases, markedly adapted to their hosts. However, there is evidence, for example, that S. intermedius can transfer from dogs to humans (35,36) and somewhat anecdotal evidence that methicillin-resistant S. aureus (MRSA) has been transferred indirectly from a dog to a patient in an intensive care unit (37). Clearly, however, this is a 2-way street, since MRSA spread from humans may have been responsible for an outbreak of MRSA in horses in a veterinary teaching hospital (38) and there is evidence that cats become colonized by S. aureus of human origin (39). The potential for spread of multiresistant Salmonella enterica serovars from companion animals to humans as a result of antimicrobial use in infected animals enhancing shedding, though not well documented, must always be recognized (32,40). The theoretical potential for owners to be infected by resistant pathogens or colonized, if only transiently, must be recognized. The potential problem is greatest in the immunocompromised owner.
In summary, owners probably are at some risk when their animals are treated with antimicrobial drugs, but, at the moment, the risk is poorly defined and needs further study.
5. Who is the enemy? Can resistance problems in companion animals be blamed on medicine or agriculture?
The widespread use of avoparcin as a growth promoter in food animals in Europe resulted in the selection of vancomycin-resistance in their intestinal enterococci, which subsequently entered the food chain or in other ways reached humans in Europe (1,2). Dogs and cats in Europe were likely also infected from this source. Vancomycin-resistant enterococci were isolated from the feces of 48% of 23 dogs and 16% of 24 cats in The Netherlands (41) and from a smaller proportion of dogs and cats in Belgium (42). This illustrates the apparent spread of resistant bacteria from food to companion animals. If vancomycin was used in companion animals, it could provide the selection pressure for the emergence of vancomycin resistance in other organisms, since the resistance genes are transposable. Recently, in Canada, Salmonella reached dogs from infected pig ear “treats,” with subsequent infection of dog owners; among the Salmonella subsequently isolated from pig ears was a multiresistant S. typhimurium DT 104 (43).
Pharmaceutical companies also bear some responsibility because of the marketing pressures they sometimes exert on veterinarians to use newer drugs in cases where older drugs are adequate. The pressures to use fluoroquinolones as general first-line antimicrobial drugs is one such example.
The resolution to the problems of antimicrobial resistance, however, will not come from blaming others. All who use antimicrobial drugs must accept responsibility for their prudent use and accept that resistance reflects use, even though the relationship is complex (Figure 1, 2).
In summary, everyone who uses antimicrobial drugs has a responsibility to use them prudently; blaming others for problems of resistance is generally an unproductive exercise.
6. What recommendations can be made for the future?
Antimicrobial resistant bacteria will always be with us. The resistance crisis in human medicine has shown how resistance can emerge in community-acquired infections in a remarkably short time. Scientific data on the development of drug resistance in companion animal bacteria barely exist, with a few notable exceptions. We also have scant data on the quantities and type of antimicrobial drug use in companion animal practice and on the ways in which these drugs are used, and no reliable data on many of the topics raised in this discussion (44). A recent survey of veterinary practitioners in Australia led to the reassuring conclusion that drug selection for different disorders was generally appropriate when compared with recommendations in recent texts; inappropriate use was, however, evident in some cases (45).
We need to continue the process of improving and fine-tuning prudent use guidelines in companion animal practice, so that we avoid creating resistance problems. As a simple example, the Angell Memorial Animal Hospital has 3 categories of antimicrobial drug use. First choice: empirical use (no pending culture and susceptibility results), amoxicillin with or without clavulanic acid; first-generation cephalosporins; trimethoprim-sulphamethazine; tetracyclines. Second choice: antimicrobials that can be used only when justified by culture and susceptibility results, amikacin, 2nd- and 3rd-generation cephalosporins, fluoroquinolones, lincosamides, oxacillin. Third choice (last resort): antimicrobials that can be used only when justified by culture and susceptibility results and in consultation with named infectious disease specialists, vancomycin, imipenem-cilastasin (R. Remillard, Angell Memorial Hospital, Boston, Massachusetts, USA, personal communication). It could be highly beneficial to reach international agreement among veterinarians on a similar, simple but effective, approach to prudent companion animal drug use. At a minimum, there should be an effort by VTHs, referral hospitals, and private companion animal practices to develop formal guidelines based on the practices outlined above.
We need active and effective infection control programs in veterinary hospitals to minimize spread of resistant organisms, or their resistance genes, from patients, especially those treated with broad-spectrum and potent antimicrobial drugs. The science of veterinary hospital infection control is hardly born yet, but the increasing number of neutropenic or immunosuppressed dogs and cats being treated in veterinary medicine means that we are going to have to embrace this topic, not just with enthusiasm but also systematically and with resources.
Veterinary clinical microbiology is also ripe for further development as a science rather than an art. As a matter of urgency, veterinary clinical microbiologists around the world should agree on standards for monitoring and reporting resistance in companion animal bacteria, perhaps as part of broader national resistance monitoring of bacteria of food animal origin. This data needs to be coupled with antimicrobial drug use data. We also need to understand the limitations of the data available and work to improve it. Canadian veterinary microbiology laboratories could, at low cost, agree to use compatible susceptibility testing methods and criteria, and share this data with veterinarians on a regular basis.
Physicians, food animal veterinarians and producers, and companion animal veterinarians need to be prudent, rational, responsible, and judicious in their antimicrobial drug use. They also need to recruit owners into partnership in this issue. We need to improve on the recently reported only 44% of owners who fully complied with the instructions for short-term oral antibacterial drug use (46), perhaps by engaging more effectively in client education on antimicrobial drug use and by better understanding our own prescribing practices.
Footnotes
Acknowledgment
We are grateful to Jeff Gruel for obtaining the clinical microbiology data and Cecily Strutt for providing the pharmacy data from the records of the Veterinary Teaching Hospital of the Ontario Veterinary College. CVJ
A shorter version of this paper was published as Conference Proceedings, Australian Veterinarians in Industry and Australian Veterinarians in Public Health, Australian Veterinary Association, Perth, Australia, July 2000, pages 29–33.
Address correspondence to Dr. John F. Prescott.
This special report was peer reviewed.
References
- 1.Wegener HC, Aarestrup FM, Gerner-Smidt P, et al. Transfer of antibiotic resistant bacteria from animals to man. Acta Vet Scand 1999;92 (Suppl):51–57. [PubMed]
- 2.Van den Bogaard AE, Stobberingh EE. Antibiotic usage in animals. Impact on bacterial resistance and public health. Drugs 1999;58: 589–607. [DOI] [PubMed]
- 3.Sternberg S. Antimicrobial resistance in bacteria from pets and humans. Acta Vet Scand 1999;92 (Suppl):37–50. [PubMed]
- 4.Schwartz S, Noble WC. Aspects of bacterial resistance to antimicrobials used in veterinary dermatological practice. Vet Dermatol 1999;10:163–176. [DOI] [PubMed]
- 5.Oluoch AO, Weisger R, Siegel AM, et al. Trends of bacterial infections in dogs: characterization of Staphylococcus intermedius isolates (1990–1992). Canine Pract 1996;21(2):12–19.
- 6.Hirsh DC, Jang SS. Antimicrobial susceptibility of selected infectious bacterial agents obtained from dogs. J Am Anim Hosp Assoc 1994;30:487–494.
- 7.Kruse H, Hofsgagen M, Thoresen SI, et al. The antimicrobial susceptibility of Staphylococcus species isolated from canine dermatitis. Vet Res Comm 1996;20:205–214. [DOI] [PubMed]
- 8.Lloyd DH, Lamport AI, Feeney C. Sensitivity to antibiotics amongst cutaneous and mucosal isolates of canine pathogenic staphylococcus in the UK, 1980–1996. Vet Dermatol 1996;7: 171–175. [DOI] [PubMed]
- 9.Normand EH, Gibson NR, Taylor DJ, Carmichael S, Reid SWJ. Trends of antimicrobial resistance in bacterial isolates from a small animal referral hospital. Vet Rec 2000;146:151–155. [DOI] [PubMed]
- 10.Walker RD, Thornsberry C. Decrease in antibiotic susceptibility or increase in resistance? J Antimicrob Chemother 1998;41:1–4. [DOI] [PubMed]
- 11.Walker AL, Jang SS, Hirsh DC. Bacteria associated with pyothorax of dogs and cats: 98 cases (1989–1998). J Am Vet Med Assoc 2000;216:359–363. [DOI] [PubMed]
- 12.Biberstein EL, Frantic CE, Jang SS, et al. Antimicrobial sensitivity patterns in Staphylococcus aureus from animals. J Am Vet Med Assoc 1974;164:1183–1186. [PubMed]
- 13.Medleau L, Long RE, Brown J, et al. Frequency and antimicrobial susceptibility of Staphylococcus species isolated from canine pyodermas. Am J Vet res 1986;47:229–231. [PubMed]
- 14.Noble WC, Kent LE. Antibiotic resistance in Staphylococcus intermedius isolated from cases of pyoderma in the dog. Vet Dermatol 1992;3:71–74.
- 15.Wegener HC, Pedersen K. Variations in antibiograms and plasmid profiles among multiple isolates of Staphylococcus intermedius from pyoderma in dogs. Acta Vet Scand 1992;33:391–394. [DOI] [PMC free article] [PubMed]
- 16.National Council on Clinical Laboratory Standards. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals: Approved standard. NACCLS Document M31-A. Wayne, Pennsylvania: National Council on Clinical Laboratory Standards.
- 17.McGowan JE. Does antibiotic restriction prevent resistance? New Horizons1996;4:370–376. [PubMed]
- 18.Naidoo J, Lloyd DH. Transmission of genes between staphylococci on skin. In: Woodbine M, ed. Antimicrobials and Agriculture. London: Butterworths, 1983:284–292.
- 19.Matthews PR, Cameron FH, Stewart PR. Occurrence of chloramphenicol acetyltransferase and Tn9 among chloramphenicol-resistant enteric bacteria from humans and animals. J Antimicrob Chemother 1983;11:535–542. [DOI] [PubMed]
- 20.Chiew Y-F, Yeo S-F, Hall LMC, et al. Can susceptibility to an antimicrobial be restored by halting its use? The case of streptomycin versus Enterobacteriaceae. J Antimicrob Chemother 1998;41:247–251. [DOI] [PubMed]
- 21.Low DE, Kellner JD, Wright GD. Superbugs: How they evolve and minimize the cost of resistance. Curr Infect Dis Rep 1999;1: 464–469. [DOI] [PubMed]
- 22.Pak SI, Han HR, Shimizu A. Characterization of methicillin-resistant Staphylococcusaureus isolated from dogs in Korea. J Vet Med Sci 1999;61:1013–1018. [DOI] [PubMed]
- 23.Tomlin J, Pead MJ, Lloyd DH, et al. Methicillin-resistant Staphylococcus aureus infection in 11 dogs. Vet Rec 1999;144: 60–64. [DOI] [PubMed]
- 24.Hirsh DC, Indiveri MC, Jang SS, et al. Changes in prevalence and susceptibility of obligate anaerobes in clinical veterinary practice. J Am Vet Med Assoc 1985;186:1086–1089. [PubMed]
- 25.Jang SS, Breher JE, Dabaco LA, et al. Organisms isolated from dogs and cats with anaerobic infections and susceptibility to selected antimicrobial agents. J Am Vet Med Assoc 1997;210: 1610–1614. [PubMed]
- 26.Murray BE. The life and times of the Enterococcus. Clin Microbiol Rev 1990;3:46–65. [DOI] [PMC free article] [PubMed]
- 27.Hunt CP. The emergence of enterococci as a cause of nosocomial infection. Brit J Biomed Sci 1998;55:149–156. [PubMed]
- 28.Francey T, Gaschen F, Nicolet J, et al. The role of Acinetobacter baumannii as a noscomial pathogen for dogs and cats in an intensive care unit. J Vet Intern Med 2000;14:177–183. [DOI] [PubMed]
- 29.Lippert AC, Fulton RB, Parr AM. Nosocomial infeciton surveillance in a small animal intensive care unit. J Am Anim Hosp Assoc 1988;24:627–636.
- 30.Glickman LT. Veterinary nosocomial (hospital-acquired) Klebsiella infections. J Am Vet Med Assoc 1981;179:1389–1392. [PubMed]
- 31.Uhaa IJ, Hird DW, Hirsh DC, et al. Case-control study of risk factors asscoiated with nosocomial Salmonella krefeld infection in dogs. Am J Vet Res 1988;49:1501–1505. [PubMed]
- 32.Tillotson K, Savage CJ, Salman MD, et al. Outbreak of Salmonella infantis infection in a large animal veterinary teaching hospital. J Am Vet Med Assoc 1997;211:1554–1557. [PubMed]
- 33.Canadian Committee on Antibiotic Resistance. Canada making headway on antibiotic resistance as drug use declines. Press release, January 25, 2000.
- 34.McGeer AJ, Trpeski L, Low DE, et al. Canadian Bacterial Surveillance Network. Decreasing antibiotic resistance in Streptococcus pneumoniae — what is the driving force? 40th Intersci Conf Antimicrob Agents Chemother, Toronto, Ontario, Sept. 17–20, 2000.
- 35.Harvey RG, Marples RR, Noble WC. Nasal carriage of Staphylococcus intermedius in humans in contact with dogs. Microb Ecol Health Dis 1994;7:225–227.
- 36.Tanner MA, Everett CL, Youvan DC. Molecular phylogenetic evidence for noninvasive zoonotic transmission of Staphylococcus intermedius from a canine pet to a human. J Clin Microbiol 2000; 38:1628–1631. [DOI] [PMC free article] [PubMed]
- 37.Cefai C, Ashurst S, Owens C. Human carriage of methicillin-resistant Staphylococcus aureus linked with pet dog. Lancet 1994;344:539–540. [DOI] [PubMed]
- 38.Seguin JC, Walker RD, Caron JP, et al. Methicillin-resistant Staphylococcus aureus outbreak in a veterinary teaching hospital: potential human-to-animal transmission. J Clin Microbiol 1999; 37:1459–1463. [DOI] [PMC free article] [PubMed]
- 39.Devriese LA, Nzuambe D, Godard C. Identification and characterization of staphylococci isolated from cats. Vet Microbiol 1984;9:279–285. [DOI] [PubMed]
- 40.Wall PG, Davis S, Threlfall EJ, et al. Chronic carriage of multidrug resistant Salmonella typhimurium in a cat. J Small Anim Pract 1995;36:279–281. [DOI] [PubMed]
- 41.Van Belkum A, Van den Braak N, Thomassen, et al. Vancomycin-resistant enterococci in cats and dogs. Lancet 1996;348:1038–1039. [DOI] [PubMed]
- 42.Devriese LA, Ieven M, Goossens H, et al. Presence of vancomycin-resistant enterococci in farm and pet animals. Antimicrob Agents Chemother 1996;40:2285–2287. [DOI] [PMC free article] [PubMed]
- 43.Laboratory Centre for Disease Control. Human health risk from exposure to natural dog treats. Canada Comm Dis Rep 2000;26:06. [PubMed]
- 44.Eriksson A. Policy, pets and horses. Acta Vet Scand 1992;92 (Suppl):102–104.
- 45.Watson ADJ, Maddisson JE. Systemic antibacterial drug use in dogs in Australia. Aust Vet J 2001;79:740–746. [DOI] [PubMed]
- 46.Grave K, Tanem H. Compliance with short-term oral antibacterial drug treatment in dogs. J Small Anim Pract 1999;40:158–162. [DOI] [PubMed]