Abstract
Background and Aim
Adipose tissue is the most abundant endocrine tissue in the body, producing leptin, a hormone important in regulating hunger, and adiponectin, a hormone involved in insulin sensitivity and inflammation. The aim of this study was to assess the impact of gastric bypass surgery (GBS) on leptin levels and its relation to adipose tissue expression of adiponectin.
Methods
Omental and subcutaneous adipose tissue and serum were obtained from 40 obese patients undergoing GBS, 13 patients one-year or more post-GBS, and 16 non-obese individuals (BMI 20-29). Adiponectin gene expression was measured by quantitative real time PCR and the gene expression was normalized for the GAPDH gene. Serum leptin and adiponectin were measured by a high-sensitivity enzymatic assay.
Results
Leptin levels were significantly lower in the post-GBS patients as compared to pre-GBS patients (19.8±6.7 vs. 59.0±5.1, p=0.0001) and similar to non-obese controls (19.8±6.7 vs. 18.2±4, p=0.8). Univariate analysis showed an inverse correlation between serum leptin levels and omental adiponectin gene expression (r=-0.32, p=0.01).
Conclusions
Gastric bypass surgery results in resolution of the leptin resistance status that characterizes obese subjects. We also demonstrated a significant correlation between leptin and adiponectin. This correlation provides preliminary evidences for studying a potential adiponectin-leptin cross-talking that may represent one of the physiological pathways responsible for the regulation of food intake in humans.
Keywords: adiponectin, leptin, gastric bypass surgery, bariatric surgery, visceral fat, adipose tissue
INTRODUCTION
Adipose tissue is the most abundant endocrine tissue in the body, producing several peptides named adipokines [1]. Among them, the two most important are leptin and adiponectin. Leptin is 16 kDa protein encoded by the ob gene in mice (LEP in humans) and secreted by adipose tissue in circadian pulsatile fashion [2]. Leptin plays a key role in regulating energy intake and energy expenditure, including appetite and metabolism [2]. Adiponectin is a 247 amino acids protein consisting of four domains, with a molecular weight of 30 kDa that have insulin-sensitizing, anti-atherogenic, and anti-inflammatory actions [3]. Circulating concentrations of adiponectin are decreased in subjects with obesity, type 2 diabetes, hypertension, dyslipidemia, and cardiovascular disease as compared with healthy individuals [3]. In 2008, Fain and collaborators, using omental adipose tissue explants determined that adiponectin release is reduced by obesity [4].
Until recently, there was no evidence of interaction between leptin and adiponectin pathways. In 2004, Minokoshi and collaborators demonstrated in mice that leptin inhibits AMPK activity in the hypothalamus and thereby reduces food intake [5]. Based on these findings, Kubota and collaborators investigated in mice the effect of adiponectin on the leptin-induced suppression of AMPK activity in the hypothalamus [6]. In their study, they demonstrated that both adiponectin receptors, AdipoR1 and AdipoR2 were found to be abundantly expressed in the hypothalamus with expression levels comparable to those in the liver. In situ hybridization revealed that adiponectin and the leptin receptors are localized in the same region of the hypothalamus. Their work was also fundamental in demonstrating that after intravenous (IV) infusion of adiponectin, the peptide can cross the hematoencephalic barrier in the hexamer and trimer forms. Their key finding was the demonstration that adiponectin and leptin modulate the AMPK phosphorylation in the hypothalamus [6]. They concluded that the central adiponectin-leptin signaling represents the physiological pathway by which hypothalamic AMPK activity and food intake are stimulated under fasting conditions and suppressed by refeeding.
Presently, there are limited evidences of interaction between leptin and adiponectin systems in humans. The only published study focused on a human disease that is the opposite of obesity: the anorexia nervosa [7]. In this study, adiponectin levels were inversely correlated with leptin levels and BMI during in hospital refeeding without reaching statistically significance [7].
In our study, we hypothesize that the central adiponectin-leptin signaling may represent the physiological pathway responsible for the anorexia observed after gastric bypass surgery. To test the hypothesis that this pathway is important in the pathogenesis of human obesity, we will assess the impact of gastric bypass surgery (GBS) on leptin levels and its relation to serum and adipose tissue adiponectin levels.
METHODS
After receiving Institutional Review Board approval, 69 study participants were enrolled in the study. The cohort of participants was divided in three groups: 1) subjects status post-GBS (with a least 12 months follow up) undergoing an elective abdominal surgical procedure (e.g. cholecystectomy, abdominal wall hernia repair, and/or abdominoplasty); 2) subjects with class II and III obesity undergoing GBS, and non-obese (BMI: 20-29) control subjects requiring an elective abdominal surgery. The study employed the following exclusion criteria: diagnosis of neoplastic disease, inflammatory bowel disease, acute cholecystitis, and pregnancy.
During the surgical procedure, immediately after entering the abdominal cavity, two <0.5-cm3 biopsies of abdominal subcutaneous and intra-abdominal omental fat tissue were obtained. The tissue samples were washed in PBS solution and then soaked in RNAlater preservative solution (Qiagen, Courtaboeuf, France) and stored at –80°C. All the samples were stored in the same -80°C freezer and then analyzed together. Total RNA was extracted from the adipose tissue biopsies using the Lipideasy total RNA Mini kit (Qiagen). The integrity of total RNA was checked by electrophoresis through an agarose gel. Adiponectin gene expression was studied by quantitative, real time RT-PCR using the specific protocol for the iCycler iQ Detection System (BioRad ,Hercules, CA, USA) with SYBR green fluorophore. Reactions were performed in a total volume of 20μL—including 10μL 2× SYBR Green PCR Master Mix (BioRad ,Hercules, CA, USA), 1μL of each primer at 5μM concentration, and 1μL of the previously reverse-transcribed cDNA template. The PCR primer sequence for Adiponectin and GAPDH was chosen based on the sequences available in GenBank (www.ncbi.nlm.nih.gov/Genbank). Melting curves were used to determine the specificity of the gene products, which was subsequently confirmed by running the PCR products on agarose gels. The threshold cycle (CT) value for each reaction, reflecting the amount of PCR needed to identify a target gene was calculated. Specimens were run in duplicate and the CT values averaged. GAPDH was used as internal control housekeeping gene to normalize the PCRs for the amount of RNA added to the reverse transcription reactions.
A fasting blood sample was also obtained from each subject immediately before the induction of general anesthesia and immediately spun in a refrigerated (4°C) centrifuge at 3,000 rpm for 10 minutes. The extracted plasma was used for adiponectin and leptin determinations by high-sensitivity enzyme-linked immunosorbent assay (ELISA).
Statistical Analysis
Comparisons of continuous variables between the groups were completed by unpaired Student's t tests. Categorical variables were compared using the chi-square test or Fisher's exact test. Variables that showed a significant univariate correlation were confirmed to be independent in a multiple stepwise regression model to adjust for potential confounding factors. The SPSS statistical software program (version 15.0, SPSS, Chicago, USA) was used for all analyses. All tests were 2-tail. P values of less than 0.05 were considered to indicate statistical significance.
RESULTS
Abdominal adipose tissues and plasma were obtained from 40 obese subjects undergoing GB surgery, 16 non-obese subjects, and 13 subjects after gastric bypass at the average follow-up of 19 months after the procedure.
As shown in Table 1, the three groups showed no significant differences in terms of mean age. The post-GBS group was characterized by a significant higher percent of female subject when compared with the non-obese group. Obviously, by study design the post-GBS and the non-obese groups had a mean BMI significantly lower (P=0.001) than the obese group.
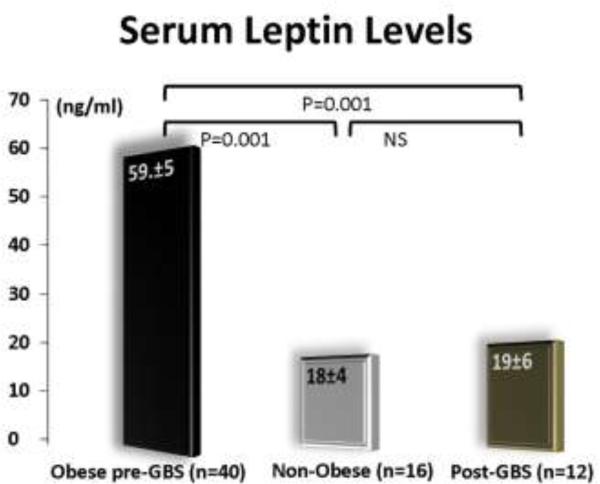
As shown in Figure 1, serum leptin levels were significantly lower (p<0.001) in the post-GBS patients as compared to the obese patients and similar to the non-obese controls.
Figure 1.
Serum leptin levels in the three groups
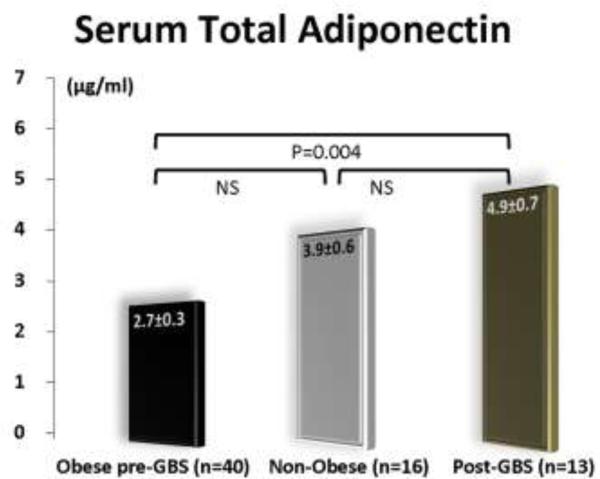
Figure 2 depicts total serum adiponectin levels in the three groups. The post-GBS group had significantly (p=0.004) higher levels of adiponectin than the obese group.
Figure 2.
Serum adiponectin levels in the three groups
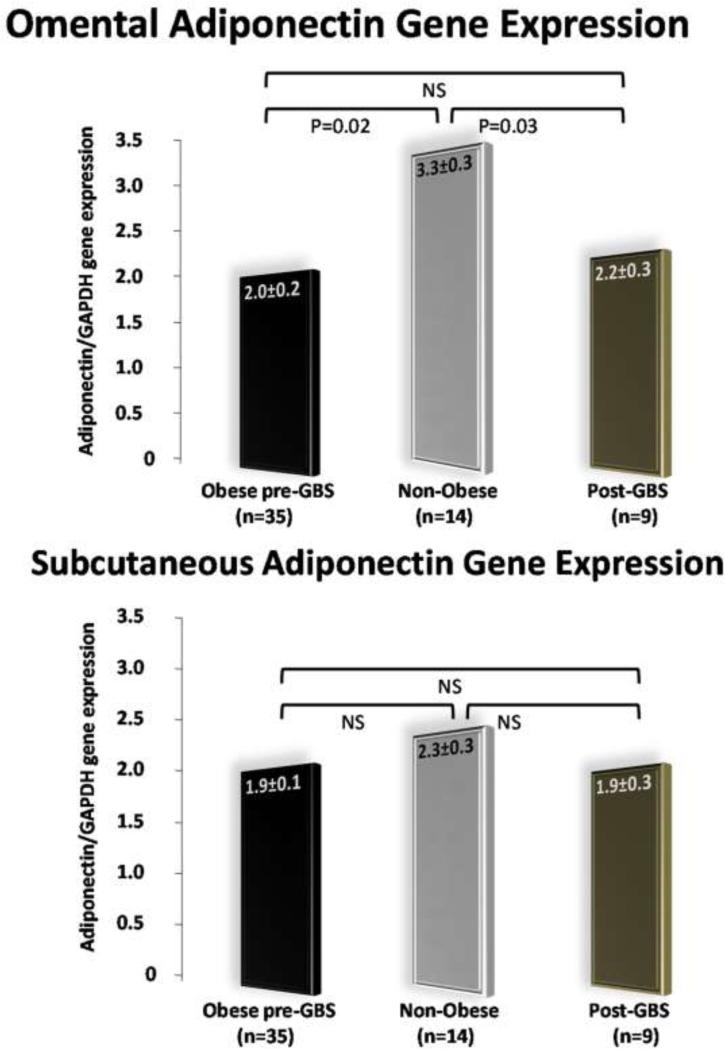
As shown in Figure 3, the adiponectin gene expression in the omental adipose tissues was significantly different among the three groups. On the contrary, the three groups showed similar levels of adiponectin gene expression in the subcutaneous adipose tissue samples.
Figure 3.
Adiponectin gene expression in subcutaneous and omental fat depots
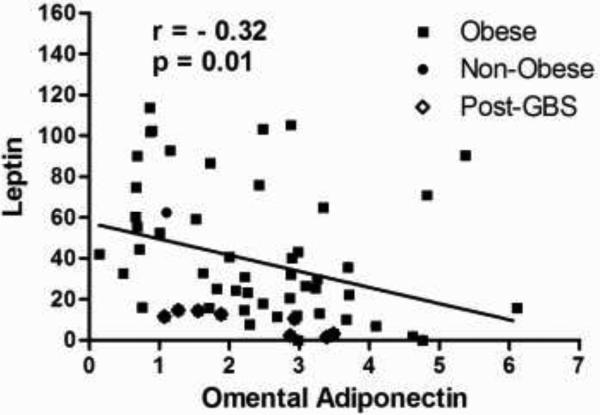
The correlation plot analysis shown in Figure 4 that includes all the three groups, demonstrated a significant inverse correlation between serum leptin levels and omental adiponectin expression (r=-0.32, p=0.01). This univariate correlation was confirmed to be independent in a multiple stepwise regression model to predict leptin after adjusting for confounding factors (BMI and age).
Figure 4.
Correlation plot between serum leptin and omental adiponectin.
Data analysis showed no significant correlation between serum leptin and subcutaneous adiponectin gene expression (r =-0.20, p=0.1). No significant correlation was also observed between serum leptin and serum adiponectin (r =-0.14, p=0.2).
DISCUSSION
In the current study, GBS results in resolution of the leptin resistance status that characterizes obese subjects and up-regulation of the adiponectin gene expression in the visceral fat. We also demonstrated in the entire cohort of patients enrolled in the study, a significant correlation between serum leptin and adiponectin gene expression in the visceral fat. This important correlation provides the basis for further investigations aimed to study the adiponectin-leptin cross-talking as one of the physiological pathways responsible for the regulation of food intake in humans.
Adipose tissue is a complex, essential, and highly active metabolic and endocrine organ. Adipose tissue not only responds to afferent signals from traditional hormone systems and the central nervous system but also expresses and secretes factors with important endocrine functions [8]. These factors include leptin and adiponectin. Fain and collaborators have demonstrated that leptin and adiponectin are mainly secreted by adipocytes whereas other adipokines are secreted by the stromal vascular fraction of the adipose tissue [9]. Until few years ago, these two proteins were thought to have no interaction, with only leptin active on the hypothalamus [10, 11]. A previous study in mice has shown that adiponectin stimulate fatty acid oxidation and enhance insulin sensitivity through the activation of AMP-activated protein kinase (AMPK) in the peripheral tissues [12]. However, the effects of adiponectin in the central nervous system was poorly understood until 2007, when Kubota and collaborators discovered that adiponectin stimulates AMPK in the hypothalamus and increases food intake [6]. Before this pivotal study, adiponectin was not thought to have a key role in regulating energy intake and energy expenditure. Because leptin has been reported to inhibit AMPK activity in the hypothalamus and to thereby suppress food intake [5], Kubota et al also investigated the effect of adiponectin on the leptin-induced suppression of AMPK activity in the hypothalamus [6]. Their key finding was that the leptin-induced AMPK phosphorylation suppression in the hypothalamus was significantly reversed by IV injection of adiponectin [6]. Recently, these results were also validated by a study from Coope and collaborators where intracerebroventricular injection of recombinant rat adiponectin promoted anorexigenic condition in rats with a 40% reduction in food intake and activates signal transduction through the classical insulin and leptin signaling pathways [13].
Preliminary evidence of interaction between the adiponectin and leptin systems was also provided by studying serum adiponectin and leptin levels in subjects with anorexia nervosa [7]. In this study serum adiponectin levels were inversely correlated with leptin levels and BMI during in hospital refeeding. This finding corroborates the results of a previous animal study suggesting that adiponectin might have a role in maintaining energy homeostasis under energy shortage conditions [14].
Several studies have shown resolution of the leptin-resistance status after bariatric surgery procedures [15-19]. Serum adiponectin is also well known to increase significantly after weight loss induced by bariatric surgery [20-22]. On the contrary, very few studies have focused on the adiponectin gene expression in adipose tissue before and after bariatric surgery [20, 23]. However, in all these studies samples were taken only from the subcutaneous fat depot. Our study was innovative in that it included a determination of adiponectin gene expression in the visceral fat before and after GBS and more important, we were able to demonstrate for the first time in humans a significant correlation between omental adiponectin and serum leptin. Our current findings support the hypothesis that high levels of circulating adiponectin combined with low level of leptin may be responsible for the anorexia observed after GBS [24]. Further studies using a rodent model of GBS are needed to confirm that increased postoperative adiponectin levels enhances AMPK activity in the arcuate hypothalamus via its receptor to regulate food intake.
ACKNOWLEDGMENTS
The authors thank Dr. Sarah Evans for comments on earlier versions of this article.
The study was supported by a National Institute of Health Grant: K23DK075907 (to AT)
Footnotes
This paper has been presented at the Plenary Session of the 2009 SAGES Annual Meeting held in Phoenix, Arizona from April 22-25.
Conflict of Interest Statement:
Drs. Jiegen Chen, Zehra Pamuklar, Anna Spagnoli have no conflicts of interest or financial ties to disclose.
Dr. Alfonso Torquati receives an honorarium from Cinemed and Allergan and research funding from Covidien.
REFERENCES
- 1.Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27:762–778. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- 2.Farooqi S, O'Rahilly S. Genetics of obesity in humans. Endocr Rev. 2006;27:710–718. doi: 10.1210/er.2006-0040. [DOI] [PubMed] [Google Scholar]
- 3.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 4.Fain JN, Buehrer B, Tichansky DS, Madan AK. Regulation of adiponectin release and demonstration of adiponectin mRNA as well as release by the non-fat cells of human omental adipose tissue. Int J Obes (Lond) 2008;32:429–435. doi: 10.1038/sj.ijo.0803745. [DOI] [PubMed] [Google Scholar]
- 5.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 6.Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Modan-Moses D, Stein D, Pariente C, Yaroslavsky A, Ram A, Faigin M, Loewenthal R, Yissachar E, Hemi R, Kanety H. Modulation of adiponectin and leptin during refeeding of female anorexia nervosa patients. J Clin Endocrinol Metab. 2007;92:1843–1847. doi: 10.1210/jc.2006-1683. [DOI] [PubMed] [Google Scholar]
- 8.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 9.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 10.Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- 11.Dridi S, Taouis M. Adiponectin and energy homeostasis: consensus and controversy. J Nutr Biochem. 2009;20:831–839. doi: 10.1016/j.jnutbio.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 13.Coope A, Milanski M, Araujo EP, Tambascia M, Saad MJ, Geloneze B, Velloso LA. AdipoR1 mediates the anorexigenic and insulin/leptin-like actions of adiponectin in the hypothalamus. FEBS Lett. 2008;582:1471–1476. doi: 10.1016/j.febslet.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 14.Saito K, Arata S, Hosono T, Sano Y, Takahashi K, Choi-Miura NH, Nakano Y, Tobe T, Tomita M. Adiponectin plays an important role in efficient energy usage under energy shortage. Biochim Biophys Acta. 2006;1761:709–716. doi: 10.1016/j.bbalip.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, Taveras C, Schrope B, Bessler M. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33:786–795. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vila G, Riedl M, Maier C, Struck J, Morgenthaler NG, Handisurya A, Prager G, Ludvik B, Clodi M, Luger A. Plasma MR-proADM correlates to BMI and decreases in relation to leptin after gastric bypass surgery. Obesity (Silver Spring) 2009;17:1184–1188. doi: 10.1038/oby.2009.22. [DOI] [PubMed] [Google Scholar]
- 17.Joao Cabrera E, Valezi AC, Delfino VD, Lavado EL, Barbosa DS. Reduction in Plasma Levels of Inflammatory and Oxidative Stress Indicators After Roux-En-Y Gastric Bypass. Obes Surg. 2009 doi: 10.1007/s11695-009-9988-2. [DOI] [PubMed] [Google Scholar]
- 18.Swarbrick MM, Stanhope KL, Austrheim-Smith IT, Van Loan MD, Ali MR, Wolfe BM, Havel PJ. Longitudinal changes in pancreatic and adipocyte hormones following Roux-en-Y gastric bypass surgery. Diabetologia. 2008;51:1901–1911. doi: 10.1007/s00125-008-1118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia de la Torre N, Rubio MA, Bordiu E, Cabrerizo L, Aparicio E, Hernandez C, Sanchez-Pernaute A, Diez-Valladares L, Torres AJ, Puente M, Charro AL. Effects of weight loss after bariatric surgery for morbid obesity on vascular endothelial growth factor-A, adipocytokines, and insulin. J Clin Endocrinol Metab. 2008;93:4276–4281. doi: 10.1210/jc.2007-1370. [DOI] [PubMed] [Google Scholar]
- 20.Savu MK, Phillips SA, Oh DK, Park K, Gerlan C, Ciaraldi TP, Henry RR. Response of adiponectin and its receptors to changes in metabolic state after gastric bypass surgery: dissociation between adipose tissue expression and circulating levels. Surg Obes Relat Dis. 2009;5:172–180. doi: 10.1016/j.soard.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Engl J, Bobbert T, Ciardi C, Laimer M, Tatarczyk T, Kaser S, Weiss H, Molnar C, Tilg H, Patsch JR, Spranger J, Ebenbichler CF. Effects of pronounced weight loss on adiponectin oligomer composition and metabolic parameters. Obesity (Silver Spring) 2007;15:1172–1178. doi: 10.1038/oby.2007.627. [DOI] [PubMed] [Google Scholar]
- 22.Swarbrick MM, Austrheim-Smith IT, Stanhope KL, Van Loan MD, Ali MR, Wolfe BM, Havel PJ. Circulating concentrations of high-molecular-weight adiponectin are increased following Roux-en-Y gastric bypass surgery. Diabetologia. 2006;49:2552–2558. doi: 10.1007/s00125-006-0452-8. [DOI] [PubMed] [Google Scholar]
- 23.Coughlin CC, Finck BN, Eagon JC, Halpin VJ, Magkos F, Mohammed BS, Klein S. Effect of marked weight loss on adiponectin gene expression and plasma concentrations. Obesity (Silver Spring) 2007;15:640–645. doi: 10.1038/oby.2007.556. [DOI] [PubMed] [Google Scholar]
- 24.Guijarro A, Kirchner H, Meguid MM. Catabolic effects of gastric bypass in a diet-induced obese rat model. Curr Opin Clin Nutr Metab Care. 2006;9:423–435. doi: 10.1097/01.mco.0000232903.04910.7b. [DOI] [PubMed] [Google Scholar]