Abstract
The proinflammatory cytokine TNF-alpha has been shown to promote activation and sensitization of primary afferent nociceptors. The downstream signaling processes that play a role in promoting this neuronal response remain however controversial. Increased TNF-alpha plasma levels during migraine attacks suggest that local interaction between this cytokine and intracranial meningeal nociceptors plays a role in promoting the headache. Here, using in vivo single unit recording in the trigeminal ganglia of anesthetized rats, we show that meningeal TNF-alpha action promotes a delayed mechanical sensitization of meningeal nociceptors. Using immunohistochemistry, we provide evidence for non-neuronal localization of the TNF receptors TNFR1 to dural endothelial vascular cells and TNFR2 to dural resident macrophages as well as to some CGRP-expressing dural nerve fibers. We also demonstrate that meningeal vascular TNFR1 is co-localized with COX-1 while the perivascular TNFR2 is co-expressed with COX-2. We further report here for the first time that TNF-alpha evoked sensitization of meningeal nociceptors is dependent upon local action of cyclooxygenase (COX). Finally, we show that local application of TNF-alpha to the meninges evokes activation of the p38 MAP kinase in dural blood vessels that also express TNFR1 and that pharmacological blockade of p38 activation inhibits TNF-alpha evoked sensitization of meningeal nociceptors. Our study suggests that meningeal action of TNF-alpha could play an important role in the genesis of intracranial throbbing headaches such as migraine through a mechanism that involves at least partly activation of non-neuronal TNFR1 and TNFR2 and downstream activation of meningeal non-neuronal COX and the p38 MAP kinase.
1. Introduction
Throbbing headaches of intracranial origin, such as migraine are mediated by primary afferent nociceptive neurons that innervate the intracranial meninges and their related large blood vessels [23,27,40]. Increased discharge of these meningeal nociceptors has been suggested to promote the ongoing headache while increases in their mechanosensitivity is believed to mediate the throbbing pain as well as the exacerbation of the headache during events that increase intracranial pressure. The endogenous processes that promote meningeal nociceptors’ activation and mechanical sensitization during a headache episode remain speculative although local sterile meningeal inflammatory response has been suggested to play a critical role [18].
One particular proinflammatory mediator, the cytokine tumor necrosis factor- α (TNF-α) has been shown to promote in vivo activation and sensitization of primary afferent nociceptors innervating the hind paw [4,14], as well as deep musculoskeletal [17] and cranial muscular tissues [11]. The finding of elevated levels of TNFα in the internal jugular blood during migraine attacks [30,34] and increases in its CSF levels in chronic migraine patients [33] point to TNF-α as a potential critical mediator of migraine pain. However, whether this cytokine affects the response properties of meningeal nociceptors is not known.
Under normal physiological conditions, TNF-α has been suggested to promote enhanced nociception primarily through the activation of the TNF receptor 1 (TNFR1) [7,20,36,38] while under persistent injurious condition, activation of a second TNF receptor (TNFR2) has been implicated [4,36]. The peripheral cell types that express TNF-α receptors and the downstream signaling processes that mediate the pro-nociceptive effect of TNF-α remain however highly controversial. In-vitro studies conducted on dorsal root and trigeminal ganglia neurons have suggested that the nociceptive effects of TNF-α are mediated through direct interaction with neuronal TNF receptors and the consequent activation of various downstream intracellular signaling cascades including among others TRPV1 channels [26], TTX-resistant sodium channels [13], voltage activated calcium channels [8], intracellular calcium mobilization-related sphingolipid signaling [31] as well as the p38 MAP Kinase [4,13]. Whether these signaling cascades also play a role in mediating nociceptor sensitization at the nerve endings level is not known at present.
In addition to its postulated action on neuronal receptors, data from behavioral studies suggests that the enhanced nociception evoked by peripheral administration of TNF-α depends upon the local release of other immune or vascular-related mediators [6, 7,28,32,43]. The findings that pain hypersensitivity evoked by peripherally administered TNF-α can be ameliorated by systemic administration of non-selective cyclooxygenase (COX) inhibitors indometacin [7] or ibuprofen [35] suggest in particular the involvement of COX-derived prostaglandins in this process. Whether the nociceptors’ sensitizing effect exerted by TNF-α is mediated by local COX action is unknown.
Given its potential role as a headache mediator, in this work we initially investigated whether TNF-α can activate or sensitize meningeal nociceptors. Using local dural application of specific inhibitors, we then examined the relative contributions of local COX activity and the p38 MAPK signaling cascade in mediating the sensitizing effect of TNF-α effects. Finally, using immunohistochemistry, we investigated cell types that express TNF-α receptors in the dura mater.
2. Materials and methods
2.1 Animals
Sprague-Dawley male rats (250–300 g) were used in compliance with the experimental protocol approved by the institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center and Harvard Medical School and adhered to the guidelines of the Committee for Research and Ethical Issues of the international association for the study of Pain [48].
2.2 Electrophysiological recordings
Rats were deeply anesthetized with an initial intraperitoneal dose of 1.5 g/kg urethane plus 0.2 g/kg supplemental doses as needed. Single-unit recordings of meningeal nociceptors in the trigeminal ganglion were conduced as described before [19,46]. Briefly, the rat’s head was mounted in a stereotaxic apparatus and a craniotomy was used to expose the left transverse sinus, as well as the adjacent dura extending approximately 4 to 6 mm rostral and 2 mm caudal to the sinus. The exposed dura was then constantly bathed with modified synthetic interstitial fluid (SIF; 135 mM NaCl, 5 mM KCl, 1 mM MgCl2, 5 mM CaCl2, 10 mM glucose and 10 mM HEPES, pH 7.2). For single unit recording, a platinum-coated tungsten microelectrode (impedance ~150 KΩ; FHC, Bowdoin, ME) was lowered into the trigeminal ganglion through a small circular opening (2-mm wide) located 2 mm caudal to Bregma and 2 mm left to the midline. Meningeal nociceptors were identified by their constant latency responses to electrical stimuli (0.5 ms pulse, 0.5–5 mA, 0.5 Hz) applied to the transverse sinus. Conduction velocities were calculated based on a conductance distance between the stimulation site on dura and the recording site in the trigeminal ganglion of 12.5 mm and units were classified as either Aδ units (CV >1.5 m/sec) or C units (CV ≤1.5 m/sec). During the experiments, action potentials were acquired using a real-time waveform discriminator (Spike 2, CED, Cambridge, UK). Only one unit was tested in each animal.
2.3 Mechanical stimulation
Mechanical receptive fields of meningeal nociceptors were initially mapped using a series of calibrated von Frey monofilaments exerting pressure stimuli in the range of 38–443 kPa. Mechanically evoked neuronal responses were then determined quantitatively using a servo force-controlled stimulator (Series 300B, Aurora Scientific, Aurora, ON) fitted with a flat-ended plastic cylinder. One of three probe diameters (0.5, 0.8, or 1.1mm) was selected for each neuron, depending on the sensitivity of the neuron. Stimulus intensity is reported in units of pressure or force per area (kPa, where 1kPa = 1mN/mm2). Stimulus trials for testing changes in mechanical sensitivity consisted of graded square-wave stimuli (100-msec rise time, 2-sec width, 60-sec inter-stimulus interval) delivered in ascending order, which included threshold and suprathreshold stimuli. Neuronal responses to mechanical stimulation of the dura as well as ongoing spontaneous activity, were recorded every 15 min throughout the experiment. Baseline measurements of spontaneous and mechanically evoked activity were obtained in at least 3 consecutive trials prior to drug administration. Only units that exhibited consistent responses were tested further.
2.4 Experimental Design
Following the establishment of baseline neuronal responsiveness, the exposed dura was bathed for 60 min with recombinant rat TNF-α (rrTNF-α, 100 ng/ml, Peprotech, Rocky Hill, NJ) followed by a wash with SIF for another 60 min. The dose of TNF-α was chosen based on previous studies [3,13,31] as well as our preliminary data. To control for non-specific effects of TNF-α, in another group of animals, baseline neuronal responses were first obtained while the dura was pretreated with a SIF solution containing also a pegylated soluble TNFR1 which binds and neutralizes TNF (PEG-sTNFR1, 1.5 mg/ml, a gift from Amgen). Changes in ongoing activity and neuronal mechano-responsiveness were then recorded while the dura was bathed for 60 min with a mixture containing TNF-α and the PEG-sTNFR1 followed by 60 min wash period with SIF.
To examine the relative contribution of COX-related mediators in the development of TNF-α evoked meningeal nociceptors’ sensitization, a mix of TNF-α and the presumed selective inhibitors of COX-1 (SC-560, 100 nM) or COX-2 (NS-398, 10 µM) was applied for 60 min while recording changes in neuronal responses. The relative contribution of the MAP kinase p38 was tested similarly by applying a mixture of the p38 inhibitor SB203580 (50 µM, Tocris, International) and TNF-α for 60 min. Drug doses chosen for this study were derived from previous reports [2,12,19,22,25]. Some additional units were tested only with the COX and p38 inhibitors to examine their effect on the ongoing discharge rate and mechanosensitivity. In these studies, the inhibitors were applied for 60 min to the dura.
2.5 Immunohistochemistry
Rats were anesthetized with urethane as above and perfused transcardially with cold heparinized solution of 0.1 M phosphate-buffered saline (PBS, pH 7.4), followed by freshly made cold 4% paraformaldehyde in 0.1 M PBS. To study the expression of TNF-α receptors, the dura was removed and processed for either single or double labeling immunohistochemistry. Following an antigen retrieval protocol (2 hr incubation with citrate buffer, pH 6.0 at room temperature), sections were further incubated for 2 h at room temperature in 0.1 M PBS containing 2% fetal bovine serum as well as a non-immune serum from the host species of the secondary antibody and 0.1% Triton-X to block non-specific binding. Following blocking, specimen were incubated for 48 h at 4 °C with primary antibodies against TNFR1 (mouse monoclonal antibody raised against amino acids 30–301 mapping within the extracellular domain of TNFR1, SC-8436, 1:250, Santa Cruz Biotechnology) or against TNFR2 (goat polyclonal antibody raised against the C-terminus of TNFR2, SC-1072, 1:500, Santa Cruz Biotechnology). To examine dural cell types that might express TNFR1 and TNFR2, double labeling were conducted in conjunction with another antibody to detect: 1) peripheral nerve fibers (Peripherin, Rabbit polyclonal antibody, 1:1000, Ab1530, Millipore, or CGRP, Rabbit polyclonal antibody, 1:1000 T-4032, bachem), 2) dural vascular endothelial cells (vimentin, Rabbit polyclonal antibody, 1:250, SC-5565, Santa Cruz biotechnology), or 3) resident macrophages (mouse monoclonal antibody, CD163, clone ED2, 1:500, MCA342, Serotec) as we described earlier [47]. For all indirect immunofluorescence, Alexa 594 and/or Alexa 488- conjugated secondary antibodies (1:200, Invitrogen,) were employed. To investigate changes in dural expression of the phosphorylated form of p38 (pp38) after TNF-α treatment, a bilateral craniotomy was made to expose the dura mater. A TNF-α solution (100 ng/ml in SIF) was then placed on the left side of dura for 30 min. Ipsilateral and contralateral dural samples were then processed for immunohistochemistry using a rabbit polyclonal antibody that recognizes the p38 MAPK when phosphorylated at Thr180 and Tyr182 (1:500, Cat# 9211, Cell Signaling). Given that TNF-α evoked pp38 expression was detected only on dural blood vessels, TNF-α incubated dura mater samples were also dually labeled with the TNF-R1 antibody (also found to be expressed in dural blood vessels) and the pp38 antibody. To examine potential changes in dural vascular pp38 expression, the number of pp38 labeled vessels were counted in 15 separate visual fields, under X200 objective. For all immunohistochemical studies, controls for non-specific staining included omission of the primary antibody. For the TNFR2 antibody, a pre adsorption protocol (2 hr incubation with a blocking peptide for TNFR2, sc-1072 P, Santa Cruz biotechnology) was also conducted. Immunofluorescence images were obtained using a Leica fluorescent microscope (Leica DM6000). For each image, a montage was created from at least 10 consecutive optical images (≤1 micron sections) by utilizing the advanced motorized Z-stacking capability of the microscope.
2.6 Statistical analyses
Data are presented as mean ± SEM. TNF-α evoked sensitization was analyzed by applying the Friedman test on all time points tested. The level of significance was set at p=0.05. The time course of sensitization was analyzed by comparing neuronal responses at baseline to those obtained at 15, 30, 45 and 60 min after treatment with TNF-α and 60 min after wash with using the wilcoxon matched pairs signed ranks test. For this subsequent post hoc test, the Bonferroni correction was applied to lower the level of significance to 0.001 (i.e., 0.05/5). To test inhibition of TNF-evoked sensitization, the wilcoxon matched pairs signed ranks test was used. To test changes in the number of p38-labled dural vessels, the Mann Whitney test was applied. For these tests, p<0.05 was considered statistically significant.
3. Results
3.1 Responses of meningeal nociceptors to exogenous application of TNF-α
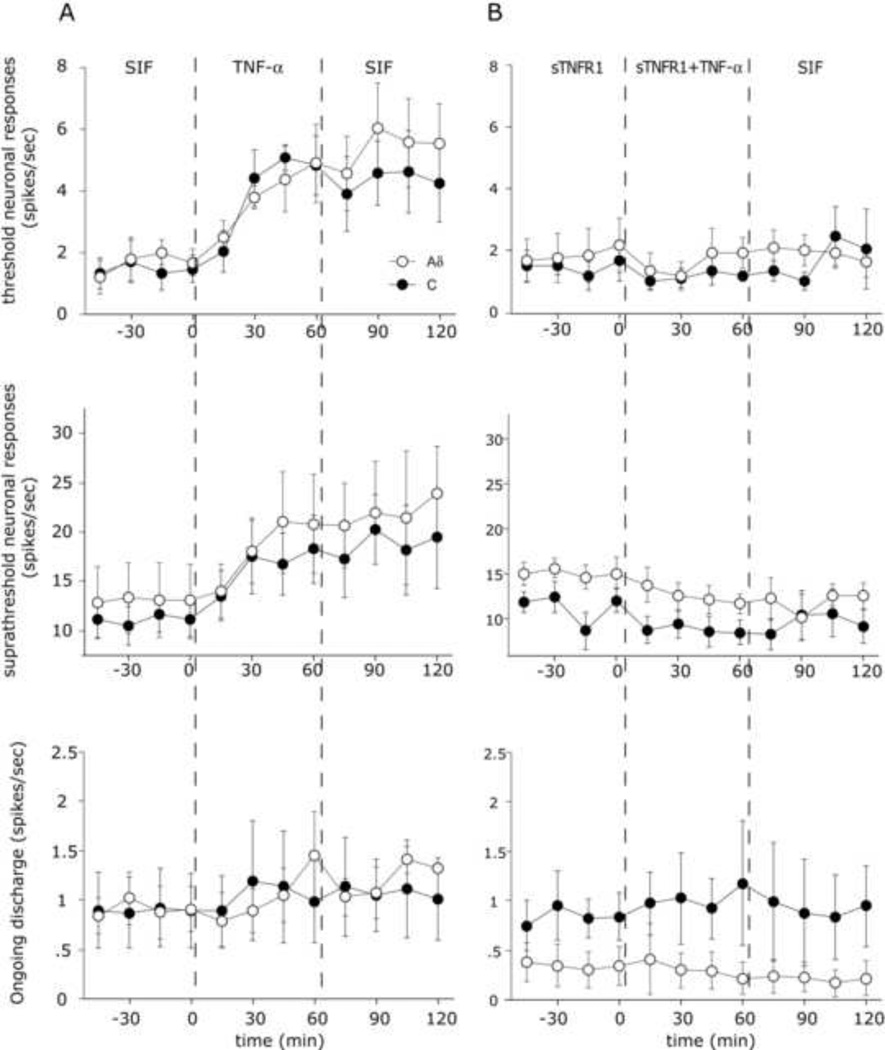
TNF-α evoked changes in ongoing discharge levels and mechanosensitivity was examined in 15 meningeal nociceptors (6 Aδ and 9 C-units). Among the units tested, neuronal activation was rare with only 2 Aδ units showing increased neuronal discharges. This increased activity returned to baseline almost immediately upon wash with SIF. As the example in Figure 1 demonstrates, in contrast to the lack of activation, dural application of TNF-α resulted in a progressive increase in mechanosensitivity. TNF-α evoked sensitization was noted in 10/15 units tested (5 Aδ and 5 C-units) with similar response profiles exhibited by both neuronal populations. Overall for the populations, there was a significant increase in threshold responses over time (Figure 2a, p<0.05 Friedman test). While some units showed sensitization beginning at 30 min, post hoc analysis revealed a statistically significant increase in threshold responses starting at 45 min post TNF-α application (p<0.05, Wilcoxon test). Following 60 minutes of wash with SIF, 8/10 units remained sensitized with overall enhanced threshold responsiveness elevated for the population (p<0.05, Wilcoxon test). Similar to the increases seen in threshold responsiveness, TNF-α application also promoted an increase in the suprathreshold responses in 9/15 units (5 Aδ and 4 C-units) tested (p<0.05, Friedman test). This increased responsiveness, similar to the increase in threshold responses, also began at 45 min (p<0.05, Wilcoxon test). The enhanced responsiveness of most units (7/9) remaining elevated (p<0.05, Wilcoxon test) even after a 60 min wash with SIF. To determine whether the effects of TNF-α were specific, changes in neuronal responses were tested in additional 10 units (5 Aδ, 5 C units) while bathing the receptive field with a mixture of TNF-α and PEG-sTNF-R1, which specifically binds and neutralizes TNF-α. As Fig 2B demonstrates, co-administration of TNF-α with PEG-sTNF-R1 completely blocked the TNF-α evoked increases in threshold and suprathreshold responsiveness. (p>0.05, Friedman test). Application of PEG-sTNF-R1 alone did not have an effect on baseline mechanosensitivity or ongoing activity (data not shown)
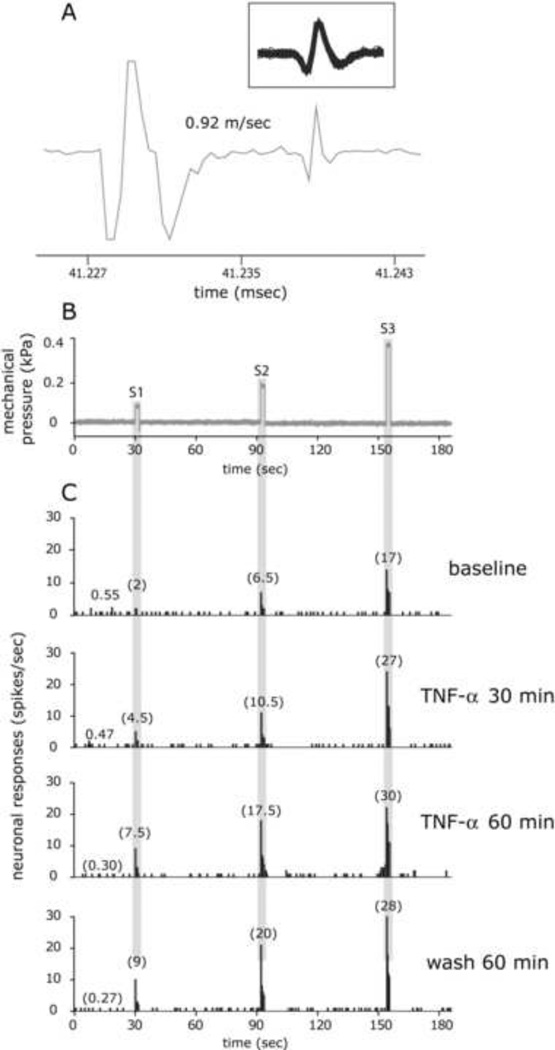
Figure 1.
TNF-α evoked sensitization of a C-unit meningeal nociceptor. A. Identification and isolation of a C-unit meningeal nociceptor based on the response latency (11 milliseconds) to electrical stimulation of the dura and a consistent waveform (insert). B. Peri-stimulus time histograms depicting the responses to threshold (S1) and suprathreshold (S2–3) mechanical stimulation of the dura at baseline, before TNF-α application, 30 and 60 min after TNF-α (100 ng/ml), and then 60 min after wash with SIF. The numbers in parenthesis indicate mean firing rate in spikes/sec. The rate of ongoing activity is depicted in all trials prior to S1. Note the persistent sensitization during the wash period.
Figure 2.
Time course changes in the average responses of Aδ and C-units meningeal nociceptors following topical application of TNF-α (100 ng/ml) and the effect of co-application of the TNF-α blocker Peg-sTNFR1 (1.5 mg/ml). A. Mean ± SEM response magnitude to mechanical stimulation of the dura using threshold (top) and suprathreshold (middle) pressure stimuli, and ongoing firing rate (bottom) at baseline with SIF, during TNF-α treatment and during the wash with SIF. B. Time course changes in the mean ± SEM responses to TNF-α in the presence of its blocker Peg-sTNFR1. Note the lack of TNF-evoked sensitization in the presence of the blocker.
3.2 The effect of local COX inhibition
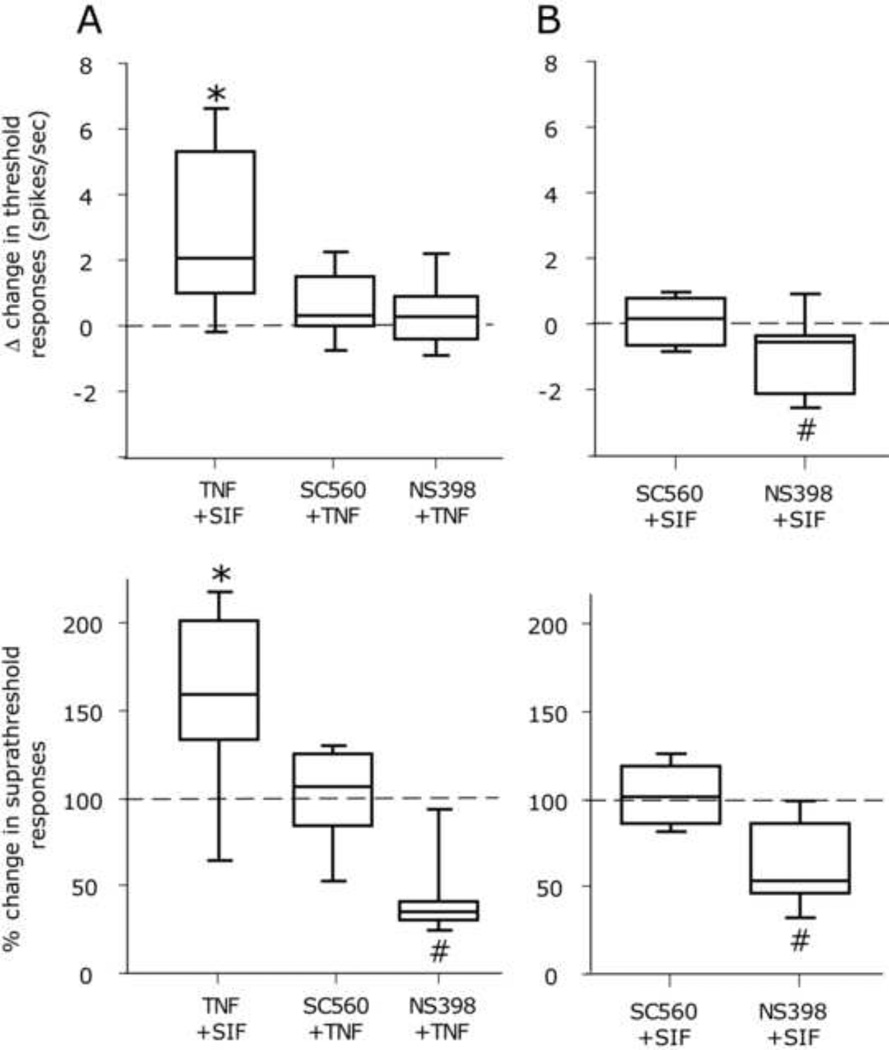
To examine the relative contribution of COX in mediating the nociceptive effect of TNF-α we tested whether SC-560 could inhibit meningeal nociceptors’ sensitization evoked by TNF-α. Application of SC-560 (5 Aδ, 6 C-units) did not affect baseline values (Fig 3 A). However, when co-administered with SC-560 (6Aδ, 6C-units), TNF-α failed to increase threshold as well as suprathreshold neuronal responsiveness (both p>0.05, Wilcoxon test, Fig 3B). When administered with NS-398 (4Aδ, 4C-units), TNF-α failed to increase threshold as well as suprathreshold neuronal responsiveness. At the suprathreshold levels, responses were actually lower than baseline (p<0.05, Wilcoxon test, Fig 3A). Application of NS-398 alone also reduced baseline threshold as well as supra-threshold response in 3 Aδ and 5 C units (both p<0.05. Wilcoxon test, Fig 3B).
Figure 3.
Effects of topical application of the COX inhibitors SC-560 (100 nM) and NS-398 (10µM) on baseline mechanical responsiveness of meningeal nociceptors and the development of TNF-α evoked mechanical sensitization. A. Median, 10th, 25th, 75th and 90th percentile changes in threshold (top) and suprathreshold (bottom) responses of meningeal nociceptors to mechanical stimulation of the dura 60 min following application of TNF-α alone (in SIF) and TNF-α in the presence of SC-560 or NS-398. Note the additional inhibition of suprathreshold values following treatment with NS-398. B. Median, 10th, 25th, 75th and 90th percentile changes in baseline threshold (top) and suprathreshold (bottom) responses of meningeal nociceptors following local application of SC-560 or NS-398. Note the inhibition in baseline responsiveness following NS-398 treatment. (* p<0.05 sensitization, # p<0.05, inhibition, Wilcoxon test, 60 min after drug vs. baseline).
3.3 The effect of local MAPK p38 inhibition
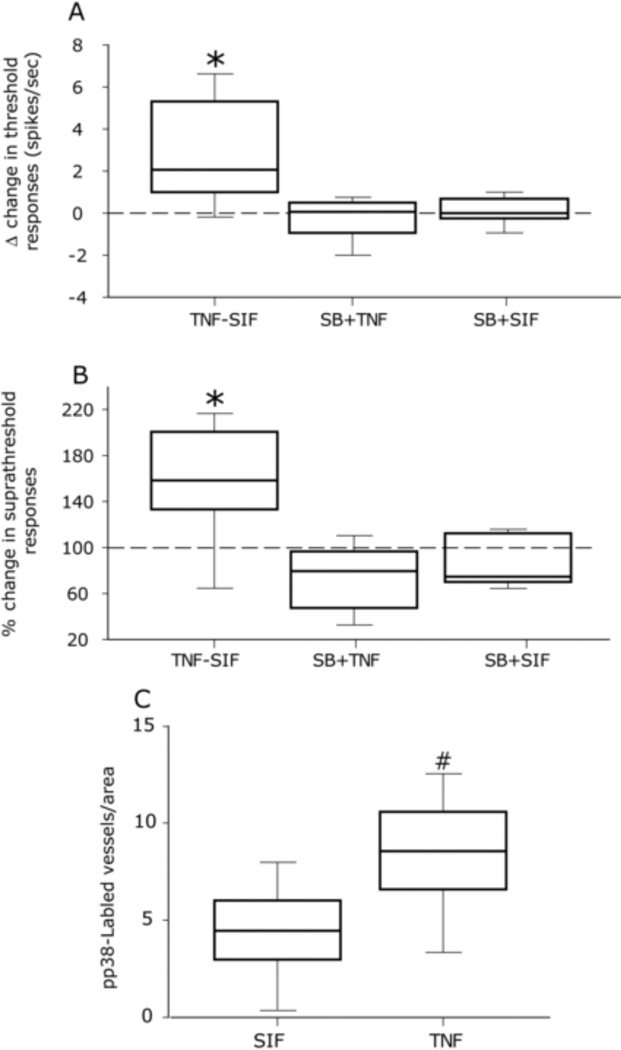
To investigate whether phosphorylation of the p38 MAPK is involved in mediating TNF-α evoked meningeal nociceptors’ sensitization, we tested in 5 Aδ and 5 C-units whether co-administration of the p38 inhibitor SB203580 could inhibit TNF-α evoked mechanical sensitization. As Figs 4A and B demonstrate, in the presence of SB203580, TNF-α did not increase threshold or suprathreshold responses (both p>0.05, Wilcoxon test). Application of SB203580 alone (4Aδ, 4-C units) did not affect baseline sensitivity (p>0.05 for both threshold and suprathreshold responses). To further address the role of p38, we examined the effect of dural application of TNF-α on the ensuing dural expression of pp38 using immunofluorescence in 4 animals. Following topical TNF-α application, pp38 immunofluorescence was detected only on dural vessels: the number of pp38 immunolabeled dural vessels at the side exposed to TNF-α was significantly higher than that observed at the contralateral SIF-only treated dura (7.65±0.3 vs 4.35±0.2, p<0.05 Mann Whitney, Fig 4C).
Figure 4.
TNF-α evoked meningeal nociceptors’ sensitization involves p38 MAP kinase. Median, 10th, 25th, 75th and 90th percentile changes in threshold (A) and suprathreshold (B) responses of meningeal nociceptors to mechanical stimulation of the dura 60 min following application of TNF-α alone (in the presence of SIF), TNF-α in the presence of the p38 inhibitor SB203580 (SB), or SB203580 alone (in the presence of SIF). Note the blockade of TNF-α evoked sensitization by SB203580 and the lack of effect of this drug on baseline values. (* p<0.05 Wilcoxon test, 60 min after drug vs. baseline). C. Average number of pp38 labeled dural blood vessels observed per visual field following topical application of TNF-α or SIF (# p<0.05, Mann Whitney test TNF-α vs. SIF).
3.4 Expression of TNF receptors in the rat’s dura mater
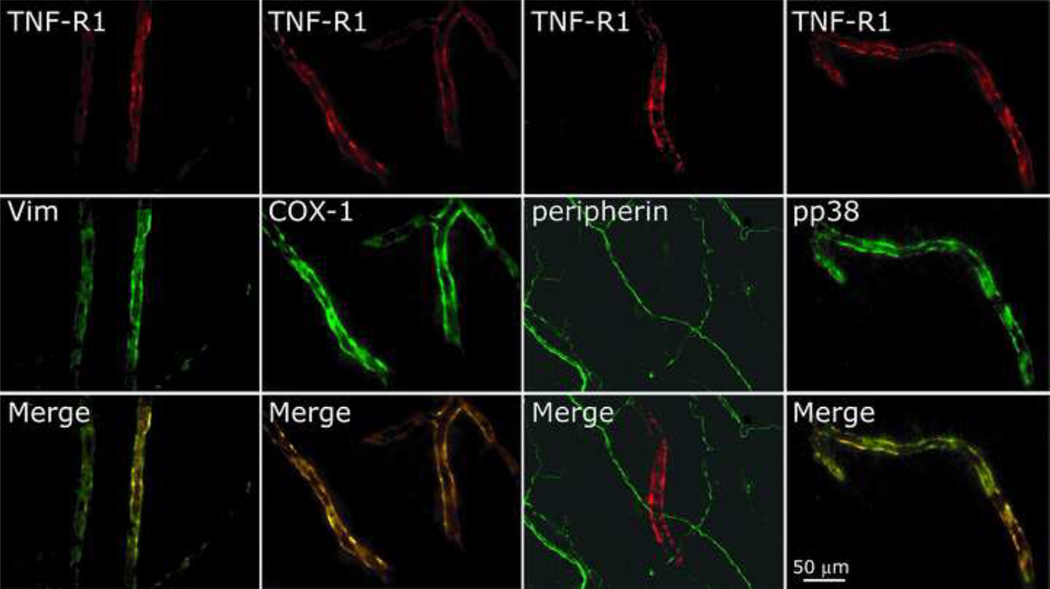
Dural TNFR1 expression was examined in 7 animals. TNFR1 immunolabeling was predominantly localized to the dural vasculature with medium and small dural blood vessels showing the highest expression. As figure 5 demonstrates, TNFR1 was co-localized in dural vessels with vimentin, indicating its presence in endothelial cells [47]. Vascular TNFR1 was also co-labeled with vascular COX-1. TNFR1 immunoreactivity was not co-localized with peripherin indicating its lack of expression in dural nerve fibers. TNFR1 was also not co-localized with COX-2 (data not shown). In animals treated with TNF-α, vascular TNFR1 was also co-localized with pp38. In control studies, omission of the TNFR1 antibody resulted in no labeling.
Figure 5.
Immunohistochemical localization of TNFR1 in dural blood vessels. Note the localization of TNFR1 with vimentin (vim), COX-1, and pp38 and its lack of co-expression in peripherin immunolabeled dural nerve fibers.
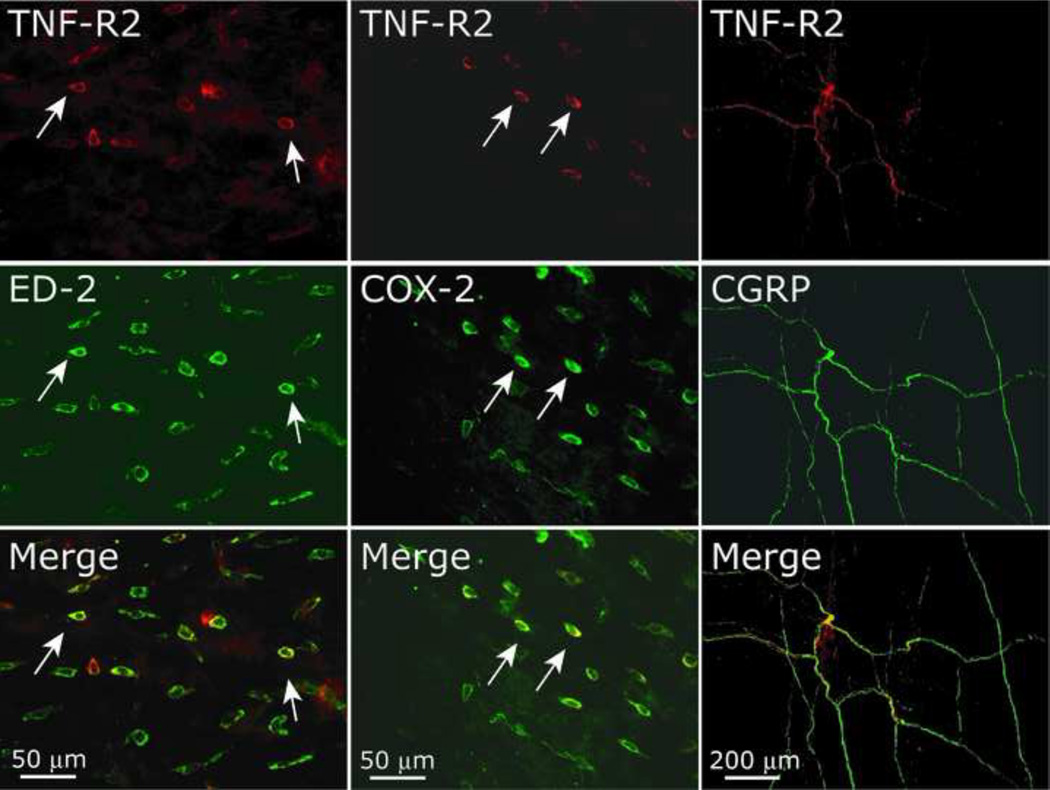
Dural TNFR2 expression was examined in 8 animals. The bulk of TNFR2 immunolabeling was found in perivascular cells. As Fig 6 demonstrates, TNFR2 was co-labeled with ED-2 positive cells, indicating its expression in dural macrophages. Additional double labeling immunohistochemistry reveled co-localization of TNFR2 with COX-2 in perivascular cells resembling those labeled with the ED-2 marker. TNFR2 was not co-localized with COX-1 (not shown). In addition to perivascular labeling, we identified a small number of dural nerve fibers expressing TNFR2, which were also labeled with antibodies against peripherin and CGRP. Incubation of the TNFR2 antibody with a blocking peptide resulted in a lack of TNFR2 expression in both perivascular cells and dural axon-like structures.
Figure 6.
Immunohistochemical localization of TNFR2 in the dura mater. Note the localization of TNFR2 with ED-2 (macrophages) and COX-2 positive cells. Also note the expression of TNFR2 in axonal like structures that are immunolabeled with CGRP.
4. Discussion
The present study provides, for the first time evidence that TNF-α evoked mechanical sensitization of nociceptors is mediated by local COX activity as well as local phosphorylation of the p38 MAP kinase. Previous in vivo electrophysiological studies have shown that local administration of TNF-α evokes a relatively rapid activation of cutaneous nociceptors and small diameter DRG cells [4,14]. TNF-α also promotes rapid nociceptor activation when applied in the vicinity of the sciatic nerve [17,39]. In contrast, our data indicates a marginal propensity of meningeal nociceptors to become activated in response to TNF-α. This is unlikely due to a dose issue given that a similar lack of responsiveness was also noted recently when much higher doses of TNF-α were applied to the receptive field of trigeminal afferents that innervate the masseter muscle [11]. We propose that such lack of responsiveness is related to differences in the responsiveness of trigeminal compared to non-trigeminal nociceptors.
Despite its lack of excitatory action, TNF-α promoted an increase in the responses of meningeal nociceptor to mechanical stimulation of their dural receptive field. The complete blockade of this action by local co-administration of a soluble TNF receptor indicates that this action is specific to TNF-α and is likely mediated by activation of peripheral dural TNF-α receptors. In rodents’ cutaneous tissues, TNF-α promotes mechanical sensitization within 15 min [14,4,39]. In our study, however sensitization was delayed in most neurons by at least 45 minutes suggesting the involvement of complex neuronal or non-neuronal downstream processes that mediate this sensitization.
Previous in-vitro studies, conducted on somata of putative nociceptors have concluded that TNF-α promotes nociceptor activation and sensitization by acting directly on neuronal TNF receptors [8,13,15,31]. However, a study conducted by Parada et al. [28] showing that administration of TNF-α to the hind paw can still promote peripheral sensitization despite specific ablation of neuronal TNFR1 receptors in DRG neurons that innervate this region suggesting the potential involvement of non-neuronal TNFR1 receptor.
In agreement with the lack of peripheral neuronal TNFR1 involvement, using immunohistochemistry, we were unable to detect TNFR1 expression in axon-like structures in the dura mater. An additional double labeling study, using peripherin, a marker of peripheral nerve fibers further confirmed the lack of TNFR1 axonal expression. While it is possible that technical limitations hampered our ability to detect TNFR1 in dural nerve fibers, the immunolabeling of vascular TNFR1 suggests otherwise. The lack of axonal TNFR1 expression in our study is in agreement with previous studies showing its lack of axonal expression in the skin [16] and sciatic nerve [41,42]. Although we do not discount the possibility that a very limited distal axonal transport of trigeminal TNFR1 hinders its immunohistochemical detection, it is tempting to speculate that TNFR1 expression in nociceptive neurons is purely somatic. It is noteworthy that a lack of peripheral transport of receptors in sensory neurons has also been described for the neuropeptide Y NPY1, which are expressed in DRG cells but do not undergo axonal transport [45]. Despite the lack of TNFR1 immunoreactivity in dural axons, we detected its expression in dural blood vessels. Dural vascular TNFR1 expression was also co-localized with vimentin, a marker that identifies dural vascular endothelial cells [47] and is in agreement with its expression on vascular endothelial cells from other tissues [21]. Dural vascular TNFR1 was also co-localized with COX-1 which we have shown recently also localized to dural vascular endothelial cells [47].
Systemic administration of COX inhibitors has been shown to inhibit the local hyperalgesic effect of TNF-α [7,35]. Our study provides for the first time direct evidence that in vivo, TNF-α evoked peripheral nociceptor sensitization involves local COX action. We have found that the sensitizing effects of TNF-α were blocked by the presumed selective inhibitors of COX-1 (SC-560) and COX-2 (NS-398). It should be noted however that a recent in vitro study has demonstrated the ability of SC-560 to abolish TNF-α evoked prostaglandin release from primary cell cultures obtained from the spinal cord of COX-1 knockout mice, suggesting its lack of COX selectivity. Data from other studies conducted either in vivo or ex-vivo suggests however that SC-560 effects are unrelated to COX-2 inhibition. For example bradykinin-mediated induction of nociceptor sensitization is inhibited by SC-560 but not NS-398 [22] and IL-1 induced hyperalgesia is prevented by NS-398 but not SC-560 [1]. In our study there was also a marked difference between the actions of SC-560 and NS-398 on the baseline responsiveness of meningeal nociceptors: local application of SC-560 did not reduce baseline mechanosensitivity while NS-398 had a marked inhibitory effect. Based on this data it is conceivable that, at least in the dura mater, SC-560 inhibits primarily the activity of COX-1, and that such action blocked the sensitizing effect of TNF-α we observed. The findings that TNFR1 is expressed on dural vascular endothelial cells that express COX-1 and that in endothelial cells, TNF-α binds specifically to TNFR1 [37] and evokes rapid release of prostaglandins in a COX-1 dependent manner [44] further suggest that dural vascular COX-1 plays, at least partly a role in mediating the sensitization of meningeal nociceptors by TNF-α
In our study, in addition to the findings of vascular TNFR1 expression, we also noted immunolabeling of TNFR2 primarily in perivascular cells that also expressed ED-2, a marker of resident macrophages. These finding are in agreement with the expression of TNFR2 on resident immune cells of the monocytes/macrophages lineage [5]. We have also noted limited immunolabeling of TNFR2 in dural nerve fibers, most of which also expressed CGRP, suggesting TNFR2 expression in peptidergic meningeal nociceptors. Although peripheral TNFR2 involvement in the development of mechanical pain hypersensitivity has been suggested only following a prolonged tissue injury [4,36], a recent study has also implicated this receptor in the development of acute sensitization of slow conducting masseter afferents [11]. Determining the relative contribution of TNFR2 (whether of neuronal or macrophage origin) in mediating the sensitization of meningeal nociceptors will require further studies.
The co-expression of TNFR2 with COX-2 (likely in ED-2 positive cells) is in agreement with previous data showing that TNF-α evoked prostaglandin release from macrophages is COX-2 dependent [10]. In order to examine the relative contribution of COX-2 in mediating the sensitizing effect of TNF-α we have tested the effect of the more selective COX-2 inhibitor NS-398. As indicated above, we observed that NS-398 had already an inhibitory effect on the baseline sensitivity of meningeal nociceptors. Although this effect remains puzzling, a recent study has shown the ability of another selective COX-2 inhibitor, celecoxib to inhibit sodium currents in rat dorsal root ganglion neurons [29]. The inhibitory effect of NS-398 at baseline thus precludes us from inferring at present a specific role for COX-2 in mediating the sensitizing effect of TNF-α that is, the inhibition of the TNF-α sensitizing effect may be related to direct neuronal inhibitory effect of COX-2 inhibition rather than the interference with the downstream signaling of TNFR (presumably TNFR2). Given the uncertainty regarding the specificity of “selective” pharmacological COX inhibitors [29] future studies examining the relative contribution of COX-1 and COX-2 in mediating the sensitizing effect of TNF-α on nociceptor nerve endings will require a knockout approach.
Our data shows for the first time that TNF-α evoked nociceptors’ sensitization at the peripheral nerve endings level involves activation of the p38 MAP kinase. Previous studies have demonstrated the ability of acute TNF-α treatment to activate (i.e. phosphorylate) the p38 MAP kinase in cultured sensory neurons including of trigeminal origin [3]. In addition, it has been shown that p38 inhibitors can block the potentiation of TTX-persistent currents [13] as well as capsaicin and heat-evoked currents in DRG neurons [4] in response to TNF-α In our in vivo experiments, however we did not detect axonal pp38 expression in response to TNF-α application to the dura mater despite a similar incubation time of TNF-α as well as the use of the same antibody as in one of the abovementioned in vitro study [13]. The possibility that TNF-α activates p38 in primary afferent nociceptive neurons cell bodies only in vitro, but not in their peripheral nerve endings in vivo is therefore plausible. Despite the lack of TNF-α evoked pp38 in dural axons, we observed that TNF-α application to the dura was associated with an increase in the number of dural vessels expressing pp38 which also expressed TNFR1. The vascular upregulation of pp38 we observed is in agreement with previous studies showing the ability of TNF-α to rapidly phophorylate p38 in vascular endothelial cells [24] and to promote in vivo increased permeability of these cells in a p38-dependent manner [9]. The exact mechanism by which vascular pp38 might contribute to the development of the mechanical sensitization of meningeal nociceptors by TNF-α remains to be established. Given that in endothelial cells TNF-α activates TNFR1 to promote a COX-1 dependent increase in prostanoid production [44], activation of vascular p38 in these cells may be a link between TNF-α, COX-1 activation and the sensitization of meningeal nociceptors.
Acknowledgments
Supported by NIH grants NS61116, NS46502 and a grant from the National Headache Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Reference
- 1.Ahn DK, Chae JM, Choi HS, Kyung HM, Kwon OW, Park HS, Youn DH, Bae YC. Central cyclooxygenase inhibitors reduced IL-1beta-induced hyperalgesia in temporomandibular joint of freely moving rats. Pain. 2005;117(1–2):204–213. doi: 10.1016/j.pain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28(52):14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowen EJ, Schmidt TW, Firm CS, Russo AF, Durham PL. Tumor necrosis factor-alpha stimulation of calcitonin gene-related peptide expression and secretion from rat trigeminal ganglion neurons. J Neurochem. 2006;96(1):65–77. doi: 10.1111/j.1471-4159.2005.03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Constantin CE, Mair N, Sailer CA, Andratsch M, Xu ZZ, Blumer MJ, Scherbakov N, Davis JB, Bluethmann H, Ji RR, Kress M. Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J Neurosci. 2008;28(19):5072–5081. doi: 10.1523/JNEUROSCI.4476-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nature reviews. 2009;9(4):271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. British journal of pharmacology. 1992;107(3):660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunha TM, Verri WA, Jr, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(5):1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czeschik JC, Hagenacker T, Schafers M, Busselberg D. TNF-alpha differentially modulates ion channels of nociceptive neurons. Neuroscience letters. 2008;434(3):293–298. doi: 10.1016/j.neulet.2008.01.070. [DOI] [PubMed] [Google Scholar]
- 9.Ferrero E, Zocchi MR, Magni E, Panzeri MC, Curnis F, Rugarli C, Ferrero ME, Corti A. Roles of tumor necrosis factor p55 and p75 receptors in TNF-alpha-induced vascular permeability. Am J Physiol Cell Physiol. 2001;281(4):C1173–C1179. doi: 10.1152/ajpcell.2001.281.4.C1173. [DOI] [PubMed] [Google Scholar]
- 10.Fournier T, Fadok V, Henson PM. Tumor necrosis factor-alpha inversely regulates prostaglandin D2 and prostaglandin E2 production in murine macrophages. Synergistic action of cyclic AMP on cyclooxygenase-2 expression and prostaglandin E2 synthesis. The Journal of biological chemistry. 1997;272(49):31065–31072. doi: 10.1074/jbc.272.49.31065. [DOI] [PubMed] [Google Scholar]
- 11.Hakim AW, Dong XD, Svensson P, Kumar U, Cairns BE. TNFalpha mechanically sensitizes masseter muscle afferent fibers of male rats. Journal of neurophysiology. 2009;102(3):1551–1559. doi: 10.1152/jn.00326.2009. [DOI] [PubMed] [Google Scholar]
- 12.Huntjens DR, Danhof M, Della Pasqua OE. Pharmacokinetic-pharmacodynamic correlations and biomarkers in the development of COX-2 inhibitors. Rheumatology (Oxford) 2005;44(7):846–859. doi: 10.1093/rheumatology/keh627. [DOI] [PubMed] [Google Scholar]
- 13.Jin X, Gereau RWt. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci. 2006;26(1):246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Junger H, Sorkin LS. Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain. 2000;85(1–2):145–151. doi: 10.1016/s0304-3959(99)00262-6. [DOI] [PubMed] [Google Scholar]
- 15.Khan AA, Diogenes A, Jeske NA, Henry MA, Akopian A, Hargreaves KM. Tumor necrosis factor alpha enhances the sensitivity of rat trigeminal neurons to capsaicin. Neuroscience. 2008;155(2):503–509. doi: 10.1016/j.neuroscience.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 16.Kristensen M, Chu CQ, Eedy DJ, Feldmann M, Brennan FM, Breathnach SM. Localization of tumour necrosis factor-alpha (TNF-alpha) and its receptors in normal and psoriatic skin: epidermal cells express the 55-kD but not the 75-kD TNF receptor. Clinical and experimental immunology. 1993;94(2):354–362. doi: 10.1111/j.1365-2249.1993.tb03457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leem JG, Bove GM. Mid-axonal tumor necrosis factor-alpha induces ectopic activity in a subset of slowly conducting cutaneous and deep afferent neurons. J Pain. 2002;3(1):45–49. doi: 10.1054/jpai.2002.27138. [DOI] [PubMed] [Google Scholar]
- 18.Levy D. Migraine pain, meningeal inflammation, and mast cells. Current pain and headache reports. 2009;13(3):237–240. doi: 10.1007/s11916-009-0040-y. [DOI] [PubMed] [Google Scholar]
- 19.Levy D, Zhang XC, Jakubowski M, Burstein R. Sensitization of meningeal nociceptors: inhibition by naproxen. Eur J Neurosci. 2008;27(4):917–922. doi: 10.1111/j.1460-9568.2008.06068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Ji A, Weihe E, Schafer MK. Cell-specific expression and lipopolysaccharide-induced regulation of tumor necrosis factor alpha (TNFalpha) and TNF receptors in rat dorsal root ganglion. J Neurosci. 2004;24(43):9623–9631. doi: 10.1523/JNEUROSCI.2392-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackay F, Loetscher H, Stueber D, Gehr G, Lesslauer W. Tumor necrosis factor alpha (TNF-alpha)-induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF-R55. The Journal of experimental medicine. 1993;177(5):1277–1286. doi: 10.1084/jem.177.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer S, Izydorczyk I, Reeh PW, Grubb BD. Bradykinin-induced nociceptor sensitisation to heat depends on cox-1 and cox-2 in isolated rat skin. Pain. 2007;130(1–2):14–24. doi: 10.1016/j.pain.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 23.Messlinger K. Migraine: where and how does the pain originate? Experimental brain research Experimentelle Hirnforschung. 2009;196(1):179–193. doi: 10.1007/s00221-009-1756-y. [DOI] [PubMed] [Google Scholar]
- 24.Modur V, Zimmerman GA, Prescott SM, McIntyre TM. Endothelial cell inflammatory responses to tumor necrosis factor alpha.Ceramide-dependent and - independent mitogen-activated protein kinase cascades. The Journal of biological chemistry. 1996;271(22):13094–13102. doi: 10.1074/jbc.271.22.13094. [DOI] [PubMed] [Google Scholar]
- 25.Morioka N, Inoue A, Hanada T, Kumagai K, Takeda K, Ikoma K, Hide I, Tamura Y, Shiomi H, Dohi T, Nakata Y. Nitric oxide synergistically potentiates interleukin-1 beta-induced increase of cyclooxygenase-2 mRNA levels, resulting in the facilitation of substance P release from primary afferent neurons: involvement of cGMP-independent mechanisms. Neuropharmacology. 2002;43(5):868–876. doi: 10.1016/s0028-3908(02)00143-0. [DOI] [PubMed] [Google Scholar]
- 26.Nicol GD, Lopshire JC, Pafford CM. Tumor necrosis factor enhances the capsaicin sensitivity of rat sensory neurons. J Neurosci. 1997;17(3):975–982. doi: 10.1523/JNEUROSCI.17-03-00975.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet neurology. 2009;8(7):679–690. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- 28.Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci. 2003;17(9):1847–1852. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- 29.Park SY, Kim TH, Kim HI, Shin YK, Lee CS, Park M, Song JH. Celecoxib inhibits Na+ currents in rat dorsal root ganglion neurons. Brain research. 2007;1148:53–61. doi: 10.1016/j.brainres.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Perini F, D'Andrea G, Galloni E, Pignatelli F, Billo G, Alba S, Bussone G, Toso V. Plasma cytokine levels in migraineurs and controls. Headache. 2005;45(7):926–931. doi: 10.1111/j.1526-4610.2005.05135.x. [DOI] [PubMed] [Google Scholar]
- 31.Pollock J, McFarlane SM, Connell MC, Zehavi U, Vandenabeele P, MacEwan DJ, Scott RH. TNF-alpha receptors simultaneously activate Ca2+ mobilisation and stress kinases in cultured sensory neurones. Neuropharmacology. 2002;42(1):93–106. doi: 10.1016/s0028-3908(01)00163-0. [DOI] [PubMed] [Google Scholar]
- 32.Poole S, Cunha FQ, Selkirk S, Lorenzetti BB, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-10. British journal of pharmacology. 1995;115(4):684–688. doi: 10.1111/j.1476-5381.1995.tb14987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rozen T, Swidan SZ. Elevation of CSF tumor necrosis factor alpha levels in new daily persistent headache and treatment refractory chronic migraine. Headache. 2007;47(7):1050–1055. doi: 10.1111/j.1526-4610.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- 34.Sarchielli P, Alberti A, Baldi A, Coppola F, Rossi C, Pierguidi L, Floridi A, Calabresi P. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache. 2006;46(2):200–207. doi: 10.1111/j.1526-4610.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- 35.Schafers M, Marziniak M, Sorkin LS, Yaksh TL, Sommer C. Cyclooxygenase inhibition in nerve-injury- and TNF-induced hyperalgesia in the rat. Experimental neurology. 2004;185(1):160–168. doi: 10.1016/j.expneurol.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Schafers M, Sommer C, Geis C, Hagenacker T, Vandenabeele P, Sorkin LS. Selective stimulation of either tumor necrosis factor receptor differentially induces pain behavior in vivo and ectopic activity in sensory neurons in vitro. Neuroscience. 2008;157(2):414–423. doi: 10.1016/j.neuroscience.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 37.Slowik MR, De Luca LG, Fiers W, Pober JS. Tumor necrosis factor activates human endothelial cells through the p55 tumor necrosis factor receptor but the p75 receptor contributes to activation at low tumor necrosis factor concentration. The American journal of pathology. 1993;143(6):1724–1730. [PMC free article] [PubMed] [Google Scholar]
- 38.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neuroscience letters. 2004;361(1–3):184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience. 1997;81(1):255–262. doi: 10.1016/s0306-4522(97)00147-4. [DOI] [PubMed] [Google Scholar]
- 40.Strassman AM, Raymond SA. On the origin of headaches. Endeavour. 1997;21(3):97–100. doi: 10.1016/s0160-9327(97)80216-5. [DOI] [PubMed] [Google Scholar]
- 41.Wagner R, Myers RR, O'Brien JS. Prosaptide prevents hyperalgesia and reduces peripheral TNFR1 expression following TNF-alpha nerve injection. Neuroreport. 1998;9(12):2827–2831. doi: 10.1097/00001756-199808240-00026. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Zhou D, Shen Q, Cheng C, Liu HO, Qin Y, Sun L, Xiao F, Zhao J, Shen A. Lipopolysaccharide-induced upregulation of tumor necrosis factor-alpha (TNF-alpha) and TNF receptors in rat sciatic nerve. J Mol Neurosci. 2007;32(3):207–216. doi: 10.1007/s12031-007-0036-1. [DOI] [PubMed] [Google Scholar]
- 43.Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. British journal of pharmacology. 1997;121(3):417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zauli G, Pandolfi A, Gonelli A, Di Pietro R, Guarnieri S, Ciabattoni G, Rana R, Vitale M, Secchiero P. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) sequentially upregulates nitric oxide and prostanoid production in primary human endothelial cells. Circulation research. 2003;92(7):732–740. doi: 10.1161/01.RES.0000067928.83455.9C. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Bao L, Xu ZQ, Kopp J, Arvidsson U, Elde R, Hokfelt T. Localization of neuropeptide Y Y1 receptors in the rat nervous system with special reference to somatic receptors on small dorsal root ganglion neurons. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(24):11738–11742. doi: 10.1073/pnas.91.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Strassman AM, Burstein R, Levy D. Sensitization and activation of intracranial meningeal nociceptors by mast cell mediators. J Pharmacol Exp Ther. 2007;322(2):806–812. doi: 10.1124/jpet.107.123745. [DOI] [PubMed] [Google Scholar]
- 47.Zhang XC, Kainz V, Jakubowski M, Burstein R, Strassman A, Levy D. Localization of COX-1 and COX-2 in the intracranial dura mater of the rat. Neuroscience letters. 2009;452(1):33–36. doi: 10.1016/j.neulet.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]