Abstract
AIM: To determine if the fraction of Nardostachys jatamansi (NJ) has the potential to ameliorate the severity of acute pancreatitis (AP).
METHODS: Mice were administered the biologically active fraction of NJ, i.e., the 4th fraction (NJ4), intraperitoneally, and then injected with the stable cholecystokinin analogue cerulein hourly for 6 h. Six hours after the last cerulein injection, the pancreas, lung, and blood were harvested for morphological examination, measurement of cytokine expression, and examination of neutrophil infiltration.
RESULTS: NJ4 administration attenuated the severity of AP and lung injury associated with AP. It also reduced cytokine production and neutrophil infiltration and resulted in the in vivo up-regulation of heme oxygenase-1 (HO-1). Furthermore, NJ4 and its biologically active fraction, NJ4-2 inhibited the cerulein-induced death of acinar cells by inducing HO-1 in isolated pancreatic acinar cells.
CONCLUSION: These results suggest that NJ4 may be a candidate fraction offering protection in AP and NJ4 might ameliorate the severity of pancreatitis by inducing HO-1 expression.
Keywords: Nardostachys jatamansi, Acute pancreatitis, Cytokines, Heme oxygenase-1
INTRODUCTION
Acute pancreatitis (AP) refers to inflammation of the pancreas and is caused by an imbalance in the factors that maintain cellular homeostasis[1]. AP could lead to distant organ damage and multiple organ dysfunction, the primary cause of morbidity and mortality in this condition[2]. The AP cascade is complex. An unknown trigger within the pancreas converts digestive pro-enzymes into their active form, initiating auto-digestion of the gland, causing necrosis, edema, and destruction of the pancreatic parenchyma[2].
Nardostachys jatamansi (NJ) is widely used in several Asian countries to treat mental disorders, insomnia, and disorders of the circulatory system[3]. It has protective effects against diabetes and sepsis[4,5]. We have previously reported that NJ protects against cerulein-induced AP[6]. Further, several studies have reported on the protective effects afforded by compounds from NJ[3,7] such as jatamansic acid and nardosinone. However, the compound in NJ that protects against AP remains to be identified.
This study aimed to identify the candidate fraction of NJ that protects against cerulein-induced AP in a mouse model. To achieve this, we fractionated NJ by using RP C-18 column chromatography and the 4th fraction (NJ4) showed more potent effects than the aqueous extract of NJ. Our results suggest that NJ4 may be a candidate fraction for reducing the severity of AP.
MATERIALS AND METHODS
Materials
Avidin peroxidase and 3,3’,5,5’-tetramethylbenzidine (TMB), cerulein, Tris-HCl, NaCl, Triton X-100, curcumin, ZnPP, and hexadecyltrimethyl ammonium bromide were purchased from Sigma-Aldrich (St. Louis, MO). Anti-mouse interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α antibodies and recombinant IL-1β, IL-6, and TNF-α were purchased from R and D Systems (Minneapolis). Phosphospecific mitogen-activated protein kinases (MAPKs), extracellular signal-regulated kinases (ERK)1/2, c-Jun NH2-terminal kinases (JNK), and p38 were purchased from Cell Signaling Technology (Beverly, MA). ERK1/2, JNK, p38, inhibitory kappa-Ba heme oxygenase-1 (HO-1), and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Plant materials
The roots of NJ were purchased from a standard commercial source (Omni Herb, Seoul, South Korea). The herb’s identity was confirmed at Wonkwang University. Voucher specimens were deposited at the College of Oriental Medicine Herbarium of Wonkwang University. The NJ roots were prepared by decocting the dried prescription of herbs (100 g) with boiling distilled water (1 L). The decoction time was approximately 2 h. The water extract was frozen at -80 °C and then freeze-dried to be powdered (7.35 g, 7.35 w/w%).
Preparation of NJ4 fraction
The water extract (3.8 g) was subjected to octadecyl functionalized silica gel flash column (5 cm × 20 cm; 63-200 μm particle size) chromatography (Figure 1). The column was eluted with a stepwise gradient with 500 mL aliquots of MeOH in H2O (starting from 10% and followed by 20%, up to 100% at 20% increments), affording 6 fractions (NJ1: 1.24 g; NJ2: 79.1 mg; NJ3: 490.7 mg; NJ4: 416.2 mg; NJ5: 273.1 mg; NJ6: 67.8 mg). NJ4 (60% MeOH) in saline was used as the main fraction (Figure 1).
Figure 1.
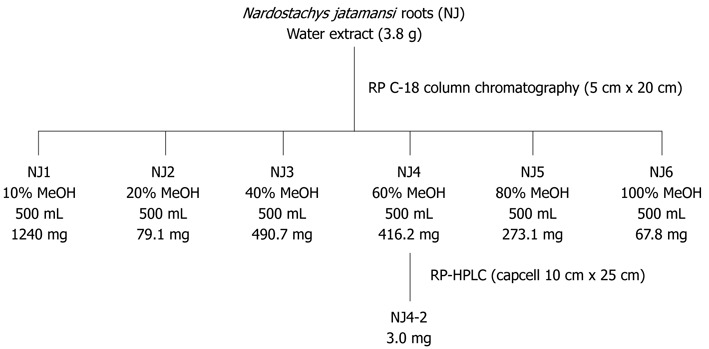
Fractionation of the aqueous extract of Nardostachys jatamansi. NJ: Nardostachys jatamansi; HPLC: High-performance liquid chromatography.
Preparation of NJ4 fraction
A portion (9.0 mg) of the fraction eluted with 60% MeOH inH2O (NJ4-2) was then purified by semi-preparative reversed-phase high-performance liquid chromatography (HPLC) [Shiseido Capcell Pak C18 column (10 mm × 250 mm; 5 μm particle size); 2 mL/min; detection at 254 nm] eluting with a gradient from 40% to 70% MeOH in H2O (0.1% formic acid) over 30 min to yield NJ4-2 (3.0 mg, tR = 28.0 min) (Figure 1).
HPLC sample preparation and HPLC conditions
For HPLC analysis, the chromatographic system consisted of a pump (3000 HPLC pump; Dionex Association, United States), a ultraviolet detector (Photodiode array detector; Dionex Association), and an autosampler (Waters Association, United States). A hydrosphere C18 column (4.6 mm × 250 mm, 5 μm) was used. Water-methanol glacial (50:50) was used as the mobile phase. Detection of the peaks was made at 254 nm and the sensitivity was set at 0.5 absorbance units full scales. The injection volume was 10 μL and the flow rate was 1.0 mL/min. A standard solution was prepared by dissolving in distilled methanol (10 μg/10 mL). The solution was filtered through a 0.45 μm membrane filter and applied to HPLC (Figure 2).
Figure 2.
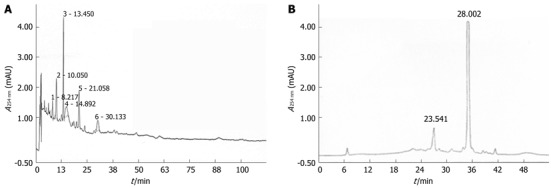
High-performance liquid chromatography findings of the aqueous extract of Nardostachys jatamansi (A) and the 4th fraction of Nardostachys jatamansi (B).
Animals
Protocols approved by the Animal Care Committee of Wonkwang University were used for all experiments. Female 6- to 8-wk-old C57BL/6 mice weighing 15-20 g were purchased from Orient Bio (Sungnam, KyungKiDo, South Korea). All animals were bred and housed in standard shoebox cages in a climate-controlled room with an ambient temperature of 23 ± 2 °C under a 12-h light-dark cycle for 7 d. The animals were fed standard laboratory chow, allowed water ad libitum, and randomly assigned to control or experimental groups. The mice were fasted for 18 h before AP was induced.
Experimental design
AP was induced by intraperitoneal injections of supramaximal concentrations of the stable cholecystokinin analogue cerulein (50 μg/kg) or saline, hourly for 6 h[6]. Prior to injecting the NJ4 treatment group with cerulein, NJ4 (1 μg/kg, 5 μg/kg, or 10 μg/kg, n = 6) or saline (control group, n = 6) were intraperitoneally administered (1 h before the first cerulein injection). Mice were killed 6 h after the last cerulein injection was administered. Blood samples were taken to determine serum amylase, lipase, and cytokine levels. For histological examination and scoring, the entire pancreas and lungs were rapidly removed from each mouse and fixed in formalin. To measure tissue myeloperoxidase (MPO) activity, an indicator of neutrophil sequestration, and for real-time reverse transcriptase polymerase chain reaction (RT-PCR) studies, the pancreas and lungs were stored at -80 °C.
Histological analysis
The pancreases from each treatment group were examined and semi-quantitatively described in terms of necrosis, vacuolization, inflammation, and edema. A tissue section representing a minimum of 100 fields was examined for each sample and scored on a scale of 0-3 (0 being normal and 3 being severe disease) on the basis of the number of necrotic acinar cells and the presence of interstitial edema and interstitial inflammation.
Measurement of serum amylase and lipase
Blood samples to determine serum amylase and lipase were obtained 6 h after inducing pancreatitis. Mice were anesthetized with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (4 mg/kg). After anesthetization, blood was withdrawn from the heart of each mouse into a syringe. Serum amylase and lipase were measured using an assay kit from BioAssay Systems (CA).
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) for IL-1β, IL-6, and TNF-α were carried out in duplicate in 96-well plates coated with 100 μL aliquots of anti-mouse IL-1β, IL-6, and TNF-α monoclonal antibodies in phosphate buffered saline (PBS) at pH 7.4 during an overnight incubation at 4 °C. The plates were washed in PBS containing 0.05% Tween-20 and blocked with PBS containing 10% FBS for 2 h. After additional washes, standards and samples were added and incubated at room temperature for 2 h. Subsequently, the wells were washed, and biotinylated anti-mouse IL-1β, IL-6, and TNF-α were added and incubated at room temperature for 1 h. The wells were washed, avidin-peroxidase was added, and the plates were incubated for 30 min at room temperature before washing again and adding the TMB substrate. Color development was measured at 450 nm by using an automated microplate ELISA reader. Standard samples were run on each assay plate using serial dilutions of recombinant IL-1β, IL-6, and TNF-α.
Messenger RNA expression
Transcription of target cytokines in mouse pancreatic tissues and acini was analyzed using RT-PCR. Total RNA was isolated from the mouse pancreas using TriZol (Invitrogen, Carlsbad, CA) and subjected to reverse transcription using SuperScript II RT (Invitrogen). TaqMan quantitative RT-PCR using the LightCycler 2.0 detection system was performed according to the manufacturer’s instructions (Roche, Basel, Switzerland). For each sample, triplicate test reactions and a control reaction lacking reverse transcriptase were analyzed for the expression of the gene of interest, and the results were normalized to those of “housekeeping” hypoxanthine-guanine phosphoribosyltransferase (HPRT) mRNA. Arbitrary expression units were calculated by dividing the expression of the gene of interest by ribosomal protein HPRT mRNA expression. The sequences of forward, reverse, and probe oligonucleotide primers for multiplex real-time TaqMan PCR were as follows: for mouse IL-1β (forward, 5’-TTG ACG GAC CCC AAA AGA T-3’; reverse, 5’-GAA GCT GGA TGC TCT CAT CTG-3’; universal probe, M15131.1, Roche Applied Science), for mouse IL-6 (forward, 5’-TTC ATT CTC TTT GCT CTT GAA TTA GA-3’; reverse, 5’-GTC TGA CCT TTA GCT TCA AAT CCT-3’; universal probe, M20572.1, Roche Applied Science), and for mouse TNF-α (forward, 5’-TCT CTT CAA GGG ACA AGG CTG-3’; reverse, 5’-ATA GCA AAT CGG CTG ACG GT-3’; probe, 5’-CCC GAC TAC GTG CTC CTC ACC CA-3’). For mouse HO-1, we purchased a custom primer from Applied Biosystems (CA).
MPO estimation
Neutrophil sequestration in the pancreas was quantified by measuring tissue MPO activity. Tissue samples were thawed, homogenized in 20 mmol/L phosphate buffer (pH 7.4), and centrifuged (13 000 rpm, 10 min, 4 °C). The pellet was resuspended in 50 mmol/L phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide (Sigma-Aldrich). The suspension was subjected to 4 cycles of freezing and thawing and further disrupted by sonication for 40 s. The sample was then centrifuged (13 000 rpm, 5 min, 4 °C), and the supernatant was used for the MPO assay. The reaction mixture consisted of the supernatant, 1.6 mmol/L TMB, 80 mmol/L sodium phosphate buffer (pH 5.4), and 0.3 mmol/L hydrogen peroxide. This mixture was incubated at 37 °C for 110 s, the reaction was terminated with 2 mol/L H2SO4, and the absorbance was measured at 450 nm.
Western blotting
Pancreatic tissues and pancreatic acini were homogenized, following which the lysates were boiled in a sample buffer (62.5 mmol/L Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate (SDS), 20% glycerol, and 10% 2-mercaptoethanol). Proteins in the cell lysates were then separated using 10% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Then, the membrane was blocked with 5% skim milk in PBS-Tween-20 for 2 h at RT and then incubated with primary antibodies overnight. After washing 3 times, each blot was incubated with peroxidase-conjugated secondary antibody for 1 h, and antibody-specific proteins were visualized using an enhanced chemiluminesence detection system (Amersham, Piscataway, NJ) according to the manufacturer’s recommended protocol.
Acinar cell isolation
Pancreatic acini were isolated from C57BL/6 mice using collagenase digestion. All experiments were performed according to protocols approved by the Animal Care Committee of Wonkwang University. Briefly, pancreatic tissue was minced with scissors and digested for 15 min in solution Q (120 mmol/L NaCl, 20 mmol/L HEPES, 5 mmol/L KCl, 1 mmol/L MgCl2, 1 mmol/L CaCl2, 10 mmol/L sodium pyruvate, 10 mmol/L ascorbate, 10 mmol/L glucose, 0.1% BSA, 0.01% soybean trypsinogen inhibitor, and 150 units of collagenase/mL). Cells were continuously shaken and gassed with 100% O2 in a 37 °C water bath and subsequently washed in fresh isolation medium. After collagenase digestion, the tissue was gently pipetted. Dispersed acini were filtered through a 150-μm nylon mesh, centrifuged 3 times (each for 90 s at 720 rpm), resuspended in Waymouth medium (Invitrogen, Gibco, CA) and incubated with 95% O2 and 5% CO2 for 4 h.
Cell viability assay
Cell viability was assayed using a modified colorimetric technique that is based on the ability of live cells to convert the tetrazolium compound 3-(4,5-dimethylthiazol)-2,5-diphenyltetrazolium bromide (MTT) into purple formazan crystals. MTT (5 mg/mL) was dissolved in Kreb’s-Henseleit buffer (115 mmol/L NaCl, 3.6 mmol/L KCl, 1.3 mmol/L KH2PO4, 25 mmol/L NaHCO3, 1 mol/L CaCl2, and 1 mol/L MgCl2), and 50 μL was added to each well. After incubating for 30 min at 37 °C, the suspension was removed, and the formazan crystals formed were dissolved in 200 μL dimethyl sulfoxide. Aliquots from each well were seeded in the wells of a 96-well plate in duplicate and assayed at 540 nm using a microplate ELISA reader. The number of viable cells was expressed as a percentage of the control.
Statistical analysis
Results were expressed as means ± SE. The significance of differences was evaluated using a two-way analysis of variance (ANOVA) with time and dose parameters. Differences between the experimental groups were evaluated using ANOVA. P values < 0.05 were considered statistically significant.
RESULTS
Effect of NJ4 on cerulein-induced AP
To examine the effect of NJ4 on the development and severity of AP, mice pretreated with saline or NJ4 were injected intraperitoneally with a supramaximal dose (50 μg/kg) of cerulein. Intraperitoneal injection of cerulein resulted in significant pancreatic parenchyma destruction, inflammation, edema, and necrosis. However, NJ4-treated mice showed reductions in damage of the pancreas and severity of AP (Table 1 and Figure 3A). As an additional assessment of the severity of the inflammatory response, we measured MPO activity, which is an indicator of neutrophil infiltration in the pancreas. Cerulein-induced MPO activity in the pancreas of NJ-treated mice was lower than that in the pancreas of saline-injected mice (Figure 3B).
Table 1.
Semi-quantitative analysis of pancreas in acute pancreatitis
| Severity score | Inflammation | Edema | Necrosis |
| Saline | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.2 |
| Saline + AP | 2.5 ± 0.5a | 2.5 ± 0.5a | 2.5 ± 0.5a |
| NJ4 1 + AP | 1.5 ± 0.3ac | 2.0 ± 0.3a | 2.0 ± 0.3a |
| NJ4 5 + AP | 1.5 ± 0.2ac | 1.5 ± 0.3ac | 1.5 ± 0.2ac |
| NJ4 10 + AP | 1.1 ± 0.1ac | 1.0 ± 0.2ac | 1.2 ± 0.15ac |
Histological sections of the pancreas were scored from 0 (normal) to 3 (severe) for inflammation, edema, and necrosis.
P < 0.05 vs the saline treatment;
P < 0.05 vs cerulein treatment alone. AP: Acute pancreatitis; NJ: Nardostachys jatamansi.
Figure 3.
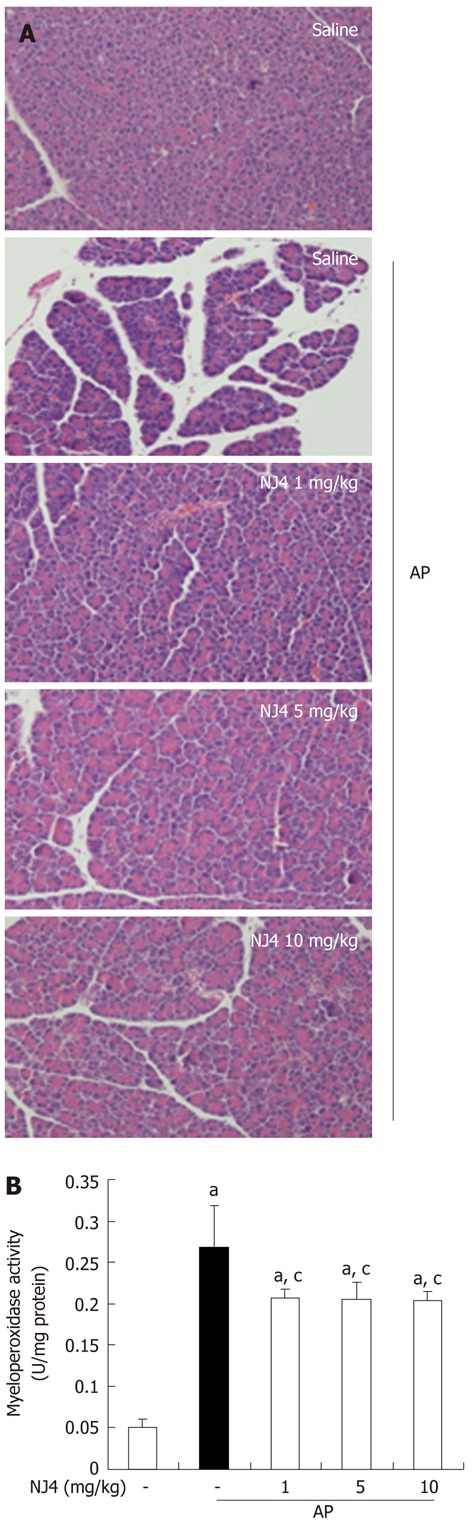
Effects of the 4th fraction of Nardostachys jatamansi on inflammatory events in the pancreas after pancreatitis. A: Representative hematoxylin-eosin stained sections of the pancreas in the control mice not administered cerulein, in mice given cerulein, and in mice given Nardostachys jatamansi (NJ) (1 mg/kg, 5 mg/kg, or 10 mg/kg) 1 h before the first cerulein injection; B: Neutrophil infiltration was assessed on the basis of myeloperoxidase activity. This figure shows representative images of 1 experiment that involved 6 mice. The results were similar in 3 additional experiments. aP < 0.05 vs the saline treatment; cP < 0.05 vs cerulein treatment alone. Original magnification: × 100. AP: Acute pancreatitis.
Effect of NJ4 on lung injury associated with AP
Acute lung injury in AP still represents a substantial problem, with a mortality rate in the range of 30%-40%[8]. The cytokines and inflammatory mediators from pancreatic acini regulate the migration and pulmonary infiltration of neutrophils to the interstitial tissue, where they cause injury and breakdown of pulmonary parenchyma[9]. As shown in Figure 4, cerulein injection resulted in lung injury, which is characterized by destruction of the lung parenchyma, edema, and inflammatory cell infiltration. However, NJ4 treatment reduced lung damage, inflammation, and edema and inhibited MPO activity in the lung in cerulein-induced AP (Table 2 and Figure 4A and B).
Figure 4.
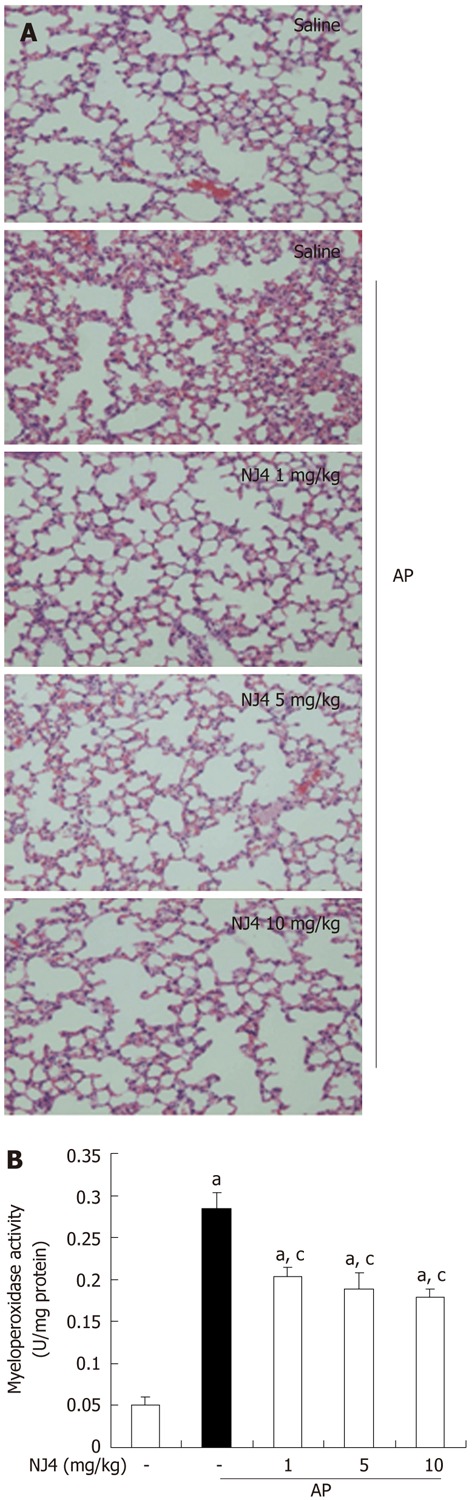
Effects of the 4th fraction of Nardostachys jatamansi on acute pancreatitis-associated lung injury. A: Representative hematoxylin-eosin stained sections of the lung in the control mice not given cerulein, in mice given cerulein, and in mice given Nardostachys jatamansi (NJ) (1 mg/kg, 5 mg/kg, or 10 mg/kg) 1 h before the first cerulein injection; B: Neutrophil infiltration was assessed on the basis of myeloperoxidase activity. This figure shows representative images from 1 experiment that involved 6 mice. The results were similar in 3 additional experiments. aP < 0.05 vs the saline treatment; cP < 0.05 vs cerulein treatment alone. Original magnification: × 100. AP: Acute pancreatitis.
Table 2.
Semi-quantitative analysis of lung in acute pancreatitis
| Severity score | Inflammation | Edema |
| Saline | 0.5 ± 0.2 | 0.5 ± 0.2 |
| Saline + AP | 2.5 ± 0.5a | 2.5 ± 0.5a |
| NJ4 1 + AP | 1.3 ± 0.4ac | 1.8 ± 0.3a |
| NJ4 5 + AP | 1.3 ± 0.3ac | 1.6 ± 0.3ac |
| NJ4 10 + AP | 1.1 ± 0.2ac | 1.2 ± 0.1ac |
Histological sections of the lung were scored from 0 (normal) to 3 (severe) for inflammation, and edema.
P < 0.05 vs the saline treatment;
P < 0.05 vs cerulein treatment alone. AP: Acute pancreatitis; NJ: Nardostachys jatamansi.
Effect of NJ4 on pancreatic weight/body weight ratio and serum digestive enzymes in cerulein-induced AP
In order to assess the effect of NJ4 on pancreatic edema, the pancreatic weight/body weight (PW/BW) was measured. As shown in Figure 5A, NJ4 administration inhibited the PW/BW ratio in a dose-dependent manner during cerulein-induced AP. When acinar cells are challenged with inflammatory responses, pro-enzymes in the pancreatic zymogen are converted to active enzymes such as amylase and lipase. Therefore, serum amylase and lipase activity were used to assess the severity of AP. The activities of amylase and lipase were up-regulated by cerulein injection. However, these activities were inhibited by NJ4 treatment (Figure 5B and C).
Figure 5.
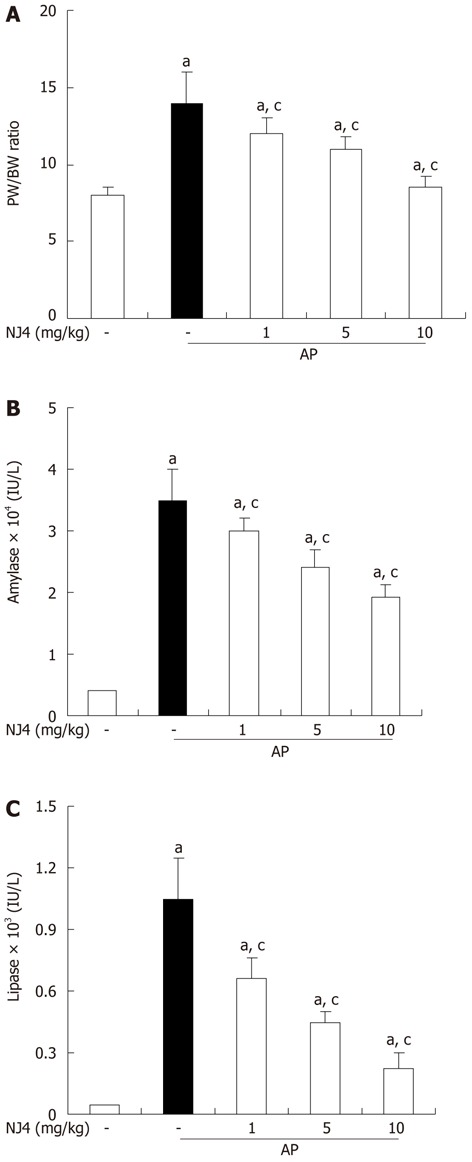
Effects of the 4th fraction of Nardostachys jatamansi pretreatment on pancreatic weight/body weight (A), serum amylase activity (B), and serum lipase activity (C) during cerulein-induced acute pancreatitis. Mice pretreated with Nardostachys jatamansi (NJ) were challenged with intraperitoneal injections of cerulein (50 μg/kg). Mice were killed 6 h after the last cerulein injection. Their serum and pancreas were harvested and (A) pancreatic weight (PW)/body weight (BW) ratio and the levels of digestive enzymes such as (B) amylase and (C) lipase were measured as indicated in the experimental protocol. Data show the mean ± SE for 6 mice in 1 group. The results were similar in 3 additional experiments. aP < 0.05 vs the saline treatment; cP < 0.05 vs cerulein treatment alone. AP: Acute pancreatitis.
Effect of NJ4 on pro-inflammatory cytokine production
Several inflammatory mediators such as ILs and TNFs have been shown to increase in AP[10]. Therefore, the levels of cytokines were examined in the serum and pancreas. Substantial increases in IL-1β, IL-6, and TNF-α were found in the serum and pancreas at 6 h after the final administration of cerulein. However, the levels of IL-1β, IL-6, and TNF-α in the serum and pancreas were significantly reduced with NJ4 treatment (Figure 6A and B).
Figure 6.
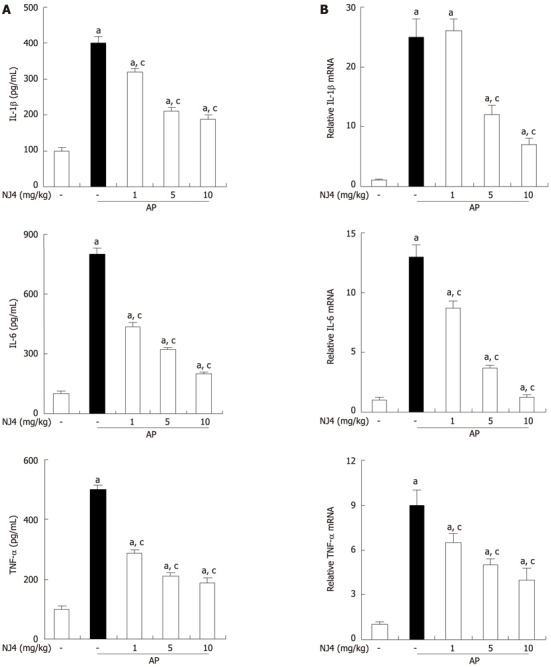
Effects of the 4th fraction of Nardostachys jatamansi on interleukin 1, interleukin 6, and tumor necrosis factor during cerulein-induced pancreatitis. Mice pretreated with Nardostachys jatamansi (NJ) were challenged with intraperitoneal injections of cerulein at a supramaximal dose (50 μg/kg). Mice were killed at 6 h after the last cerulein injection. Levels of serum cytokines were measured by enzyme-linked immunosorbent assay (A). Levels of pancreatic mRNA for interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α were quantified using real-time reverse transcriptase polymerase chain reaction (B). Data show the mean ± SE for 6 mice in 1 group. The results were similar in 3 additional experiments. aP < 0.05 vs the saline treatment; cP < 0.05 vs cerulein treatment alone. AP: Acute pancreatitis.
Effect of NJ4 on in vivo HO-1 induction
To evaluate the protective mechanisms of NJ4, we determined the levels of HO-1, which exhibits certain biological properties, including anti-inflammatory[11,12] and antioxidant[13,14] properties. Therefore, NJ4 (10 mg/kg) was administered intraperitoneally at the indicated time point. Administration of NJ4 was followed by significant up-regulation of pancreatic HO-1 after 1 h (Figure 7A and B). Pancreatic HO-1 induction also occurred in a dose-dependent manner at 6 h after the injection of NJ4 (Figure 7C). Curcumin, an HO-1 inducer, was loaded as the positive control. The pancreatic mRNA levels of HO-1 were correlated with the pancreatic protein levels of HO-1 (Figure 7D).
Figure 7.
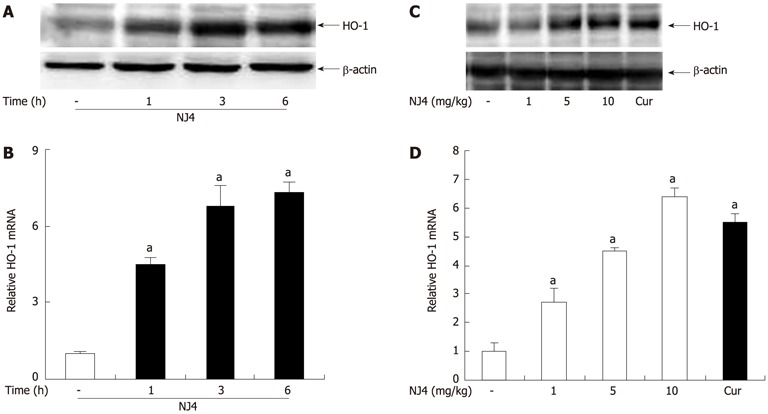
Effects of the 4th fraction of Nardostachys jatamansi on heme oxygenase-1 expression in the pancreas. Mice were treated with saline, or Nardostachys jatamansi (NJ) (10 mg/kg). Then, the pancreas was harvested at the indicated time. A: The protein level of heme oxygenase-1 (HO-1) in the pancreas was measured using Western blotting. β-actin was used as the loading control; B: The mRNA expression of HO-1 was measured using real-time reverse transcriptase polymerase chain reaction (RT-PCR). Mice were treated with the indicated dose of NJ4. At 6 h after NJ4 injection, the pancreas was harvested; C: The protein level of HO-1 in the pancreas was measured using Western blotting. β-actin was used as the loading control; D: The mRNA expression of HO-1 was measured using real-time RT-PCR. The results were similar in 3 additional experiments. aP < 0.05 vs the saline treatment. Cur: Curcumin.
Effect of NJ4 on in vitro HO-1 induction and acinar cell death
To examine the effect of NJ4 on HO-1 expression in isolated pancreatic acinar cells, HO-1 expression was measured. As shown in Figure 8A and B, NJ4 treatment increased the HO-1 expression after 1 h, but significantly at 3 h. The expression was increased in time dependant manner. HO-1 induction in pancreatic acinar cells occurred in a dose-dependent manner at 6 h after NJ4 treatment (Figure 8C and D).
Figure 8.
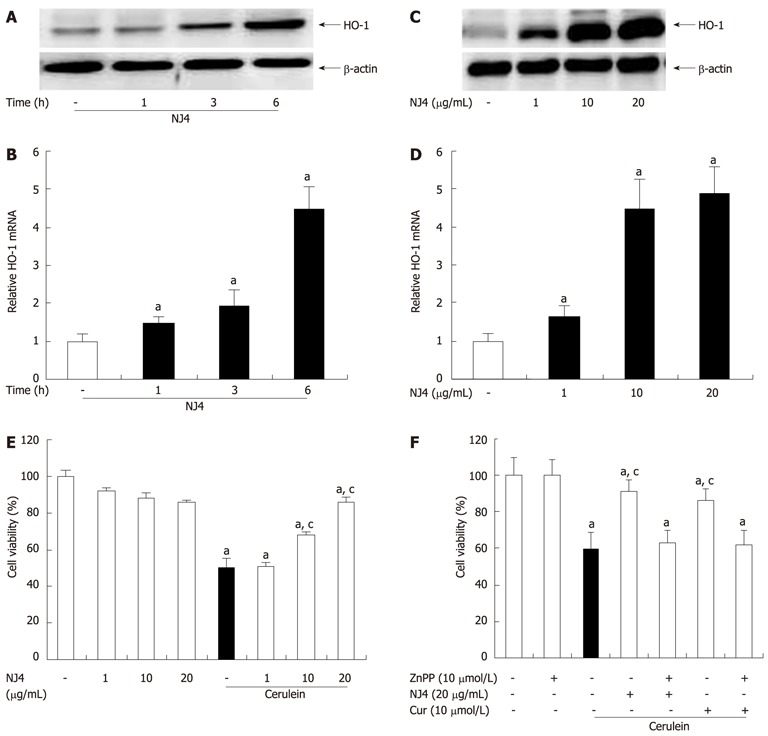
Effects of the 4th fraction of Nardostachys jatamansi on heme oxygenase-1 expression in acinar cells and cerulein-induced acinar cell death. Pancreatic acinar cells were pretreated with Nardostachys jatamansi (NJ) (20 μg/mL), and then the cells were harvested at the indicated time. A, B: The protein level (A) and mRNA level (B) of heme oxygenase-1 (HO-1) in pancreatic acini were measured. Pancreatic acinar cells were pretreated with the indicated dose of NJ4. Then, the cells were harvested at 6 h; C, D: The protein level (C) and mRNA level (D) of HO-1 in the pancreatic acini were detected. The pancreatic acinar cells were pretreated with the indicated dose of NJ4 and then stimulated with cerulein (10 nmol/L); E: After 6 h of cerulein stimulation, cell viability was measured as described in the experimental protocol. Pancreatic acinar cells were pretreated with ZnPP (10 μmol/L), an HO-1 inhibitor, for 1 h and then treated with NJ4 (20 μg/mL), curcumin (10 μmol/L); F: At 1 h after treatment, cerulein (10 nmol/L) was added; After 6 h of cerulein stimulation, cell viability was measured. The results were similar in 3 additional experiments. aP < 0.05 vs the saline treatment; cP < 0.05 vs cerulein treatment alone. Cur: Curcumin.
AP is characterized by acinar cell death in the pancreas. Therefore, we examined the effect of NJ4 on pancreatic acinar cell death. The cells were pretreated with NJ4 for 1 h and then stimulated with cerulein for 6 h. NJ4 significantly increased the viability of pancreatic acinar cells in a dose-dependent manner (Figure 8E). We also investigated whether NJ4 could regulate cerulein-induced acinar cell death through the induction of HO-1. The HO-1 inhibitor ZnPP inhibited the effect of NJ4 on cerulein-induced acinar cells (Figure 8F). Furthermore, the HO-1 inducer curcumin also inhibited cerulein-induced acinar cell death (Figure 8F).
Effect of NJ4-2 on in vitro acinar cell death
To examine the effect of NJ4-2 on HO-1 expression in isolated pancreatic acinar cells, HO-1 expression was measured. As shown in Figure 9A and B, NJ4-2 treatment increased the HO-1 expression after 1 h. The expression was increased in time dependant manner. HO-1 induction in pancreatic acinar cells occurred in a dose-dependent manner at 6 h after NJ4-2 treatment (Figure 9C and D).
Figure 9.
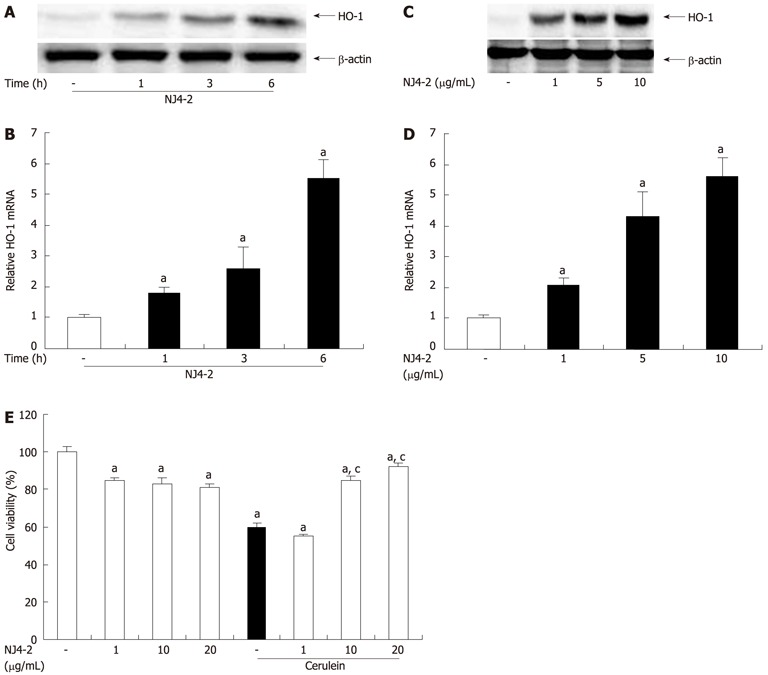
Effects of Nardostachys jatamansi-2 on heme oxygenase-1 expression in acinar cells and cerulein-induced acinar cell death. Pancreatic acinar cells were pretreated with Nardostachys jatamansi (NJ4)-2 (10 μg/mL), and then the cells were harvested at the indicated time. A, B: The protein level (A) and mRNA level (B) of heme oxygenase-1 (HO-1) in pancreatic acini were measured. Pancreatic acinar cells were pretreated with the indicated dose of NJ4-2; C, D: Then, the cells were harvested at 6 h, the protein level (C) and mRNA level (D) of HO-1 in the pancreatic acini were detected. The pancreatic acinar cells were pretreated with the indicated dose of NJ4 and then stimulated with cerulein (10 nmol/L); E: After 6 h of cerulein stimulation, cell viability was measured as described in the experimental protocol. The results were similar in 3 additional experiments. aP < 0.05 vs the saline treatment; cP < 0.05 vs cerulein treatment alone.
The cells were pretreated with NJ4-2 for 1 h and then stimulated with cerulein for 6 h. NJ4-2 significantly increased the viability of pancreatic acinar cells in a dose-dependent manner (Figure 9E).
DISCUSSION
Various studies have investigated candidates that could treat AP[6,15-18]. However, specific and effective candidates to treat AP remain unknown. Our previous report showed that the total extract of NJ reduces the severity of mild acute pancreatitis[6]. In this study, we report that the biological main fraction of NJ, i.e., NJ4, has a protective effect against cerulein-induced AP. To identify the fraction responsible for the main effect of NJ, we fractionated NJ and obtained 6 fractions. The effects of the 6 fractions on lipopolysaccharide (LPS)-induced inflammatory responses were examined in peritoneal macrophages. NJ4 significantly inhibited the LPS-induced production of inflammatory mediators such as nitric oxide (NO), IL-1, IL-6, and TNF-α (unpublished data). Therefore, NJ4 was chosen to examine its effect on pancreatitis. Also among the 3 fractions from NJ4, NJ4-2 inhibited the inflammatory mediators significantly. Thus, we also choose NJ4-2 as NJ4’s candidate. NJ4 ameliorated the severity of AP and AP associated with lung injury and inhibited neutrophil infiltration, digestive enzymes, and cytokines. NJ4 also increased cell viability against cerulein challenge. In the present study, we showed that the effect of NJ4 on pancreatitis was comparable with the effect of the total aqueous extract of NJ.
AP remains a challenging clinical problem in which mortality is significantly increased depending on its severity[19]. Despite a few clinical trials with pharmacological agents, no effective treatment exists for AP. AP is commonly caused by excessive alcohol consumption, biliary tract disease, hereditary factors, certain medications, and invasive procedures of the biliary and pancreatic ducts[20-23]. In this experiment, we used a well-established murine model. Mice with AP showed destruction in acinar cell structure and increased neutrophil infiltration. The NJ4-pretreated mice with AP showed reduced acinar cell destruction and neutrophil infiltration (Figure 3).
The mortality rate in AP is approximately 20%-30%, and the major cause of AP-induced death is acute lung injury, referred to as acute respiratory distress syndrome. After cytokines, or the digestive enzymes from the pancreas, attack the lung alveolar cells, inflammation and vascular injury occurs in the lung[8]. Cells that have infiltrated the pulmonary tissues cause breakdown of the pulmonary parenchyma[24]. This lung attack is particularly damaging in the elderly, and if they survive an attack, their quality of life is impaired[25-27]. Therefore, it is important to protect against lung injury caused by AP. In the present study, we showed that NJ4 treatment reduces lung injury and inflammation in AP (Figure 4).
Experimental pancreatitis models and pancreatitis patients showed increased IL and TNF-α levels[28-30]. IL-1β, IL-6, and TNF-α play a pivotal role in AP, acting early in the disease course in addition to transitioning the acute inflammatory response to a chronic response[31,32]. We have previously reported that the total aqueous extract of NJ could inhibit cytokine production in mice with AP and LPS-induced cytokine production in macrophages[5,6]. Similar to the total aqueous extract of NJ, NJ4 inhibited serum and pancreatic cytokine production in mice with AP.
HO-1 is a stress-inducible enzyme, whereas its isoform HO-2 is constitutively expressed[33]. HO-1 catabolizes heme into a free iron, carbon monoxide, and bilirubin/biliverdin[34]. Studies have shown that HO-1 has protective and anti-inflammatory effects on AP[35,36]. In the above experiment, the administration of HO-1 inducers resulted in significant induction of HO-1 in a specific target organ, the pancreas, and reduction in pro-inflammatory cytokines, a major factor in AP regulation. We have already reported that the protective mechanism of the total water extract of NJ on AP involves the inhibition of MAPKs. Similarly, NJ4 inhibited the activation of MAPKs, but the inhibition was not significant (data not shown). Therefore, we hypothesize that the main protective mechanism of NJ4 is via HO-1 upregulation. HO-1 was up-regulated 1 h after NJ4 injection, and the upregulation was dose-dependent. The HO-1 up-regulation by NJ4 was comparable with that observed with curcumin. Furthermore, NJ4 induced expression of HO-1 in isolated pancreatic acinar cells at 3 h significantly; this finding is different from that of the in vivo experiment. In the in vivo experiment, NJ4 induced pancreatic HO-1 expression at 1 h significantly; however, in the in vitro experiment, NJ4 treatment led to the significant up-regulation of HO-1 at 3 h. We speculate that differences in metabolism between tissues and cells might be the reason for the difference in the expression time of HO-1 in the in vivo and in vitro experiments.
In this study, we suggest and evaluate the possible protective effect of NJ4 against AP. NJ4 treatment attenuated the severity of AP and lung injury associated with AP via the up-regulation of HO-1. The biological fraction of the total aqueous extract of NJ (NJ4) showed similar effects to those obtained with the total extract, which means that NJ4 might contain a compound useful for treating AP. Further fractionation and analyses are needed to identify this novel compound from NJ4.
COMMENTS
Background
Acute pancreatitis (AP) is a serious, unpredictable clinical problem, whose pathophysiology remains poorly understood. Therefore, drugs and therapies need to be developed.
Research frontiers
The paper previously reported that the total water extract of Nardostachys jatamansi (NJ) could protect against AP. This study aimed to determine if the fraction of NJ has the potential to ameliorate the severity of AP.
Innovations and breakthroughs
Nowadays, the treatment of acute pancreatitis is limited to protease inhibitors, and the pathogenesis is not well-understood. In this paper, the authors studied a possible candidate to develop as drug treatment for acute pancreatitis, in line with the previous report. Also the authors provided the regulating mechanisms in AP. This finding could strengthen up further studies of acute pancreatitis.
Applications
The papers, here, firstly provided the possible candidate of NJ. These results could provide the clinical basis for development of a drug or compound to treat acute pancreatitis.
Terminology
Acute pancreatitis: a sudden inflammation of the pancreas. It can have severe complications and high mortality despite treatment. While mild cases are often successfully treated with conservative measures and aggressive intravenous fluid rehydration, severe cases may require admission to the intensive care unit or even surgery to deal with complications of the disease process.
Peer review
This submission shows interesting results.
Footnotes
Supported by The Ministry of Education, Science and Technology at Wonkwang University, No. MEST 2010-0017094
Peer reviewer: Juei-Tang Cheng, Professor, Department of Pharmacology, National Cheng Kung University, No. 1 University Road, Tainan 70101, Taiwan, China
S- Editor Cheng JX L- Editor O’Neill M E- Editor Zhang DN
References
- 1.Shrivastava P, Bhatia M. Essential role of monocytes and macrophages in the progression of acute pancreatitis. World J Gastroenterol. 2010;16:3995–4002. doi: 10.3748/wjg.v16.i32.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogawa M. Acute pancreatitis and cytokines: “second attack” by septic complication leads to organ failure. Pancreas. 1998;16:312–315. [PubMed] [Google Scholar]
- 3.Arora RB. Nardostachys jatamansi: a chemical, pharmacological and clinical appraisal. Spec Rep Ser Indian Counc Med Res. 1965;51:1–117. [PubMed] [Google Scholar]
- 4.Song MY, Bae UJ, Lee BH, Kwon KB, Seo EA, Park SJ, Kim MS, Song HJ, Kwon KS, Park JW, et al. Nardostachys jatamansi extract protects against cytokine-induced beta-cell damage and streptozotocin-induced diabetes. World J Gastroenterol. 2010;16:3249–3257. doi: 10.3748/wjg.v16.i26.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae GS, Seo SW, Kim MS, Park KC, Koo BS, Jung WS, Cho GH, Oh HC, Yun SW, Kim JJ, et al. The roots of Nardostachys jatamansi inhibits lipopolysaccharide-induced endotoxin shock. J Nat Med. 2011;65:63–72. doi: 10.1007/s11418-010-0458-x. [DOI] [PubMed] [Google Scholar]
- 6.Bae GS, Park HJ, Kim DY, Song JM, Kim TH, Oh HJ, Yun KJ, Park RK, Lee JH, Shin BC, et al. Nardostachys jatamansi protects against cerulein-induced acute pancreatitis. Pancreas. 2010;39:520–529. doi: 10.1097/MPA.0b013e3181bd93ce. [DOI] [PubMed] [Google Scholar]
- 7.Li P, Yamakuni T, Matsunaga K, Kondo S, Ohizumi Y. Nardosinone enhances nerve growth factor-induced neurite outgrowth in a mitogen-activated protein kinase- and protein kinase C-dependent manner in PC12D cells. J Pharmacol Sci. 2003;93:122–125. doi: 10.1254/jphs.93.122. [DOI] [PubMed] [Google Scholar]
- 8.Zhou MT, Chen CS, Chen BC, Zhang QY, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16:2094–2099. doi: 10.3748/wjg.v16.i17.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elder AS, Saccone GT, Bersten AD, Dixon DL. Caerulein-induced acute pancreatitis results in mild lung inflammation and altered respiratory mechanics. Exp Lung Res. 2011;37:69–77. doi: 10.3109/01902148.2010.516307. [DOI] [PubMed] [Google Scholar]
- 10.Mota RA, Sánchez-Bueno F, Saenz L, Hernández-Espinosa D, Jimeno J, Tornel PL, Martínez-Torrano A, Ramírez P, Parrilla P, Yélamos J. Inhibition of poly(ADP-ribose) polymerase attenuates the severity of acute pancreatitis and associated lung injury. Lab Invest. 2005;85:1250–1262. doi: 10.1038/labinvest.3700326. [DOI] [PubMed] [Google Scholar]
- 11.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 12.Sheikh SZ, Hegazi RA, Kobayashi T, Onyiah JC, Russo SM, Matsuoka K, Sepulveda AR, Li F, Otterbein LE, Plevy SE. An anti-inflammatory role for carbon monoxide and heme oxygenase-1 in chronic Th2-mediated murine colitis. J Immunol. 2011;186:5506–5513. doi: 10.4049/jimmunol.1002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turkseven S, Kruger A, Mingone CJ, Kaminski P, Inaba M, Rodella LF, Ikehara S, Wolin MS, Abraham NG. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. Am J Physiol Heart Circ Physiol. 2005;289:H701–H707. doi: 10.1152/ajpheart.00024.2005. [DOI] [PubMed] [Google Scholar]
- 14.Taillé C, El-Benna J, Lanone S, Dang MC, Ogier-Denis E, Aubier M, Boczkowski J. Induction of heme oxygenase-1 inhibits NAD(P)H oxidase activity by down-regulating cytochrome b558 expression via the reduction of heme availability. J Biol Chem. 2004;279:28681–28688. doi: 10.1074/jbc.M310661200. [DOI] [PubMed] [Google Scholar]
- 15.Seo SW, Jung WS, Lee SE, Choi CM, Shin BC, Kim EK, Kwon KB, Hong SH, Yun KJ, Park RK, et al. Effects of bee venom on cholecystokinin octapeptide-induced acute pancreatitis in rats. Pancreas. 2008;36:e22–e29. doi: 10.1097/MPA.0b013e31815a396b. [DOI] [PubMed] [Google Scholar]
- 16.Jung WS, Chae YS, Kim DY, Seo SW, Park HJ, Bae GS, Kim TH, Oh HJ, Yun KJ, Park RK, et al. Gardenia jasminoides protects against cerulein-induced acute pancreatitis. World J Gastroenterol. 2008;14:6188–6194. doi: 10.3748/wjg.14.6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo SW, Bae GS, Kim SG, Yun SW, Kim MS, Yun KJ, Park RK, Song HJ, Park SJ. Protective effects of Curcuma longa against cerulein-induced acute pancreatitis and pancreatitis-associated lung injury. Int J Mol Med. 2011;27:53–61. doi: 10.3892/ijmm.2010.548. [DOI] [PubMed] [Google Scholar]
- 18.Carvalho KM, Morais TC, de Melo TS, de Castro Brito GA, de Andrade GM, Rao VS, Santos FA. The natural flavonoid quercetin ameliorates cerulein-induced acute pancreatitis in mice. Biol Pharm Bull. 2010;33:1534–1539. doi: 10.1248/bpb.33.1534. [DOI] [PubMed] [Google Scholar]
- 19.Rau B, Uhl W, Buchler MW, Beger HG. Surgical treatment of infected necrosis. World J Surg. 1997;21:155–161. doi: 10.1007/s002689900208. [DOI] [PubMed] [Google Scholar]
- 20.Beger HG, Rau B, Mayer J, Pralle U. Natural course of acute pancreatitis. World J Surg. 1997;21:130–135. doi: 10.1007/s002689900204. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser AM, Saluja AK, Steer ML. Repetitive short-term obstructions of the common bile-pancreatic duct induce severe acute pancreatitis in the opossum. Dig Dis Sci. 1999;44:1653–1661. doi: 10.1023/a:1026687632370. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med. 1994;330:1198–1210. doi: 10.1056/NEJM199404283301706. [DOI] [PubMed] [Google Scholar]
- 23.Heath DI, Cruickshank A, Gudgeon M, Jehanli A, Shenkin A, Imrie CW. Role of interleukin-6 in mediating the acute phase protein response and potential as an early means of severity assessment in acute pancreatitis. Gut. 1993;34:41–45. doi: 10.1136/gut.34.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumann B, Zantl N, Veihelmann A, Emmanuilidis K, Pfeffer K, Heidecke CD, Holzmann B. Mechanisms of acute inflammatory lung injury induced by abdominal sepsis. Int Immunol. 1999;11:217–227. doi: 10.1093/intimm/11.2.217. [DOI] [PubMed] [Google Scholar]
- 25.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 26.Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, Bion J, Romand JA, Villar J, Thorsteinsson A, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004;30:51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- 27.Angus DC, Clermont G, Linde-Zwirble WT, Musthafa AA, Dremsizov TT, Lidicker J, Lave JR. Healthcare costs and long-term outcomes after acute respiratory distress syndrome: A phase III trial of inhaled nitric oxide. Crit Care Med. 2006;34:2883–2890. doi: 10.1097/01.CCM.0000248727.29055.25. [DOI] [PubMed] [Google Scholar]
- 28.de Beaux AC, Ross JA, Maingay JP, Fearon KC, Carter DC. Proinflammatory cytokine release by peripheral blood mononuclear cells from patients with acute pancreatitis. Br J Surg. 1996;83:1071–1075. doi: 10.1002/bjs.1800830811. [DOI] [PubMed] [Google Scholar]
- 29.Gukovskaya AS, Gukovsky I, Zaninovic V, Song M, Sandoval D, Gukovsky S, Pandol SJ. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Invest. 1997;100:1853–1862. doi: 10.1172/JCI119714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norman JG, Fink GW, Denham W, Yang J, Carter G, Sexton C, Falkner J, Gower WR, Franz MG. Tissue-specific cytokine production during experimental acute pancreatitis. A probable mechanism for distant organ dysfunction. Dig Dis Sci. 1997;42:1783–1788. doi: 10.1023/a:1018886120711. [DOI] [PubMed] [Google Scholar]
- 31.Papachristou GI. Prediction of severe acute pancreatitis: current knowledge and novel insights. World J Gastroenterol. 2008;14:6273–6275. doi: 10.3748/wjg.14.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malleo G, Mazzon E, Genovese T, Di Paola R, Muià C, Crisafulli C, Siriwardena AK, Cuzzocrea S. Effects of thalidomide in a mouse model of cerulein-induced acute pancreatitis. Shock. 2008;29:89–97. doi: 10.1097/shk.0b013e318067df68. [DOI] [PubMed] [Google Scholar]
- 33.Durante W. Heme oxygenase-1 in growth control and its clinical application to vascular disease. J Cell Physiol. 2003;195:373–382. doi: 10.1002/jcp.10274. [DOI] [PubMed] [Google Scholar]
- 34.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 35.Nakamichi I, Habtezion A, Zhong B, Contag CH, Butcher EC, Omary MB. Hemin-activated macrophages home to the pancreas and protect from acute pancreatitis via heme oxygenase-1 induction. J Clin Invest. 2005;115:3007–3014. doi: 10.1172/JCI24912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Habtezion A, Kwan R, Akhtar E, Wanaski SP, Collins SD, Wong RJ, Stevenson DK, Butcher EC, Omary MB. Panhematin provides a therapeutic benefit in experimental pancreatitis. Gut. 2011;60:671–679. doi: 10.1136/gut.2010.217208. [DOI] [PMC free article] [PubMed] [Google Scholar]


