Abstract
AIM: To determine the precise incidence and clinical features of endoscopic ulcers following gastrectomy.
METHODS: A consecutive series of patients who underwent endoscopic examination following gastrectomy between 2005 and 2010 was retrospectively analyzed. A total of 78 patients with endoscopic ulcers and 759 without ulcers following gastrectomy were enrolled. We analyzed differences in patient age, sex, size of the lesions, method of operation, indications for gastric resection, and infection rates of Helicobacter pylori (H. pylori) between the nonulcer and ulcer groups.
RESULTS: The incidence of endoscopic ulcers after gastrectomy was 9.3% and that of marginal ulcers was 8.6%. Ulcers were more common in patients with Billroth II anastomosis and pre-existing conditions for peptic ulcer disease (PUD). Infection rates of H. pyloridid not differ significantly between the two groups. The patients who underwent operations to treat PUD had lower initial levels of hemoglobin and higher rates of hospital admission.
CONCLUSION: H. pylori was not an important factor in ulcerogenesis following gastrectomy. For patients who underwent surgery for PUD, clinical course of marginal ulcers was more severe.
Keywords: Gastrectomy, Marginal ulcer, Helicobacter pylori
INTRODUCTION
Total or partial gastrectomy is reserved for patients with peptic ulcer disease (PUD) who have failed to respond to therapy or individuals with gastric malignancies. The need to perform gastrectomy on PUD patients has decreased since the discovery of Helicobacter pylori (H. pylori) and development of proton pump inhibitors[1,2]. Nevertheless, the total frequency of performing gastrectomy has increased due to the frequent detection of early gastric cancer and increasing morbid obesity[3-6]. The complications of gastrectomy are recurrent ulcers, nutritional disturbances, dumping syndrome, de novo cancer of the gastric remnant [so-called “gastric stump cancer” (GSC)], and alkaline reflux gastritis[7].
During follow-up endoscopic examination, the detection of ulcerative lesions including marginal ulcers as well as cancer recurrence and development of GSC are of clinical interest. Therefore, information about the distinct features of these different ulcerative lesions will help discriminate these lesions from one another so that appropriate treatment strategies can be identified. Marginal ulcers are defined as ulceration around anastomosis following gastrectomy. It has been reported that the incidence of marginal ulcers varies from 0.6% to 16%[8,9]. Although the etiology of marginal ulcers remains obscure, several mechanisms have been postulated. Until now, there are no convincing results, and the exact link between H. pylori and the development of marginal ulcers is unclear. GSC was originally defined as a gastric cancer that arises in the remnant stomach > 5 years after primary surgery for benign diseases such as PUD. A minimal latency of 5 years is included in the definition to avoid misdiagnosis[10,11]. Until now, many features of these lesions were controversial.
The goal of this study was to determine the precise incidence of endoscopic ulcers in patients with a history of gastrectomy. Clinical features of these lesions and patients were evaluated including location, size, types of reconstruction following gastrectomy, causative disease for operation, and history of H. pylori infection. In addition, features of GSC were evaluated including frequency, site, time of appearance following gastrectomy, and pathological characteristics of cancer.
MATERIALS AND METHODS
Patients
This study protocol was approved by the Ethics Committee of the Catholic University of Korea. The study was conducted at St. Vincent Hospital, a teaching hospital of the Catholic University of Korea. The medical records, charts, and digitized archived images of consecutive patients who had a history of gastrectomy and underwent diagnostic esophago-gastroduodenoscopy between January 2005 and December 2010 were reviewed.
Each case was classified as H. pylori-positive or -negative according to the histological results [rapid urease test (CLO test®, Kimberly-Clark, Utah, United States) or silver staining] for two biopsy specimens taken from the remnant stomach. Exclusion criteria were endoscopic evaluation within 1 year following gastrectomy and comorbidity of serious systemic disease. Individuals with conditions that might have substantial effects on our study results (e.g., serum creatinine > 2.5 mg/dL, total bilirubin > 3.0 mg/dL), pregnant women, patients with psychiatric diseases, and patients who did not sign a consent form were excluded. Patients were also excluded if they had cancer recurrence.
Study design
The endoscopic, histological, and clinical features of the ulcerative lesions were analyzed. Subsequently, the ulcerative lesions were classified as neoplastic or non-neoplastic lesions. Cases of recurrent cancer were excluded; the remaining lesions included marginal ulcer and GSC. We analyzed differences in patient age, sex, size of lesions, method of operation, indications for gastric resection, and infection rates of H. pylori between the non-ulcer and ulcer groups. For patients with marginal ulcers, we analyzed baseline characteristics, clinical manifestation, and rates of H. pylori infection according to the location of ulcers. In addition, we evaluated the clinical features of GSC.
Statistical analysis
Continuous variables were expressed as the mean ± SD and compared using Student’s t-test. Categorical variables were expressed as percentages and compared using a χ2 test. Statistical analyses were conducted with SPSS version 12.0 software (SPSS, Chicago, IL, United States). P < 0.05 was considered to be statistically significant.
RESULTS
Demographic features
Data for a consecutive series of patients who underwent gastrectomy between 2005 and 2010 were retrospectively analyzed. A total of 2862 endoscopic examinations were performed and 918 patients were enrolled in our study. Among these, endoscopic examinations were performed for 512 patients within 1 year of gastrectomy and were excluded from our study. Each patient underwent two or three endoscopic procedures during the study period. Eighty-one patients were excluded due to underlying chronic illnesses including: liver cirrhosis, heart failure, chronic obstructive pulmonary disease, chronic renal failure (n = 37), simple closure (n = 12), gastrojejunostomy without gastrectomy (n = 7), and recurrence of previous cancer (n = 25). The remaining 837 patients were endoscopically examined and included in our study. A total of 78 patients with endoscopic ulcers and 759 without ulcers following gastrectomy were enrolled (Figure 1). Six (0.7%) out of all 837 patients who underwent gastrectomy were diagnosed with GSC.
Figure 1.
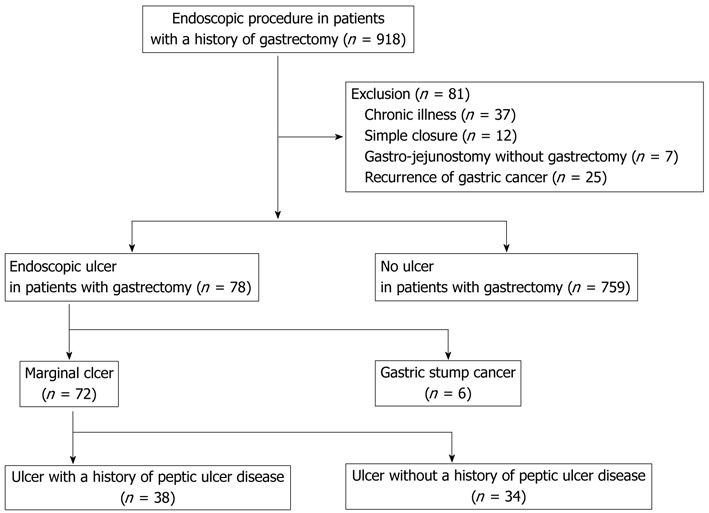
Study design.
Basal characteristics of endoscopic ulcers following gastrectomy
The clinical features of patients in both groups are shown in Table 1. There were no significant differences in age or sex ratio in the group of patients with or without ulcers following gastrectomy. Patients with Billroth II (B-II) gastrojejunal anastomosis were more prone to endoscopic ulceration (P < 0.01) compared to those with Billroth I (B-I) anastomosis (Figure 2). The formation of endoscopic ulcers was more frequent in patients who had undergone gastrectomy for PUD complications than for other reasons (P < 0.01). In patients treated with gastrectomy for PUD complications, the incidence of ulcers was 30.1% (44/146). When patients with GSC were excluded, the incidence of marginal ulcers was 27.1% (38/140). The incidence of ulcers in patients treated with gastrectomy for non-PUD diseases was 4.9% (34/691). The incidence of H. pylori infection did not differ significantly between the two groups (34.5% vs 36.5%, P > 0.05).
Table 1.
Comparison of patients with and without endoscopic ulcers following gastrectomy
| Patients with endoscopic ulcers after gastrectomy (n = 78) | Patients without endoscopic ulcers after gastrectomy (n = 759) | P value | |
| Age (yr) | 62.26 ± 12.89 | 61.00 ± 12.50 | 0.92 |
| Gender (male/female) | 59:19 | 523:236 | 0.20 |
| Anastomosis | |||
| Billroth-I | 12 | 237 | < 0.01 |
| Billroth-II | 63 | 477 | |
| Other method | 3 | 45 | |
| Causative diseases | |||
| Malignancy | 31 | 645 | < 0.01 |
| Complication of PUD | 44 | 102 | |
| Others | 3 | 12 | |
| H. pylori infection (%) | 20/58 (34.5) | 145/397 (36.5) | 0.76 |
H. pylori: Helicobacter pylori; PUD: Peptic ulcer disease.
Figure 2.

Endoscopic finding of marginal ulcer. In patients with B-I (A) and B-II (B) anastomosis, ovoid shaped ulcerations were observed on the efferent side.
Clinical features of marginal ulcers
A total of 72 patients had marginal ulcers that were located on the efferent side or anastomosis. Most of these patients (86.1%, 62/72) complained of specific gastrointestinal symptoms, and 41.7% (30/72) experienced episodes of bleeding or developed anemia. The most frequent symptom was abdominal pain. However, there were no differences of clinical manifestation according to ulcer location. The rate of patients undergoing therapeutic endoscopy to stop or prevent bleeding was 19.4% (14/72) and the rate of patients receiving admission care was 54.2% (39/72). The recurrence of marginal ulcers was observed in 29.2% (21/72) of the patients. There were no differences in the rate of patients receiving therapeutic endoscopy or recurrence of ulcers according to ulcer location (Table 2).
Table 2.
Comparison of marginal ulcers according to the location
| Ulcers on the efferent side (n = 36) | Both (n = 4) | Ulcers at anastomosis (n = 32) | |
| Age (yr) | 63.45 ± 14.03 | 62.50 ± 10.25 | 61.15 ± 12.49 |
| Sex (male/female) | 26:10 | 2:2 | 25:7 |
| Tobacco use | 15 | 2 | 10 |
| Alcohol consumption | 14 | 2 | 12 |
| Clinical symptoms | |||
| Bleeding episode | 6 | 1 | 10 |
| Anemia evaluation | 9 | 2 | 2 |
| Abdominal pain | 11 | 1 | 10 |
| Dyspepsia | 5 | 0 | 5 |
| Routine check | 5 | 0 | 5 |
| Anastomosis | |||
| Billroth-I | 7 | 0 | 6 |
| Billroth-II | 27 | 4 | 25 |
| Total gastrectomy with RY | 1 | 0 | 0 |
| Whipple | 1 | 0 | 1 |
| Causative disease | |||
| Malignancy | 14 | 1 | 16 |
| Complication of PUD | 20 | 3 | 15 |
| Others | 2 | 0 | 1 |
| Ulcer multiplicity | 15 | 4 | 6 |
| Number of therapeutic intervention | 5 | 1 | 8 |
| Initial hemoglobin (g/dL) | 9.70 ± 3.38 | 6.70 ± 2.53 | 9.87 ± 3.79 |
| Admission | 21 | 4 | 1 |
| Ulcer recurrence | 12 | 2 | 7 |
| H. pylori infection (%) | 7/25 (28) | 1/2 (50) | 8/25 (32) |
RY: Roux-en-Y anastomosis; H. pylori: Helicobacter pylori; PUD: Peptic ulcer disease.
The patients were divided into two groups according to ulcer location: individuals with ulcers on the efferent side and ulcers at anastomosis sites. The most common location of marginal ulcers was the efferent side (55.6%, 40/72). There were no differences in patient age, sex ratio, tobacco use, alcohol consumption, types of reconstruction, causative disease, initial hemoglobin levels, recurrence rates of marginal ulcers, or H. pylori infection rates. Ulcers on the efferent side compared to those at anastomosis sites had a tendency of multiplicity, but a number of marginal ulcers appeared as a single lesion (69.4%, 50/72).
Comparison of marginal ulcers according to causative disease
Thirty-eight patients with marginal ulcers previously underwent an operation to control PUD, whereas 34 patients underwent operations to treat malignancies or other conditions (e.g., hemoperitoneum due to trauma). Clinical features of these two groups are shown in Table 3. There was no significant difference in the clinical characteristics including age, sex, types of reconstruction, or H. pylori infection rates. The patients who underwent operations to treat PUD had a higher rate of admission (25/38, 65.8%) than the other group (13/34, 38.2%; P = 0.01). Initial hemoglobin levels were significantly lower in the PUD group. However, the incidence of rebleeding after primary hemostasis and rate of ulcer recurrence were not different (Table 3).
Table 3.
Comparison of marginal ulcers according to causative disease
| Marginal ulcers in patients with a history of PUD (n = 38) | Marginal ulcers in patients without a history of PUD (n = 34) | P value | |
| Age (yr) | 62.87 ± 13.67 | 63.47 ± 13.03 | 0.85 |
| Sex (male/female) | 31:7 | 22:12 | 0.10 |
| Anastomosis | |||
| B-I/B-II/others | 3/35/0 | 6/25/3 | 0.06 |
| Clinical symptoms | |||
| Bleeding episode | 10 | 7 | 0.04 |
| Severe anemia | 10 | 3 | |
| Location | |||
| Efferent side/ anastomosis/both | 20/15/3 | 16/17/1 | 0.50 |
| Initial hemoglobin (g/dL) | 8.83 ± 2.83 | 11.04 ± 3.73 | 0.03 |
| Admission (case) | 25 | 13 | 0.01 |
| Rebleeding during admission | 4 | 3 | 0.87 |
| Ulcer recurrence | 9 | 11 | 0.41 |
| H. pylori infection (%) | 9/25 (36) | 7/24 (29.2) | 0.61 |
H. pylori: Helicobacter pylori; PUD: Peptic ulcer disease; B: Billroth.
Clinical features of GSC
GSC was found in six (0.7%) out of 837 patients who underwent gastrectomy. The clinicopathological features of GSC are shown in Table 4. All of these patients were male, and the median interval between the initial gastrectomy and treatment of GSC was 298.8 mo (range, 141-480). Half of these patients underwent gastric reconstruction by B-I anastomosis and the remaining patients underwent reconstruction by B-II. In two-thirds of all patients, the location of the ulcerative lesion was the gastric stump (Figure 3). Most of these individuals underwent total gastrectomy and only one patient survived the operation.
Table 4.
Case profile of gastric stump cancer
| Case number | 1 | 2 | 3 | 4 | 5 | 6 |
| Sex | M | M | M | M | M | M |
| Age (yr) | 73 | 73 | 58 | 61 | 61 | 44 |
| Tobacco use | Yes | Yes | No | No | Yes | No |
| Alcohol consumption | No | Yes | No | No | No | No |
| Anastomosis | B-II | B-I | B-I | B-I | B-II | B-II |
| Latency period (mo) | 360 | 480 | 360 | 152 | 432 | 228 |
| Reason for operation | Pyloric obstruction | GU perforation | GU bleeding | Pyloric obstruction | GU perforation | DU perforation |
| Chief complaint | Melena | Soreness | Pain | Routine | Pain | Pain |
| Tumor location | Anastomosis | Anastomosis | Stump | Stump | Stump | Stump |
| Tumor type | Adenoca | Adenoca | Adenoca | Adenoca | Adenoca | Adenoca |
| Differentiation | Moderate | Poor | Poor | Poor | Moderate | Poor |
| H. pylori infection | Negative | Negative | Positive | Positive | Positive | Negative |
| TNM stage | ||||||
| T | T4a | T3 | T3 | T2b | pT3 | T3 |
| N | N2 | N0 | N0 | N1 | N1 | N0 |
| M | M1 (Liver) | M0 | M0 | M0 | M0 | M0 |
| Treatment | None | Total gastrectomy | Total gastrectomy | Total gastrectomy | Total gastrectomy | Total gastrectomy |
| Survival (mo) | No (3) | No (10) | No (10)1 | Yes (36) | No (8) | No (18) |
| DFS (mo) | No (0) | Yes (9) | Yes (10) | Yes (36) | No (0) | Yes (15) |
Expired due to leukopenia and pneumonia. GU: Gastric ulcer; DU: Duodenal ulcer; Adenoca: Adenocarcinoma; DFS: Disease-free survival; H. pylori: Helicobacter pylori; B: Billroth.
Figure 3.
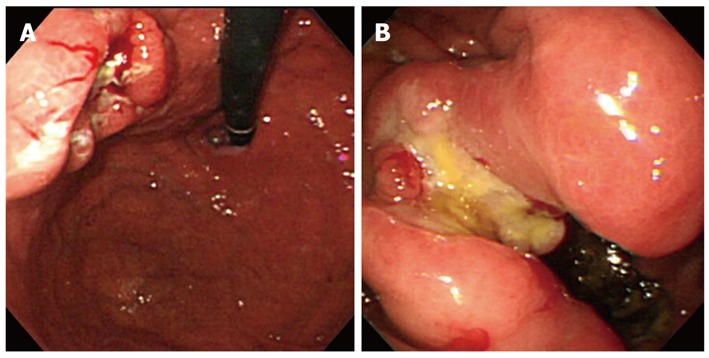
Endoscopic findings of gastric stump cancer. A: An ulcerating lesion of the gastric stump was observed; B: After gastrectomy with Billroth-II reconstruction, a large ulcerating mass was found at the anastomosis site.
DISCUSSION
The etiology of ulcer formation following gastrectomy is often multifactorial. Possible contributing factors include local ischemia, anastomotic tension, increased gastric acidity, H. pylori infection, tobacco use, and nonsteroidal anti-inflammatory drug (NSAID) use[12-14]. Chronic inflammation caused by the suture materials at the anastomosis may lead to ulcer formation. Earlier studies have demonstrated that most marginal ulcers develop in the first 3 mo postoperatively due to prolonged irritation by foreign material, however, no ulcers have been found after the first postoperative year[9,15].
In this study, we included patients who developed ulcers 1 year or more after surgery, and analyzed true marginal ulcers that appeared after gastrectomy. Our results showed that the incidence of endoscopic ulcers after gastrectomy was 9.3% and marginal ulcers was 8.6%. In particular, the incidence of marginal ulcers in patients who had undergone gastrectomy to treat PUD-associated complications was 27.1%. Furthermore, it is noteworthy that most of the patients with marginal ulcers (86.1%) complained of specific gastrointestinal symptoms. Half of these individuals experienced bleeding episodes or developed severe anemia. There was a limitation in this study and we did not consider whether vagotomy was performed. We focused on ulcer development after gastrectomy. Previously, it has been demonstrated that vagotomy prevents ulcer recurrence[16,17], and most of the enrolled patients had gastrectomy with vagotomy as peptic ulcer surgery.
Marginal ulcer is a well-known complication of partial gastrectomy. It has been reported that marginal ulcer is evidence of inadequate acid reduction. However, incomplete suppression of gastric acid production is not the sole cause of marginal ulcers. In our endoscopic survey, marginal ulcer was formed following total gastrectomy. On the basis of this result, impaired blood supply or use of NSAIDs can be another mechanism of ulcer development after gastrectomy. In our study, risk factors for ulcer formation after gastrectomy were B-II anastomosis and previous gastrectomy for treating PUD complications. Generally, B-I anastomosis has several advantages. This technique can be performed more easily and rapidly than a B-II anastomosis because fewer steps are required. Normal continuity of the gastrointestinal tract is maintained, thus promoting the mixture of food with bile and pancreatic secretions. The remaining duodenum offers more resistance to recurrent ulceration than does the jejunum and plays a role in preventing the recurrence of ulcers after B-I anastomosis[18]. These factors explained why ulcerative lesions developed less frequently following B-I anastomosis.
Infection with H. pylori has been shown to be a predictive marker of PUD in the general population, but the exact link between H. pylori and the development of marginal ulcers following gastrectomy is unclear. Previously, uncontrolled data have suggested that the frequency of symptomatic marginal ulcers can be reduced by preoperative screening and treatment of H. pylori infection in patients undergoing gastric bypass surgery[19,20]. Although these studies were performed in bariatric patients who have undergone gastric bypass surgery, the results indicate that infection with H. pylori may promote marginal ulcer formation. On the contrary, our investigation showed that the rate of H. pylori infection did not differ significantly between the ulcer and non-ulcer groups. This suggests that H. pylori infection does not play an important role in the pathogenesis of recurrent ulceration after gastrectomy.
However, our study had several limitations associated with our investigation of H. pylori infection. First, we did not determine whether patients enrolled in this study were treated when H. pylori was diagnosed before they underwent endoscopic examination. In fact, our patients were not routinely tested nor treated for H. pylori before gastrectomy. Second, spontaneous clearance of H. pylori might occur in a certain number of patients who undergo distal gastrectomy[21]. Third, the number of patients with B-II was more than twice that with B-I anastomosis. A previous study has shown that resection with B-I has an incidence of H. pylori infection of approximately 70%, followed by a significantly lower rate of infection in B-II anastomosis of 45%[22]. The lower rate of H. pylori infection found in B-II may reflect the role of bile reflux, which is more common in B-II than B-I, and may interfere with colonization by H. pylori.
To date, many studies have shown that GSC develops after distal gastrectomy, at frequencies of 0.4-2.5%, with an increased incidence 15 years after surgery[23-25]. Some authors have postulated that patients who have undergone B-II gastrojejunal anastomosis are more prone to developing carcinomas in the gastric remnant[26,27]. However, GSC has been reported in patients following B-I anastomosis. Our results revealed that GSC was found in three patients after B-I and three after B-II anastomosis. GSC is often described as a tumor with a poor prognosis, with low resectability rates because of extended lymph node metastases and infiltration of adjacent organs[24,28]. The reported 5-year disease-free overall survival ranges from 7% to 20%[29,30]. In our study, all patients had advanced stage disease at diagnosis and surgical treatment (total gastrectomy with radical lymph node dissection) was performed in 80% of cases. However, only one patient had 5-year disease-free survival.
Although specific factors responsible for the pathogenesis of GSC have not been identified, chronic inflammation of remnant gastric mucosa is considered to be a risk factor. Enterogastric reflux of bile acids results in increased proliferation of epithelial cells, and hypochlorhydria with subsequent bacterial overgrowth promotes this condition[31,32]. It has been previously reported that the gastric mucosa undergoes marked morphological changes in most patients after gastrectomy[27,33]. Other mechanisms underlying GSC development are effects of hormonal regulation following vagotomy, and H. pylori infection[34-37]. Although H. pylori infection may also be a causative factor for GSC, it has reported that the rate of H. pylori infection in patients with GSC is lower than in those with primary gastric cancer. The reason is that the gastric stump may be an unsuitable environment for H. pylori colonization due to increased bile reflux[38,39]. However, the exact effect of H. pylori is unclear in clinical practice because of the small number of studies on this subject.
In conclusion, we found that endoscopic ulcers following gastrectomy were more common in patients who have undergone B-II anastomosis and had pre-existing cases of PUD. Infection with H. pylori did not appear to play an important role in the formation of ulcers following gastrectomy. Patients who underwent operations to treat PUD had lower initial levels of hemoglobin and a higher frequency of admission. Our study demonstrated that patients who undergo operations to treat PUD and subsequently develop marginal ulcer are likely to have a more severe clinical course. Further prospective and detail studies are needed to confirm our findings.
COMMENTS
Background
The total frequency of performing gastrectomy has increased due to the frequent detection of early gastric cancer and increasing morbid obesity. During follow-up endoscopic examination, the detection of ulcerative lesions including marginal ulcers, as well as cancer recurrence, is of clinical interest. Until now, there are limited data currently available on the incidence of marginal ulcer, and the exact link between Helicobacter pylori (H. pylori) and the development of marginal ulcers is unclear.
Research frontiers
Earlier studies have demonstrated that most marginal ulcers develop in the first 3 mo postoperatively due to prolonged irritation by foreign material. In this study, we excluded the possibility of chronic inflammation caused by the suture materials, and included patients who developed ulcers 1 year or more after surgery. Therefore, true marginal ulcers were analyzed. It was more common in patients in who had undergone Billroth II (B-II) anastomosis and had pre-existing cases of peptic ulcer disease (PUD). It is noteworthy that the incidence of marginal ulcers in patients who had undergone gastrectomy to treat PUD-associated complications was 27.1%. This provided information about the precise incidence and clinical features of marginal ulcers.
Innovations and breakthroughs
The results showed that the incidence of endoscopic ulcers after gastrectomy was 9.3% and that of marginal ulcers was 8.6%. When the patients were divided into two groups according to the presence of ulcer, infection rates of H. pylori did not differ significantly between the two groups (34.5% vs 36.5%, P > 0.05). Ulcers were more common in patients with B-II anastomosis and pre-existing conditions for PUD. The patients who underwent operations to treat PUD had lower initials levels of hemoglobin and higher rates of hospital admission.
Applications
The researchers drew a conclusion that H. pylori infection did not play an important role in the formation of ulcers following gastrectomy. For the patients who underwent operations for PUD, the clinical course of marginal ulcers was more severe.
Peer review
The paper is well written and the findings are of values for gastrectomy evaluation.
Footnotes
Peer reviewers: Javier San Martín, Chief, Gastroenterology and Endoscopy, Sanatorio Cantegril, Av. Roosevelt y P 13, Punta del Este 20100, Uruguay; Dr. Jianyuan Chai, PhD, MS, BS, Assistant Professor, Research (09-151), VA Long Beach Healthcare System, 5901 E. 7th St, Long Beach, CA 90822, United States
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
References
- 1.Nikolopoulou VN, Thomopoulos KC, Theocharis GI, Arvaniti VA, Vagianos CE. Acute upper gastrointestinal bleeding in operated stomach: outcome of 105 cases. World J Gastroenterol. 2005;11:4570–4573. doi: 10.3748/wjg.v11.i29.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walan A, Bader JP, Classen M, Lamers CB, Piper DW, Rutgersson K, Eriksson S. Effect of omeprazole and ranitidine on ulcer healing and relapse rates in patients with benign gastric ulcer. N Engl J Med. 1989;320:69–75. doi: 10.1056/NEJM198901123200201. [DOI] [PubMed] [Google Scholar]
- 3.Jeong O, Park YK. Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer. 2011;11:69–77. doi: 10.5230/jgc.2011.11.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hur H, Song KY, Park CH, Jeon HM. Follow-up strategy after curative resection of gastric cancer: a nationwide survey in Korea. Ann Surg Oncol. 2010;17:54–64. doi: 10.1245/s10434-009-0676-1. [DOI] [PubMed] [Google Scholar]
- 5.Katai H. Function-preserving surgery for gastric cancer. Int J Clin Oncol. 2006;11:357–366. doi: 10.1007/s10147-006-0613-2. [DOI] [PubMed] [Google Scholar]
- 6.Runkel N, Colombo-Benkmann M, Hüttl TP, Tigges H, Mann O, Sauerland S. Bariatric surgery. Dtsch Arztebl Int. 2011;108:341–346. doi: 10.3238/arztebl.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passaro E, Gordon HE, Stabile BE. Marginal ulcer: a guide to management. Surg Clin North Am. 1976;56:1435–1444. doi: 10.1016/s0039-6109(16)41096-0. [DOI] [PubMed] [Google Scholar]
- 8.Higa KD, Boone KB, Ho T. Complications of the laparoscopic Roux-en-Y gastric bypass: 1,040 patients--what have we learned? Obes Surg. 2000;10:509–513. doi: 10.1381/096089200321593706. [DOI] [PubMed] [Google Scholar]
- 9.Sacks BC, Mattar SG, Qureshi FG, Eid GM, Collins JL, Barinas-Mitchell EJ, Schauer PR, Ramanathan RC. Incidence of marginal ulcers and the use of absorbable anastomotic sutures in laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2006;2:11–16. doi: 10.1016/j.soard.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Helsingen N, Hillestad L. Cancer development in the gastric stump after partial gastrectomy for ulcer. Ann Surg. 1956;143:173–179. doi: 10.1097/00000658-195614320-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanigawa N, Nomura E, Lee SW, Kaminishi M, Sugiyama M, Aikou T, Kitajima M. Current state of gastric stump carcinoma in Japan: based on the results of a nationwide survey. World J Surg. 2010;34:1540–1547. doi: 10.1007/s00268-010-0505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sapala JA, Wood MH, Sapala MA, Flake TM. Marginal ulcer after gastric bypass: a prospective 3-year study of 173 patients. Obes Surg. 1998;8:505–516. doi: 10.1381/096089298765554061. [DOI] [PubMed] [Google Scholar]
- 13.Schneider BE, Villegas L, Blackburn GL, Mun EC, Critchlow JF, Jones DB. Laparoscopic gastric bypass surgery: outcomes. J Laparoendosc Adv Surg Tech A. 2003;13:247–255. doi: 10.1089/109264203322333575. [DOI] [PubMed] [Google Scholar]
- 14.MacLean LD, Rhode BM, Nohr C, Katz S, McLean AP. Stomal ulcer after gastric bypass. J Am Coll Surg. 1997;185:1–7. doi: 10.1016/s1072-7515(01)00873-0. [DOI] [PubMed] [Google Scholar]
- 15.Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359:114–117. doi: 10.1016/S0140-6736(02)07369-5. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy T, Kelly JM, George JD. Vagotomy for gastric ulcer. Br Med J. 1972;2:371–373. doi: 10.1136/bmj.2.5810.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YC, Wang HP, Yang CS, Yang TH, Chen JH, Lin CC, Tsai CY, Chang LY, Huang SP, Wu MS, et al. Endoscopic hemostasis of a bleeding marginal ulcer: hemoclipping or dual therapy with epinephrine injection and heater probe thermocoagulation. J Gastroenterol Hepatol. 2002;17:1220–1225. doi: 10.1046/j.1440-1746.2002.02875.x. [DOI] [PubMed] [Google Scholar]
- 18.Johnston JW, Weinberg JA. Billroth I resection for peptic ulcer. Calif Med. 1956;84:157–161. [PMC free article] [PubMed] [Google Scholar]
- 19.Schirmer B, Erenoglu C, Miller A. Flexible endoscopy in the management of patients undergoing Roux-en-Y gastric bypass. Obes Surg. 2002;12:634–638. doi: 10.1381/096089202321019594. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen JJ, Fuller W, Ali MR. Marginal ulceration after laparoscopic gastric bypass: an analysis of predisposing factors in 260 patients. Surg Endosc. 2007;21:1090–1094. doi: 10.1007/s00464-007-9285-x. [DOI] [PubMed] [Google Scholar]
- 21.Bair MJ, Wu MS, Chang WH, Shih SC, Wang TE, Chen CJ, Lin CC, Liu CY, Chen MJ. Spontaneous clearance of Helicobacter pylori colonization in patients with partial gastrectomy: correlates with operative procedures and duration after operation. J Formos Med Assoc. 2009;108:13–19. doi: 10.1016/S0929-6646(09)60027-9. [DOI] [PubMed] [Google Scholar]
- 22.Tomtitchong P, Onda M, Matsukura N, Tokunaga A, Kato S, Matsuhisa T, Yamada N, Hayashi A. Helicobacter pylori infection in the remnant stomach after gastrectomy: with special reference to the difference between Billroth I and II anastomoses. J Clin Gastroenterol. 1998;27 Suppl 1:S154–S158. doi: 10.1097/00004836-199800001-00025. [DOI] [PubMed] [Google Scholar]
- 23.Tokudome S, Kono S, Ikeda M, Kuratsune M, Sano C, Inokuchi K, Kodama Y, Ichimiya H, Nakayama F, Kaibara N. A prospective study on primary gastric stump cancer following partial gastrectomy for benign gastroduodenal diseases. Cancer Res. 1984;44:2208–2212. [PubMed] [Google Scholar]
- 24.Viste A, Bjørnestad E, Opheim P, Skarstein A, Thunold J, Hartveit F, Eide GE, Eide TJ, Søreide O. Risk of carcinoma following gastric operations for benign disease. A historical cohort study of 3470 patients. Lancet. 1986;2:502–505. doi: 10.1016/s0140-6736(86)90368-5. [DOI] [PubMed] [Google Scholar]
- 25.Takeno S, Noguchi T, Kimura Y, Fujiwara S, Kubo N, Kawahara K. Early and late gastric cancer arising in the remnant stomach after distal gastrectomy. Eur J Surg Oncol. 2006;32:1191–1194. doi: 10.1016/j.ejso.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 26.Inokuchi K, Tokudome S, Ikeda M, Kuratsune M, Ichimiya H, Kaibara N, Ikejiri T, Oka N. Mortality from carcinoma after partial gastrectomy. Gann. 1984;75:588–594. [PubMed] [Google Scholar]
- 27.Safatle-Ribeiro AV, Ribeiro U, Reynolds JC. Gastric stump cancer: what is the risk? Dig Dis. 1998;16:159–168. doi: 10.1159/000016860. [DOI] [PubMed] [Google Scholar]
- 28.Sasako M, Maruyama K, Kinoshita T, Okabayashi K. Surgical treatment of carcinoma of the gastric stump. Br J Surg. 1991;78:822–824. doi: 10.1002/bjs.1800780718. [DOI] [PubMed] [Google Scholar]
- 29.Thorban S, Böttcher K, Etter M, Roder JD, Busch R, Siewert JR. Prognostic factors in gastric stump carcinoma. Ann Surg. 2000;231:188–194. doi: 10.1097/00000658-200002000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ovaska JT, Havia TV, Kujari HP. Retrospective analysis of gastric stump carcinoma patients treated during 1946-1981. Acta Chir Scand. 1986;152:199–204. [PubMed] [Google Scholar]
- 31.Muscroft TJ, Deane SA, Youngs D, Burdon DW, Keighley MR. The microflora of the postoperative stomach. Br J Surg. 1981;68:560–564. doi: 10.1002/bjs.1800680813. [DOI] [PubMed] [Google Scholar]
- 32.Reed PI, Smith PL, Summers K, Haines K, Burgess BA, House FR, Walters CL. The influence of enterogastric reflux on gastric juice bacterial growth, nitrite, and N-nitroso compound concentrations following gastric surgery. Scand J Gastroenterol Suppl. 1984;92:232–234. [PubMed] [Google Scholar]
- 33.Sons HU, Borchard F. Gastric carcinoma after surgical treatment for benign ulcer disease: some pathologic-anatomic aspects. Int Surg. 1987;72:222–226. [PubMed] [Google Scholar]
- 34.Lundegårdh G, Adami HO, Helmick C, Zack M, Meirik O. Stomach cancer after partial gastrectomy for benign ulcer disease. N Engl J Med. 1988;319:195–200. doi: 10.1056/NEJM198807283190402. [DOI] [PubMed] [Google Scholar]
- 35.Kaminishi M, Shimizu N, Shimoyama S, Yamaguchi H, Tsuji E, Aoki F, Nomura S, Yoshikawa A, Kuramoto S, Oohara T, et al. Denervation promotes the development of cancer-related lesions in the gastric remnant. J Clin Gastroenterol. 1997;25 Suppl 1:S129–S134. doi: 10.1097/00004836-199700001-00022. [DOI] [PubMed] [Google Scholar]
- 36.Asaka M, Dragosics BA. Helicobacter pylori and gastric malignancies. Helicobacter. 2004;9 Suppl 1:35–41. doi: 10.1111/j.1083-4389.2004.00252.x. [DOI] [PubMed] [Google Scholar]
- 37.Sugiyama T. Development of gastric cancer associated with Helicobacter pylori infection. Cancer Chemother Pharmacol. 2004;54 Suppl 1:S12–S20. doi: 10.1007/s00280-004-0881-3. [DOI] [PubMed] [Google Scholar]
- 38.Baas IO, van Rees BP, Musler A, Craanen ME, Tytgat GN, van den Berg FM, Offerhaus GJ. Helicobacter pylori and Epstein-Barr virus infection and the p53 tumour suppressor pathway in gastric stump cancer compared with carcinoma in the non-operated stomach. J Clin Pathol. 1998;51:662–666. doi: 10.1136/jcp.51.9.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Rees BP, Musler A, Caspers E, Drillenburg P, Craanen ME, Polkowski W, Chibowski D, Offerhaus GJ. K-ras mutations in gastric stump carcinomas and in carcinomas from the non-operated stomach. Hepatogastroenterology. 1999;46:2063–2068. [PubMed] [Google Scholar]


