Abstract
AIM: To investigate preoperative factors associated with poor short-term outcome after resection for multinodular hepatocellular carcinoma (HCC) and to assess the contraindication of patients for surgery.
METHODS: We retrospectively analyzed 162 multinodular HCC patients with Child-Pugh A liver function who underwent surgical resection. The prognostic significance of preoperative factors was investigated by univariate analysis using the log-rank test and by multivariate analysis using the Cox proportional hazards model. Each independent risk factor was then assigned points to construct a scoring model to evaluate the indication for surgical intervention. A receiver operating characteristics (ROC) curve was constructed to assess the predictive ability of this system.
RESULTS: The median overall survival was 38.3 mo (range: 3-80 mo), while the median disease-free survival was 18.6 mo (range: 1-79 mo). The 1-year mortality was 14%. Independent prognostic risk factors of 1-year death included prealbumin < 170 mg/L [hazard ratio (HR): 5.531, P < 0.001], alkaline phosphatase > 129 U/L (HR: 3.252, P = 0.005), α fetoprotein > 20 μg/L (HR: 7.477, P = 0.011), total tumor size > 8 cm (HR: 10.543; P < 0.001), platelet count < 100 × 109/L (HR: 9.937, P < 0.001), and γ-glutamyl transpeptidase > 64 U/L (HR: 3.791, P < 0.001). The scoring model had a strong ability to predict 1-year survival (area under ROC: 0.925, P < 0.001). Patients with a score ≥ 5 had significantly poorer short-term outcome than those with a score < 5 (1-year mortality: 62% vs 5%, P < 0.001; 1-year recurrence rate: 86% vs 33%, P < 0.001). Patients with score ≥ 5 had greater possibility of microvascular invasion (P < 0.001), poor tumor differentiation (P = 0.003), liver cirrhosis with small nodules (P < 0.001), and intraoperative blood transfusion (P = 0.010).
CONCLUSION: A composite preoperative scoring model can be used as an indication of prognosis of HCC patients after surgical resection. Resection should be considered with caution in patients with a score ≥ 5, which indicates a contraindication for surgery.
Keywords: Hepatectomy, Hepatocellular carcinoma, Multinodular, Prognosis, Treatment outcome
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer deaths worldwide, with the highest incidence rates reported in East Asia[1,2]. Multinodular HCC accounts for a large number of HCC cases. For these patients, hepatic resection may lead to an increased risk of postoperative liver failure and the recurrence rates after resection are higher than those for a single HCC. Hence, multinodular HCC have been considered a controversial indication for hepatic resection, especially for cases involving liver cirrhosis[3].
However, the fact that patients with multinodular HCC have poorer survival than those with a single small tumor should not be considered a sufficient reason for excluding them from undergoing resection. Although liver transplantation is considered another curative treatment, its application is hampered by the lack of liver donors and high drop-out rates[4]. The efficacy of radiofrequency ablation (RFA) is greatly limited by tumor size and location. Chemoembolization has been regarded as a palliative method with lower rates of tumor necrosis[5,6]. With developments in surgical techniques and perioperative treatment, the safety of hepatic resection has markedly improved. Surgical mortality rates have been reduced to less than 5.0%[7]. Many previous studies have demonstrated that resection can provide survival benefits for patients with multinodular HCC[4,8-12]. Therefore, hepatic resection remains the widely accepted mainstay of curative treatment for multinodular HCC.
Better patient selection plays a crucial role in the improvement of postoperative outcome[7]. Although it is widely accepted that Child-Pugh A cirrhosis can be treated safely in elective surgery, poor short-term survival still exists in these patients. It is reported that patients with multinodular HCC have a 1-year mortality ranging from 14% to 26% after hepatectomy[4,13]. Several previous studies reported that HCC patients undergoing non-surgical therapy had a total 1-year survival rate of 40%-62%[2,14-16], and patients without adverse factors who only received supportive care had a 1-year survival rate of 80%[16], thus patients who die in the first year after surgery may not have gained a benefit from hepatic resection. Hepatectomy should not be indicated for these patients even though there is acceptable perioperative safety.
Several recent studies showed that traditional scoring systems of liver function such as the Child-Pugh score have limitations[17,18], and routine parameters may not describe hepatic impairment sufficiently. Most current staging systems for HCC focus on predicting perioperative or long-term survival but neglect the short-term outcome. The risk factors for poor short-term survival in patients with satisfactory liver function remain unclear. The present study was designed to investigate the independent predictive factors that are associated with poor short-term outcome after resection of multinodular HCC. We only focused on the preoperative clinical data that were most helpful in choosing the optimal initial treatment. The results may supplement the classical estimation system for surgical indication.
MATERIALS AND METHODS
Patients
Between January 2004 and December 2008, we retrospectively accumulated 208 consecutive patients with multinodular HCC underwent hepatic resection at the Department of Surgery, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University (Shanghai, China). We regarded a tumor with a surrounding co-nodule(s) as a single tumor only when the co-nodule(s) was attached to the main tumor[4,13]. Multinodular HCC was diagnosed when the tumor number was ≥ 2. Fifteen patients with extrahepatic metastasis and 31 patients with macroscopic cancerous emboli were excluded. The remaining 162 patients (28 female, 134 male; mean age, 50.16 years; range: 27-76 years) who underwent curative hepatic resection were enrolled in this study. The baseline clinical features are listed in Table 1. All patients had liver function of Child-Pugh class A. Among them, 44 patients were Barcelona Clinic Liver Cancer (BCLC) stage A and 118 BCLC stage B. A total of 156 patients were diagnosed with hepatitis B virus (HBV) infection, which was defined as positivity of hepatitis B surface antigen (HBsAg) in the serum. No patient had a background of other chronic liver disease. The study protocol was approved by the clinical research ethics committee of the hospital. Written informed consent was obtained from all patients according to the policies of the committee.
Table 1.
The baselines of clinical features at the time of diagnosis of hepatocellular carcinoma
| Variable | mean/n |
| Age (yr) | 50.16 ± 10.65 (range: 27-76) |
| Gender (male/female) | 134:28 |
| HBsAg-positive | 156 (96.30%) |
| HCV-Ab positive | 0 |
| HBV-DNA | |
| > 104/L | 88 (140, 62.85%) |
| > 106/L | 32 (140, 22.86%) |
| Child-Pugh score | |
| 5 | 148 (97.53%) |
| 6 | 10 (2.47%) |
| TBIL (μmol/L) | 15.76 ± 5.72 (range: 4.9-33) |
| DBIL (μmol/L) | 5.51 ± 2.26 (range: 1.5-15.5) |
| IBIL (μmol/L) | 10.28 ± 4.09 (range: 1-23) |
| ALB (g/L) | 41.32 ± 3.59 (range: 32-53) |
| Prealbumin (mg/L) | 217.73 ± 65.83(range:110-500) |
| PT > 13 s (n) | 41(25.31%) (range: 10.4-17.6) |
| ALT > 44U/L (n) | 48 (29.63%) (range: 12.9-235) |
| AST > 38 U/L (n) | 88 (54.32%) (range: 12.8-207) |
| AFP > 20 μg/L | 102 (62.96%) (range: 2.5-4537) |
| AFP > 400 μg/L | 59 (36.42%) |
| Diabetes | 14 (8.64%) |
| Cardiovascular hypertension | 16 (9.88%) |
| History of smoke | 50 (30.86%) |
| History of alcoholism | 24 (14.81%) |
| History of other operations | 20 (12.35%) |
| BCLC Staging | |
| Stage A | 44 (27.16%) |
| Stage B | 118 (72.84%) |
| With Milan criteria | 44 (27.16%) |
| With UCSF criteria | 86 (53.09%) |
| TNM staging (6th, 2002 ) | |
| T2 | 103 (63.58%) |
| T3 | 59 (36.42%) |
| Total tumor size (cm) | 7.58 ± 4.66 (range: 1.4-23) |
| The largest tumor size (cm) | 5.37 ± 4.00 (range: 0.7-21) |
| Number of tumors | |
| 2 | 110 |
| 3 | 36 |
| 4 | 10 |
| > 4 | 6 |
| Serum creatinine (mmol/L) | 68.65 ± 14.67 (range: 36-119) |
| Serum urea nitrogen (μmol/L) | 5.86 ± 5.92 (range: 2.6-57) |
| WBC (× 109/L) | 5.23 ± 1.69 (range: 1.66-11.6) |
| RBC (× 1012/L) | 4.53 ± 0.52 (range: 3.3-6.59) |
| HGB (g/L) | 142.8 ± 21.32 (range: 94-146) |
| PLT (× 109/L) | 148.3 ± 75.93 (range: 31-476) |
| PLT < 100 × 109/L (n) | 44 (27.16%) |
| Intraoperative transfusion | 14 (8.64%) |
| Postoperative transfusion | 6 (3.70%) |
| Death in 3 mo after operation | 4 (2.47%) |
HBsAg: Hepatitis B surface antigen; HBV: Hepatitis B virus; HCV: Hepatitis C virus; AFP: α fetoprotein; TBIL: Total bilirubin; DBIL: Direct bilirubin; IBIL: Indirect bilirubin; ALB: Albumin; PT: Prothrombin time; ALT: Alanine transaminase; AST: Aspartate aminotransferase; BCLC: Barcelona Clinic Liver Cancer; UCSF: University of California, San Francisco; WBC: White blood cell; RBC: Red blood cell; PLT: Platelet; HGB: Hematoglobulin; TNM: Tumor, nodes, metastasis.
Diagnosis of hepatocellular carcinoma and preoperative assessment
The preoperative diagnosis of multinodular HCC was based on the findings of focal lesions with signs of early hyperenhancement and delayed hypoenhancement on 2 different imaging modalities such as enhanced spiral computed tomography (CT) and magnetic resonance imaging (MRI), or on the combination of imaging findings and α fetoprotein (AFP) level[5,13,19]. Tumor size was defined as the maximal diameter. Routine biopsy of the lesions was not performed before resection if the lesion had typical characteristics of HCC. No patient enrolled in this study received a fine-needle biopsy. The diagnoses of all patients were confirmed definitively by pathological examination after resection. Histological tumor differentiation was determined using the Edmonson-Steiner grading.
Each patient underwent a complete blood count. Liver impairment was evaluated in all patients by liver biochemistry, including serum total bilirubin (TBIL), direct bilirubin, indirect bilirubin, albumin (ALB), globulin, prealbumin, alanine transaminase, aspartate aminotransferase (AST), alkaline phosphatase (ALP) and γ-glutamyl transpeptidase (GGT). Prothrombin time (PT) and activated partial thromboplastin time were also determined to evaluate liver function and surgical safety. Tumor markers, including AFP, carcinoembryonic antigen, carbohydrate antigen 19-9 and α fucosidase (AFU), were used to identify the tumor origin. The parameters of HBV infection were tested. We evaluated kidney function by measuring serum urea nitrogen, creatinine, uric acid and electrolytes. All patients received an upper gastrointestinal endoscopic examination for portal hypertension and hemorrhage. Patients also underwent thoracic X-ray to examine metastasis to the lungs. Abdominal ultrasonography and enhanced CT or MRI were used to evaluate the size and location of the tumors. For patients older than 60 years or who had a relevant disease history, pulmonary function tests and cardiovascular Doppler ultrasound were performed to determine any contraindications to resection.
Hepatic resection
Hepatic resection was considered the first-line therapy for patients with Child-Pugh A liver function. The indications for hepatic resection included the technically feasibility of resection, the absence of macroscopic cancerous thrombi in vessels, absence of distant metastasis and sufficient future remnant liver volume in the preoperative evaluation. Patients with resectable multinodular HCC received resection immediately without any preoperative anti-HCC therapy. We defined curative hepatic resection as complete removal of all the detectable tumors combined with tumor-free margins confirmed by histopathology[4,8,13]. In China, most HCC patients had underlying liver disease such as hepatitis, fibrosis and cirrhosis, which result in limited capacity for regeneration. Surgery must balance resectability with preservation of hepatic function to reduce the risk of hepatic failure[20]. For multinodular HCC, concomitant resection may result in more blood loss, a longer Pringle time, and a significant change in blood perfusion and drainage. The limits for safe resection of multinodular HCC should be smaller than that of a single tumor. Therefore, all patients received local nonanatomic resection. The surgical procedure has been reported previously[21]. The tumors of 44 patients were resected en bloc (27.16%), and 118 patients underwent multinodular hepatic resections (72.84%). Intraoperative ultrasonography was always used to detect non-visible, nonpalpable nodules and to check the resection plane. Resection margins were examined by a microscopic histological test.
Follow-up
All patients were regularly followed up for recurrence by determination of AFP, liver enzymes, complete blood count, and CT or MRI scan monthly for the first 3 mo after resection. If there was no recurrence, the frequency of routine examination was changed to once every 3 mo. Tumor recurrence was identified by new lesions on imaging with appearances typical of HCC, a rising AFP level, or rapid enlargement of lesions without typical HCC characteristics. If tumor recurrence was diagnosed, patients received a second hepatectomy, chemoembolization and locoregional ablation, such as RFA or percutaneous ethanol rejection.
Statistical analysis
Patient demographics, tumor parameters, liver function and hepatitis-associated characteristics were evaluated. Continuous data are expressed as mean ± SD. Categorical data were compared using the χ2 test and Fisher’s exact test as appropriate. Survival analyses were performed using the Kaplan-Meier method and compared using the log-rank test. For continuous data with statistical significance in the univariate analysis, a series of receiver operating characteristics (ROC) curves were used to identify the cutoff values with optimal discriminatory ability for 1-year survival. Multivariate analysis was performed using the Cox proportional hazard ratio model to identify independent prognostic factors. The factors with a P-value less than 0.1 in univariate analysis were included in the multivariate Cox regression analysis. In order to estimate the clinical value of the independent factors, a prognostic scoring system was constructed. We assigned points to each independent predictor according to the value of the partial regression coefficient, because each factor had different importance in the final Cox model[22]. Each patient’s score was the total points of 6 factors. A ROC curve was constructed to estimate the prognostic ability of the new scoring model. Overall survival was the time from the day of surgery to the day of death or to the most recent follow-up visit. Disease-free survival was the time from the day of surgery to the most recent follow-up visit at which evidence of a tumor was clear. The deaths caused by non-HCC-associated factors were included in the overall survival analysis, but not in the disease-free survival analysis. A P value < 0.05 was considered statistically significant. All statistical processing was performed by SPSS 18.0 (SPSS Inc., Chicago, IL, United States).
RESULTS
Survival, outcome and morbidity after liver resection
The mean postoperative hospitalization period was 10.6 d (range: 5-28 d). The overall morbidity was 25.31% (n = 41). Pleural effusion (n = 24) and ascites (n = 14), which required diuretics or paracentesis, were the most common sequelae, with both occurring in 7 patients. Two patients developed bile leakage and one developed transitory arrhythmia. The median overall survival was 38.3 mo (range: 3-80 mo); overall survival rates at 1 year, 3 years and 5 years were 86%, 51% and 35%, respectively; the median disease-free survival was 18.6 mo (range: 1-79 mo); 1-year, 3-year and 5-year disease-free survival rates were 56%, 40% and 31%, respectively. The survival outcome was similar to that in 2 previous retrospective studies[4,8]. During the entire follow-up, a total of 100 (61.73%) patients were diagnosed with tumor recurrence during the follow-up period. Among them, twelve patients underwent a second hepatic resection, 65 received transarterial chemoembolization (TACE) alone, 18 received TACE combined with locoregional ablation and 5 received locoregional ablation alone. A total of 32 patients died within the first year after resection. Four patients died at the 3rd month, one died of acute severe hepatitis and 3 of unrecovered liver impairment after chemoembolization for recurrence. Of the remaining 28 patients, one died of incidental hemorrhage of the upper digestive tract at the 7th month after resection, 27 died of liver failure, including 4 patients within 4-6 mo, 2 within 7-9 mo, and 9 within 10-12 mo. All 27 patients had diffuse tumor recurrence leading to liver failure.
Predictive factors for 1-year survival
In the evaluation of preoperative factors that may have potential prognostic ability for short-term outcome, we limited the endpoint of follow-up to 1 year. The univariate Kaplan-Meier analysis showed that TBIL, prealbumin, AST, AFP, HBeAg, HBV-DNA, GGT, AFU, total tumor size, largest tumor size, ALP, and platelet count were significantly associated with 1-year mortality after hepatic resection (Table 2).
Table 2.
Univariate Cox analysis of potential prognostic factors
| Variables | n | The 1-year survival rates % | P value |
| Age | < 0.001 | ||
| > 40 yr | 128 | 85.9 | |
| ≤ 40 yr | 34 | 64.7 | |
| Parameters of liver function | |||
| TBIL | < 0.001 | ||
| > 17.1 μmol/L | 49 | 58.33 | |
| ≤ 17.1 μmol/L | 113 | 91.22 | |
| ALB | 0.530 | ||
| < 35 g/L | 8 | 75.00 | |
| ≥ 35 g/L | 154 | 81.82 | |
| GLB | 0.565 | ||
| > 30 g/L | 79 | 79.48 | |
| ≤ 30 g/L | 83 | 83.33 | |
| Prealbumin | < 0.001 | ||
| < 170 mg/L | 33 | 58.3 | |
| ≥170 mg/L | 129 | 90.6 | |
| ALT | 0.281 | ||
| > 38 U/L | 82 | 78.04 | |
| ≤ 38 U/L | 80 | 85.00 | |
| ALT | 0.993 | ||
| > 76 U/L | 10 | 80.00 | |
| ≤ 76 U/L | 152 | 81.58 | |
| AST | 0.001 | ||
| > 38 U/L | 88 | 75.00 | |
| ≤ 38 U/L | 74 | 89.21 | |
| AFU | 0.004 | ||
| > 40 U/L | 31 | 64.31 | |
| ≤ 40 U/L | 131 | 85.89 | |
| ALP | 0.001 | ||
| > 129 U/L | 27 | 58.33 | |
| ≤ 129 U/L | 135 | 85.50 | |
| GGT | < 0.001 | ||
| > 64 U/L | 82 | 65.00 | |
| ≤ 64 U/L | 80 | 97.56 | |
| PT | 0.428 | ||
| > 13 s | 41 | 75.00 | |
| ≤ 13 s | 121 | 82.00 | |
| APTT | 0.566 | ||
| > 37 s | 72 | 77.80 | |
| ≤ 37 s | 90 | 82.20 | |
| Parameters of blood test | |||
| WBC | 0.389 | ||
| < 4 × 109/L | 35 | 76.5 | |
| ≥ 4 × 109/L | 127 | 82.5 | |
| PLT | < 0.001 | ||
| < 100 × 109/L | 44 | 59.1 | |
| ≥100 × 109/L | 118 | 89.7 | |
| Parameters of tumors | |||
| Total tumor size | 0.010 | ||
| > 5 cm | 108 | 75.9 | |
| ≤ 5 cm | 54 | 92.6 | |
| Total tumor size | < 0.001 | ||
| > 8 cm | 54 | 66.7 | |
| ≤ 8 cm | 108 | 88.9 | |
| Largest tumor size | 0.050 | ||
| > 5 cm | 58 | 72.41 | |
| ≤ 5 cm | 104 | 84.61 | |
| Largest tumor size | 0.001 | ||
| > 8 cm | 22 | 54.54 | |
| ≤ 8 cm | 140 | 84.2 | |
| AFP | < 0.001 | ||
| > 20 μg/L | 102 | 72.00 | |
| ≤ 20 μg/L | 60 | 96.78 | |
| AFP | 0.014 | ||
| > 400 μg/L | 59 | 72.41 | |
| ≤ 400 μg/L | 103 | 86.54 | |
| CA19-9 | 0.287 | ||
| > 39 U/L | 35 | 72.22 | |
| ≤ 39 U/L | 127 | 83.87 | |
| Location in same segment | |||
| Yes | 20 | 80.00 | 0.924 |
| No | 142 | 81.96 | |
| Location in same lobe | |||
| Yes | 34 | 88.2 | 0.238 |
| No | 128 | 79.7 | |
| Location in same hemiliver | |||
| Yes | 74 | 83.8 | 0.530 |
| No | 88 | 79.5 | |
| Number of tumors | 0.529 | ||
| ≤ 3 | 146 | 80.82 | |
| > 3 | 16 | 87.50 | |
| Parameters of hepatitis B | |||
| HBsAg | 0.517 | ||
| Positive | 156 | 80.81 | |
| Negative | 6 | 100.00 | |
| HBsAb | 0.803 | ||
| Positive | 8 | 100.0 | |
| Negative | 154 | 80.52 | |
| HBeAg | |||
| Positive | 64 | 71.88 | 0.005 |
| Negative | 98 | 87.76 | |
| HBeAb | |||
| Positive | 101 | 84.31 | 0.041 |
| Negative | 61 | 74.19 | |
| HBV-DNA1 | 0.228 | ||
| > 104/L | 88 | 79.54 | |
| ≤ 104/L | 50 | 88.00 | |
| HBV-DNA1 | 0.019 | ||
| > 106/L | 32 | 68.75 | |
| ≤ 106/L | 106 | 86.79 | |
There were only 138 patients received HBV-DNA examinations. GLB: Globulin; ALP: Alkaline phosphatase; AFU: α fucosidase; GGT: γ-glutamyl transpeptidase; PLT: Platelet; HBV: Hepatitis B virus; HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B e antigen; HBsAb: Hepatitis B surface antibody; HBeAb: Hepatitis B e antibody; CA19-9: Carbohydrate antigen 19-9; PT: Prothrombin time; APTT: Activated partial thromboplastin time; TBIL: Total bilirubin; ALB: Albumin; ALT: Alanine transaminase; AST: Aspartate aminotransferase; WBC: White blood cell.
For the above factors, we analyzed continuous data by ROC curves to determine cutoff values that had the optimal sensitivity and specificity (i.e., the largest sum of sensitivity and specificity). The cutoff values of platelet count, AST, TBIL, AFU, AFP, GGT and ALP (92.5 × 109/L, 41.8 U/L, 17.25 μg/L, 36 U/L, 32.6 μg/L, 75.5 U/L, 119.5 U/L, respectively) were similar to the limits of normal values, thus we defined the normal upper or low limitations as the best cutoff points (Table 3).
Table 3.
Area under the receiver operating characteristics curves and cut-off points
| Test variable(s) | Area | SE | Asym-ptotic Sig | Lower bound | Upper bound | Cut-off point 11 | Cut-off point 22 |
| TBIL | 0.694 | 0.054 | 0.001 | 0.588 | 0.801 | 17.25 | 17.1 |
| Prealbumin | 0.817 | 0.043 | < 0.001 | 0.099 | 0.266 | 170 | 170 |
| AST | 0.707 | 0.048 | 0.001 | 0.613 | 0.802 | 41.8 | 38 |
| ALP | 0.695 | 0.056 | 0.001 | 0.586 | 0.804 | 119.5 | 129 |
| GGT | 0.792 | 0.040 | < 0.001 | 0.714 | 0.870 | 75.5 | 64 |
| AFU | 0.615 | 0.064 | 0.058 | 0.489 | 0.740 | 36 | 40 |
| Total tumor size | 0.693 | 0.057 | 0.001 | 0.581 | 0.805 | 8.1 | 8 |
| AFP | 0.626 | 0.050 | 0.037 | 0.528 | 0.725 | 32.6 | 20 |
| PLT | 0.721 | 0.058 | < 0.001 | 0.166 | 0.393 | 92.5 | 100 |
| Largest tumor size | 0.597 | 0.064 | 0.089 | 0.472 | 0.722 | 5.9 | 6 |
The actual results defined by the ROC curves;
The values adopted for multivariate analysis. AFP: α fetoprotein; TBIL: Total bilirubin; AST: Aspartate aminotransferase; PLT: Platelet; ALP: Alkaline phosphatase; AFU: α fucosidase; GGT: γ-glutamyl transpeptidase; Sig: Significance.
Multivariate analysis showed that 6 variables were significant predictive factors for 1-year survival status after resection: prealbumin < 170 mg/L [hazard ratio (HR): 5.531, P < 0.001]; ALP > 129 U/L (HR: 3.252, P = 0.005); AFP > 20 μg/L (HR: 7.477, P = 0.011); total tumor size > 8 cm (HR: 10.543, P < 0.001); platelet count < 100 × 109/L (HR: 9.937, P < 0.001), and GGT > 64 U/L (HR: 3.791, P < 0.001) (Table 4). Although factors of tumor invasiveness cannot be detected before surgery, the data showed that microvascular invasion and poor tumor differentiation were associated with tumor size (Figure 1).
Table 4.
Results of multivariate Cox analysis
| Variables | β | SE | Sig | HR | 95% CI for HR | Assigned points |
| Prealbumin < 170 mg/L | 1.711 | 0.420 | < 0.001 | 5.531 | 3.122-13.196 | 1 |
| ALP > 129 U/L | 1.132 | 0.420 | 0.005 | 3.252 | 1.413-7.480 | 1 |
| AFP > 20 μg/L | 2.014 | 0.792 | 0.011 | 7.477 | 1.419-31.234 | 2 |
| Total tumor size > 8 cm | 2.334 | 0.542 | < 0.001 | 10.543 | 3.611-30.157 | 2 |
| PLT < 100 × 109/L | 2.310 | 0.484 | < 0.001 | 9.937 | 3.770-26.121 | 2 |
| GGT > 64 U/L | 1.291 | 0.460 | < 0.001 | 3.791 | 1.476-9.960 | 1 |
β: Partial regression coefficient; SE: Standard error; Sig: Significance; HR: Hazard ratio; CI: Confidence interval; AFP: α fetoprotein; PLT: Platelet; ALP: Alkaline phosphatase; GGT: γ-glutamyl transpeptidase.
Figure 1.
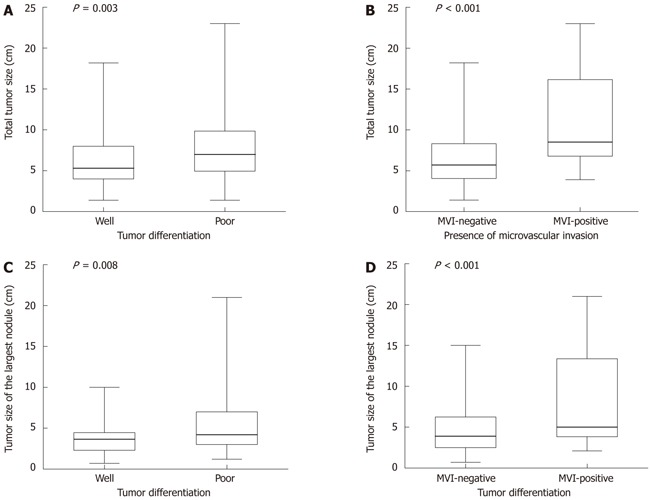
Distribution of tumor size according to tumor differentiation and presence of microvascular invasion, showing median, 25th-75th percentile box and complete range of measurements. A: Distribution of total tumor size according to tumor differentiation (P = 0.003); B: Distribution of total tumor size according to presence of microvascular invasion (P < 0.001); C: Distribution of the largest tumor size according to tumor differentiation (P = 0.008); D: Distribution of total tumor size according to tumor differentiation and presence of microvascular invasion (P < 0.001). The tumor size was associated with presence of microvascular invasion and poor tumor differentiation. MVI: Microvascular invasion.
Construction of a scoring system to determine indication for surgery
We assigned points for each prognostic factor (Table 4). The theoretical range of the prognostic score was 0 to 9. In the total population in our study, 10 patients scored 0, 16 patients scored 1, 44 patients scored 2, 32 patients scored 3, 18 patients scored 4, 22 patients scored 5, 10 patients scored 6, 6 patients scored 7, and 4 patients scored 8. A higher score implied a lower chance of 1-year survival. We assessed the prognostic value of the score on 1-year survival using a ROC curve (Figure 2). The area under the curve was 0.925 [95% confidence interval (CI): 0.864-0.985, P < 0.001]. The data indicated that, for patients with multinodular HCC, the scoring system had a satisfactory ability to predict 1-year mortality after hepatic resection. A score of 5 was the cut-off value with optimal specificity and sensitivity. For patients with a score of 5 or more, the 1-year mortality was 62% which was similar to that of patients who received nonsurgical treatment[2,15-17], while patients with a score of 0-4 had 1-year mortality of only 5%.
Figure 2.
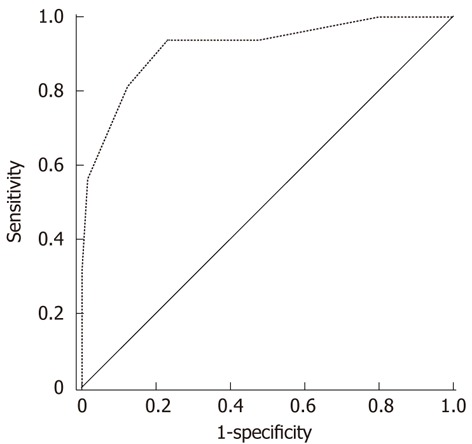
Receiver operating characteristics curve of the predictive scoring system. The area under the curve was 0.925, 95% confidence interval was 0.864-0.985 (P < 0.001). The data indicated that the scoring system had a strong ability to predict the 1-year outcome of patients with multiple hepatocellular carcinoma after hepatic resection.
We also compared the long-term survival of patients with a score 0-4 (n = 120, group 1) and those with a score of 5-8 (n = 42, group 2). In group 1, the median survival was 53.55 mo (range: 6-80), the median disease-free survival was 37.14 mo (range: 1-79). In group 2, the median survival was 12.86 mo (range: 3-51), the median disease-free survival was 7.00 mo (range: 1-45). The 1-year and 3-year survival rates of group 1 were 95% and 60%, respectively, while the 1-year and 3-year survival rates of group 2 were 38% and 8%, respectively. The 1-year and 3-year disease-free survival rates of group 1 were 67% and 35%, respectively, while the 1-year and 3-year disease-free survival rates of group 2 were 14% and 12%, respectively. Patients in group 2 had much poorer long-term overall and disease-free survival (P < 0.001 for both, Figure 3). Table 5 shows the comparison of pathological status between group 1 and group 2. Patients in group 2 had a greater possibility of microvascular invasion (P < 0.001) and poor tumor differentiation (P = 0.003). Liver cirrhosis with small nodules was more likely to be detected in group 2 (P < 0.001). In addition, group 2 patients were more likely to receive intraoperative blood transfusion (P = 0.010).
Figure 3.
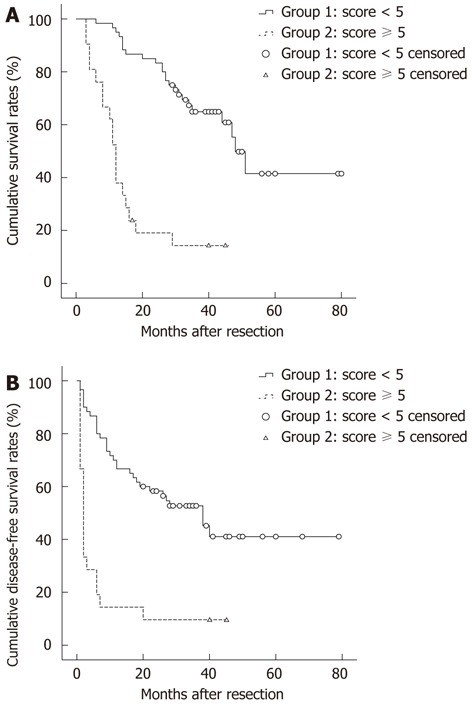
Results of long-term survival of group 1 (score < 5) and group 2 (score ≥ 5). The overall survival and disease-free survival in group 2 were significant poorer than those in group 1 (both P < 0.001).
Table 5.
The comparison of surgical data and tumor biological characteristics between two groups n (%)
| Variables | Group 1 (n = 120) | Group 2 (n = 42) | P value |
| Blood transfusion | 6 (5.00) | 8 (19.05) | 0.010 |
| Pringle time > 20 min | 52 (43.33) | 24 (57.14) | 0.151 |
| Liver cirrhosis | 68 (56.67) | 24 (57.14) | 0.854 |
| Type of liver cirrhosis | < 0.001 | ||
| Small nodules | 0 (0) | 6 (25.00) | |
| Large nodules | 52 (76.47) | 8 (33.33) | |
| Mixed nodules | 16 (23.53) | 10 (41.65) | |
| Differentiation of tumor | 0.003 | ||
| High differentiation (I-II) | 30 (25.00) | 2 (4.76) | |
| Low differentiation (III-IV) | 90 (75.00) | 40 (95.23) | |
| Microvascular invasion | 16 (13.33) | 18 (42.89) | < 0.001 |
| Completed capsule of largest tumor | 0.468 | ||
| Yes | 72 (60.00) | 22 (52.38) | |
| No | 48 (40.00) | 20 (47.62) |
DISCUSSION
This study evaluated the short-term prognosis of patients with multinodular HCC after curative resection and developed a scoring system to determine contraindication to surgery. In patients with well-preserved liver function, hepatic resection remains the optimal and effective treatment. Although liver transplantation provides an alternative curative treatment option for small HCC, organ shortages and long waiting times have prohibited it as initial treatment[4,5]. Several studies have proved that the benefit of resection was similar to that of transplantation. However, patients who received transplantation had undergone a natural selection process in which patients with more aggressive tumor phenotypes, such as the presence of microvascular invasion and microscopic metastasis, had been rules out because of tumor progression[23]. The efficacy of RFA is limited by several factors, such as subcapsular location, tumor size and tumor differentiation, which have been proved to be associated with increased risk of bleeding, peritoneal seeding and residual vital tumor tissue[5,6,24]. Several studies have demonstrated that patients who underwent resection had better survival than those who received RFA[25,26]. Chemoembolization, as a palliative treatment, results in a high incidence of residual viable tumor tissue, even after repeated treatment[27,28]. Its poor performance on blood-deficient and larger tumors restricts its application in HCC treatment. Previous studies have demonstrated that resection brought more benefit to patients of intermediate stage than TACE[4,10]. As surgical techniques have advanced, many studies have explored the possibility of broadening the surgical criteria for HCC. Ng et al[4] demonstrated the safety and effectiveness of hepatic resection for multinodular HCC, although the survival rate was lower than for a single tumor. Ishizawa et al[8] concluded that resection can provide a survival benefit for patients with multinodular HCC on the background of Child-Pugh A cirrhosis, as well as for those with portal hypertension. Torzilli et al[9] confirmed that patients with BCLC stage B and stage C HCC can tolerate hepatic resection with low mortality, acceptable morbidity, and a survival benefit, if resection is performed under strict intraoperative ultrasonographic guidance.
However, the benefit of hepatic resection in the treatment of multinodular HCC remains controversial. In spite of the exciting conclusions above, we can also observe in clinical practice that there are patients who have satisfactory liver function and seemingly optimistic prognosis empirically, but have poor short-term outcome after resection. This indicates that the classical evaluation systems have their limitations, and that they cannot detect mild liver impairment, which would markedly influence outcome. In our study, no patient died during the hospital stay, and only 4 (2.47%) died in the 3 mo after resection, indicating that the preoperative safety assessment according to the established guidelines was acceptable. However, 32 of the patients died in the first year. For these patients, surgical resection brought no benefit and may even have adversely affected survival. Therefore, identification of these patients may determine that surgical treatment should be contraindicated.
Multivariate analysis showed that 6 variables were independent predictive factors for poor short-term survival. Among them, prealbumin < 170 mg/L, ALP > 129 U/L, GGT >64 U/L and platelet count < 100 × 109/L were factors associated with liver impairment. Their roles as indicators of liver damage have been reported previously[29-32]. Platelet count may also be used as a potential marker of portal hypertension[33]. Conventional blood parameters of liver function, such as TBIL and PT, as well as scoring systems using these values (such as the Child-Pugh score and Model for End-stage Liver Disease score) have been widely accepted in clinical circles. Many staging systems also adopt them in preoperative estimation of surgical safety. However, decreased ALB and prolonged PT indicate marked liver damage[29], while ascites and encephalopathy indicate liver failure and serious portal hypertension. Previous studies reported that most patients who underwent elective hepatic resection were classified as Child-Pugh class A, but postoperative liver failure and mortality existed even in this group of patients[17,18]. This phenomenon indicated that most hepatic impairment in surgical candidates is slight and might not be fully evaluated by classical estimation systems. Our results disclosed that platelet count, ALP, prealbumin and GGT may be considered as supplemental factors for routine liver function scoring systems. Patients who have satisfactory liver function reserve according to the traditional estimation system, but abnormalities in these additional parameters above should be considered with caution for surgery.
Factors associated with tumor burden also have a crucial influence on the short-term survival of patients. The majority of our patients who died in the first year had tumor recurrence and metastasis. Early tumor recurrence and fast hyperplasia adversely affected liver function recovery. Our results included AFP > 20 μg/L and total tumor size > 8 cm as independent risk factors of first year death. AFP > 200 μg/L is the diagnostic level indicating HCC, but only one-third of patients with HCC have AFP levels higher than 100 μg/L, and even mild elevation predicts a worse prognosis[14]. Hence, the 20 μg/L as cutoff point in the ROC curve was reasonable. The AFP level and total tumor size are widely accepted risk factors affecting surgical outcome[34]. Several studies have reported that tumor size is related to the presence of microvascular invasion and poor tumor differentiation[35,36], which are strongly associated with intrahepatic metastasis and greatly increase the risk of tumor recurrence[5,35,36]. Our analysis observed a similar phenomenon. These results implied that clinicians could estimate tumor invasiveness by preoperative examination. Other tumor-associated factors were not included in our results as our study focused on short-term (1-year) survival rather than the long-term outcome.
To apply these risk factors in clinical practice, we constructed a scoring model. A total of 42 patients with high score (≥ 5, group 2) had 1-year outcome similar to that of patients who received non-surgical treatment, and also had significantly poorer long-term overall and disease-free survival. Our analysis also showed that these patients had a greater possibility of microvascular invasion and poor tumor differentiation. They also had a higher percentage of liver cirrhosis with small nodularity, which has been proved to be an independent predictor of clinically significant portal hypertension[37]. Intraoperative blood transfusion, which significantly influenced short-term survival[22], was more common in patients with a score ≥ 5, indicating greater surgical difficulty. Although we resected all visible and palpable nodules and a tumor-free margin under guidance of intraoperative ultrasonography, the 3-mo recurrence rate in group 2 patients was 71.4%, indicating the greater possibility of preoperative existence of nondetectable micrometastasis. For these patients, seemingly curative resection does not achieve radical efficacy. This series of data confirmed that this group of patients may derive little benefit from resection and surgery should be considered a contraindication in spite of satisfactory liver function estimated by classical scoring systems.
Major limitation of our study is that this study was a retrospective analysis with a small sample size in a single center. The potential selective bias that accompanied with this setup was hard to avoid. Patients with hepatitis C virus infection or other chronic liver disease were not included in the analysis. Other limitations of this study were that different types of recurrence were not taken into consideration because of the small sample size. Therefore, further study is needed before a final conclusion is made.
In summary, hepatic resection has been proved to be a safe and effective treatment for some patients with multinodular HCC. However, some patients with good liver function as estimated by traditional scoring systems have poor short-term outcome. Our study focused on these patients and indicated that other factors, namely prealbumin < 170 mg/L, ALP > 129 U/L, GGT > 64 U/L, platelet count < 100 × 109/L, AFP > 20 μg/L and total tumor size > 8 cm are independent risk factors for short-term mortality. For patients with these characteristics, 1-year mortality was significantly increased, and resection was associated with an adverse outcome.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is the third leading cause of cancer mortality worldwide. Multiple lesions are detected in about 40% patients once HCC is diagnosed. The treatment strategies for multiple HCC remain in controversy.
Research frontiers
Several recent studies reported that surgical resection improves the survival of patients with multiple HCC. However, the indication and contraindication of surgical resection for multiple HCC remain unclear.
Innovations and breakthroughs
Patients with poor short-term (1 year) survival after surgery benefit little from hepatic resection. They should be considered surgical contraindication. The risk factors of poor short-term survival for patients with satisfactory liver function remain unclear. The authors analyzed the preoperative data of 162 multiple HCC patients received surgical resection and found that factors, namely prealbumin < 170 mg/L, alkaline phosphatase (ALP) > 129 U/L, γ-glutamyl transpeptidase (GGT) > 64 U/L, platelet count < 100 × 109/L, α fetoprotein (AFP) > 20 μg/L, total tumor size > 8 cm are independent risk factors of short-term mortality. The authors then assigned points to each factor according to its partial regression coefficient and construct a score system. For the patients with score ≥ 5, 1-year mortality was 62% while only 5% for patients with score < 5.
Applications
The study results suggest that more factors namely ALP, GGT, platelet, prealbumin, AFP and total tumor size should be enrolled in preoperative estimation. Patients with score ≥ 5 should be considered with more cautious attitude towards resection.
Terminology
HCC is the most common primary malignant tumor of the liver which originated from liver parenchymal cells. Multiple HCC refers to the HCC with more than one lesion.
Peer review
The authors analyzed the clinical data of patients with multiple HCCs treated by surgery for detecting prognostic factors of 1 year survival. They determined six prognostic factors from preoperative clinical data. Based on the multivariate analysis, they proposed a prognostic scoring system. This scoring system well predicted poor short outcome after hepatic resection. The study was carefully designed and the evaluation of the results was also appropriate. The manuscript was well organized and well written.
Footnotes
Peer reviewers: Dr. Silvio Nadalin, General Surgery and Transplantation, University Hospital Tuebingen, 72076 Tuebingen, Germany; Dr. Kaye M Reid Lombardo, General Surgery, Mayo Clinic, 200 First St. SW, Rochester, MI 55905, United States; Dr. Masayuki Ohta, Department of Surgery I, Oita University Faculty of Medicine, 1-1 Idaigaoka, Hasama-machi, Oita 879-5593, Japan; Kenji Miki, MD, Department of Surgery, Showa General Hospital, 2-450 Tenjin-cho, Tokyo 187-8510, Japan
S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002;122:1609–1619. doi: 10.1053/gast.2002.33411. [DOI] [PubMed] [Google Scholar]
- 4.Ng KK, Vauthey JN, Pawlik TM, Lauwers GY, Regimbeau JM, Belghiti J, Ikai I, Yamaoka Y, Curley SA, Nagorney DM, et al. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann Surg Oncol. 2005;12:364–373. doi: 10.1245/ASO.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 6.Jarnagin WR. Management of small hepatocellular carcinoma: a review of transplantation, resection, and ablation. Ann Surg Oncol. 2010;17:1226–1233. doi: 10.1245/s10434-010-0978-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406; discussion 406-407. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908–1916. doi: 10.1053/j.gastro.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 9.Torzilli G, Donadon M, Marconi M, Palmisano A, Del Fabbro D, Spinelli A, Botea F, Montorsi M. Hepatectomy for stage B and stage C hepatocellular carcinoma in the Barcelona Clinic Liver Cancer classification: results of a prospective analysis. Arch Surg. 2008;143:1082–1090. doi: 10.1001/archsurg.143.11.1082. [DOI] [PubMed] [Google Scholar]
- 10.Lin CT, Hsu KF, Chen TW, Yu JC, Chan DC, Yu CY, Hsieh TY, Fan HL, Kuo SM, Chung KP, et al. Comparing hepatic resection and transarterial chemoembolization for Barcelona Clinic Liver Cancer (BCLC) stage B hepatocellular carcinoma: change for treatment of choice? World J Surg. 2010;34:2155–2161. doi: 10.1007/s00268-010-0598-x. [DOI] [PubMed] [Google Scholar]
- 11.Koniaris LG, Levi DM, Pedroso FE, Franceschi D, Tzakis AG, Santamaria-Barria JA, Tang J, Anderson M, Misra S, Solomon NL, et al. Is surgical resection superior to transplantation in the treatment of hepatocellular carcinoma? Ann Surg. 2011;254:527–537; discussion 537-538. doi: 10.1097/SLA.0b013e31822ca66f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho CM, Lee PH, Chen CL, Ho MC, Wu YM, Hu RH. Long-term outcomes after resection versus transplantation for hepatocellular carcinoma within UCSF criteria. Ann Surg Oncol. 2012;19:826–833. doi: 10.1245/s10434-011-1975-x. [DOI] [PubMed] [Google Scholar]
- 13.Vivarelli M, Guglielmi A, Ruzzenente A, Cucchetti A, Bellusci R, Cordiano C, Cavallari A. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240:102–107. doi: 10.1097/01.sla.0000129672.51886.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grieco A, Pompili M, Caminiti G, Miele L, Covino M, Alfei B, Rapaccini GL, Gasbarrini G. Prognostic factors for survival in patients with early-intermediate hepatocellular carcinoma undergoing non-surgical therapy: comparison of Okuda, CLIP, and BCLC staging systems in a single Italian centre. Gut. 2005;54:411–418. doi: 10.1136/gut.2004.048124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koom WS, Seong J, Han KH, Lee do Y, Lee JT. Is local radiotherapy still valuable for patients with multiple intrahepatic hepatocellular carcinomas? Int J Radiat Oncol Biol Phys. 2010;77:1433–1440. doi: 10.1016/j.ijrobp.2009.07.1676. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder RA, Marroquin CE, Bute BP, Khuri S, Henderson WG, Kuo PC. Predictive indices of morbidity and mortality after liver resection. Ann Surg. 2006;243:373–379. doi: 10.1097/01.sla.0000201483.95911.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seyama Y, Kokudo N. Assessment of liver function for safe hepatic resection. Hepatol Res. 2009;39:107–116. doi: 10.1111/j.1872-034X.2008.00441.x. [DOI] [PubMed] [Google Scholar]
- 19.Torzilli G, Minagawa M, Takayama T, Inoue K, Hui AM, Kubota K, Ohtomo K, Makuuchi M. Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology. 1999;30:889–893. doi: 10.1002/hep.510300411. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka K, Shimada H, Matsumoto C, Matsuo K, Nagano Y, Endo I, Togo S. Anatomic versus limited nonanatomic resection for solitary hepatocellular carcinoma. Surgery. 2008;143:607–615. doi: 10.1016/j.surg.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Yang T, Zhang J, Lu JH, Yang GS, Wu MC, Yu WF. Risk factors influencing postoperative outcomes of major hepatic resection of hepatocellular carcinoma for patients with underlying liver diseases. World J Surg. 2011;35:2073–2082. doi: 10.1007/s00268-011-1161-0. [DOI] [PubMed] [Google Scholar]
- 22.Yau T, Yao TJ, Chan P, Ng K, Fan ST, Poon RT. A new prognostic score system in patients with advanced hepatocellular carcinoma not amendable to locoregional therapy: implication for patient selection in systemic therapy trials. Cancer. 2008;113:2742–2751. doi: 10.1002/cncr.23878. [DOI] [PubMed] [Google Scholar]
- 23.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Difference in tumor invasiveness in cirrhotic patients with hepatocellular carcinoma fulfilling the Milan criteria treated by resection and transplantation: impact on long-term survival. Ann Surg. 2007;245:51–58. doi: 10.1097/01.sla.0000225255.01668.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 26.Poon RT, Ngan H, Lo CM, Liu CL, Fan ST, Wong J. Transarterial chemoembolization for inoperable hepatocellular carcinoma and postresection intrahepatic recurrence. J Surg Oncol. 2000;73:109–114. doi: 10.1002/(sici)1096-9098(200002)73:2<109::aid-jso10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 27.Trevisani F, De Notariis S, Rossi C, Bernardi M. Randomized control trials on chemoembolization for hepatocellular carcinoma: is there room for new studies? J Clin Gastroenterol. 2001;32:383–389. doi: 10.1097/00004836-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005;172:367–379. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishizawa T, Hasegawa K, Kokudo N, Sano K, Imamura H, Beck Y, Sugawara Y, Makuuchi M. Risk factors and management of ascites after liver resection to treat hepatocellular carcinoma. Arch Surg. 2009;144:46–51. doi: 10.1001/archsurg.2008.511. [DOI] [PubMed] [Google Scholar]
- 30.Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, Visa J, Bru C, Rodés J. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–1022. doi: 10.1016/s0016-5085(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 31.Tajiri K, Shimizu Y. Practical guidelines for diagnosis and early management of drug-induced liver injury. World J Gastroenterol. 2008;14:6774–6785. doi: 10.3748/wjg.14.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maithel SK, Kneuertz PJ, Kooby DA, Scoggins CR, Weber SM, Martin RC, McMasters KM, Cho CS, Winslow ER, Wood WC, et al. Importance of low preoperative platelet count in selecting patients for resection of hepatocellular carcinoma: a multi-institutional analysis. J Am Coll Surg. 2011;212:638–648; discussion 648-650. doi: 10.1016/j.jamcollsurg.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang CC, Iyer SG, Low JK, Lin CY, Wang SH, Lu SN, Chen CL. Perioperative factors affecting long-term outcomes of 473 consecutive patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2009;16:1832–1842. doi: 10.1245/s10434-009-0448-y. [DOI] [PubMed] [Google Scholar]
- 34.Cucchetti A, Piscaglia F, Grigioni AD, Ravaioli M, Cescon M, Zanello M, Grazi GL, Golfieri R, Grigioni WF, Pinna AD. Preoperative prediction of hepatocellular carcinoma tumour grade and micro-vascular invasion by means of artificial neural network: a pilot study. J Hepatol. 2010;52:880–888. doi: 10.1016/j.jhep.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 35.Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229–235. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, Chung AY, Ooi LL, Tan SB. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254:108–113. doi: 10.1097/SLA.0b013e31821ad884. [DOI] [PubMed] [Google Scholar]
- 37.Kumar M, Sakhuja P, Kumar A, Manglik N, Choudhury A, Hissar S, Rastogi A, Sarin SK. Histological subclassification of cirrhosis based on histological-haemodynamic correlation. Aliment Pharmacol Ther. 2008;27:771–779. doi: 10.1111/j.1365-2036.2008.03653.x. [DOI] [PubMed] [Google Scholar]


